Published online Feb 28, 2015. doi: 10.3748/wjg.v21.i8.2568
Peer-review started: September 15, 2014
First decision: October 14, 2014
Revised: October 18, 2014
Accepted: November 11, 2014
Article in press: November 11, 2014
Published online: February 28, 2015
Processing time: 166 Days and 18.7 Hours
Sorafenib, a potent multikinase inhibitor, lead to a significant improvement in progression free survival and overall survival in patients with advanced hepatocellular carcinoma (HCC). Though sorafenib has proven its efficacy in advanced stage HCC, there are limited reports on the role of sorafenib allowing for curative treatment by down-staging. We herein report a case of advanced HCC with vascular invasion, which showed treatment response by sorafenib therapy as to allow for radiofrequency ablation as curative treatment. The patient was followed-up for 6 mo without recurrence with continued sorafenib therapy.
Core tip: Though sorafenib is well known to efficacy in advanced hepatocellular carcinoma (HCC), the consensus of its role as down-staging is limited. Depending on response after sorafenib therapy, active strategy should be needed to offer chance for cure in advanced stage HCC.
- Citation: Park JG, Park SY, Lee HW. Complete remission of advanced hepatocellular carcinoma by radiofrequency ablation after sorafenib therapy. World J Gastroenterol 2015; 21(8): 2568-2572
- URL: https://www.wjgnet.com/1007-9327/full/v21/i8/2568.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i8.2568
Hepatocellular carcinoma (HCC) ranks fifth most common malignant tumor globally accounting for third most common cause of cancer-related death[1]. However, only 30% to 40% of patients are diagnosed in the early stage of HCC, which is eligible for curative treatment such as surgery, radiofrequency ablation (RFA), percutaneous ethanol injection, and liver transplantation[2]. Majority of HCC patients are still diagnosed late in advanced stage, in which only sorafenib is regarded as a standard therapy[3]. Although sorafenib therapy has shown significant survival benefit in patients with advanced HCC, overall survival is still unsatisfactory, especially in Asian countries[4]. However, as a potent multi-tyrosine-kinase inhibitor, sorafenib showed remarkable treatment response in selected patients[4-6]. Currently there are no treatment strategies for patients who are downstaged by sorafenib as to get allowed for locoregional therapies as curative treatment. We herein report a case of advanced HCC with vascular invasion, which were completely treated by RFA after downstaging by sorafenib therapy.
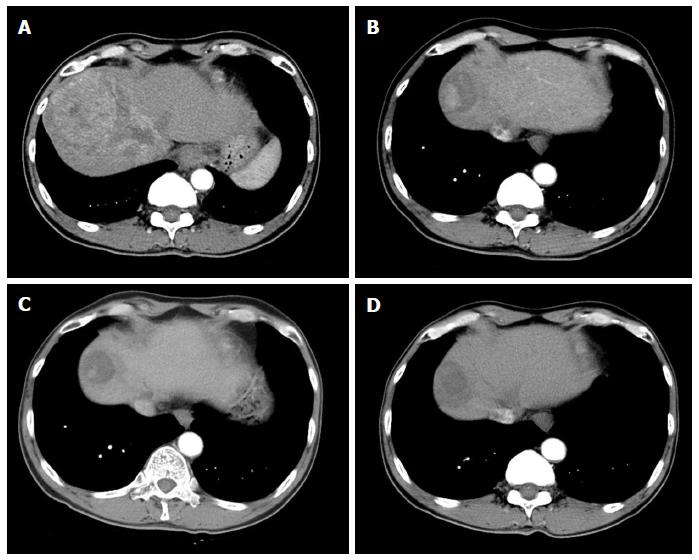
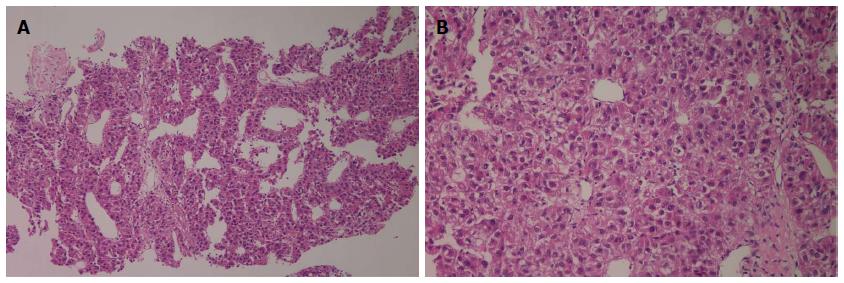
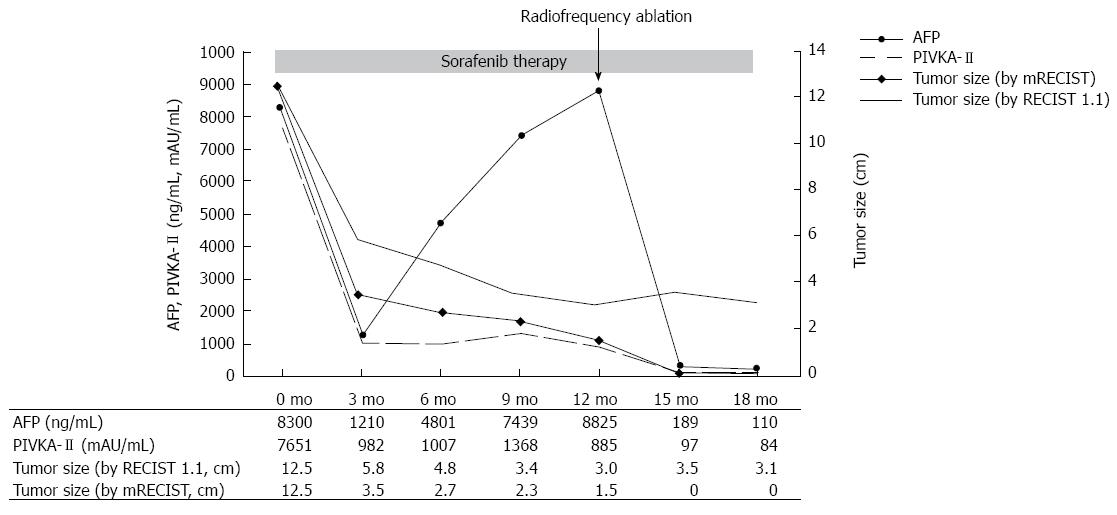
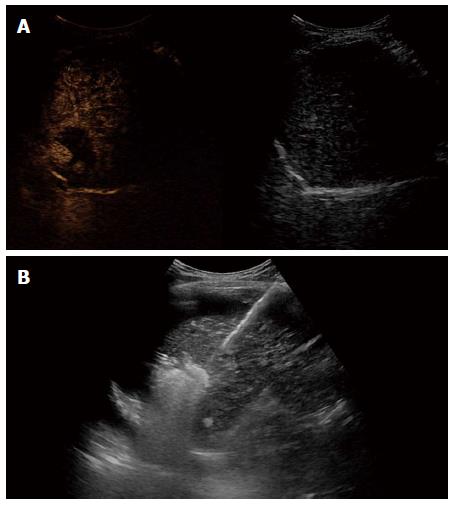
A 59-year-old male patient was referred to Kyungpook National University Hospital for evaluation of liver mass on abdominal ultrasound. He had history of chronic hepatitis B, which wasnever evaluated or treated. Laboratory findings were as follows: White blood cells, 3900/mm3, hemoglobin, 11.4 g/dL, platelet, 200000/μL, aspartate aminotransferase, 54 IU/L, alanine aminotransferase, 77 IU/L, total bilirubin, 0.22 mg/dL, albumin, 3.2 g/dL, prothrombin time, 11.8 s. Virologic tests revealed positive HBsAg and HBeAg with hepatitis B virus (HBV) DNA 718742 IU/mL by real-time polymerase chain reaction (Roche diagnostics, Basel, Switzerland). Serum alpha-fetoprotein (AFP) level and protein induced by vitamin K absence or antagonist-II (PIVKA-II) level were 8300 ng/mL and 7651 mAU/mL, respectively. Dynamic multiphasic abdominal computed tomogram (CT) scans revealed a 12.5 cm sized huge arterial enhancing mass with tumor thrombus in right and middle hepatic vein extending to intrahepatic inferior vena cava (Figure 1). We performed ultrasound guided needle biopsy of hepatic mass and confirmed HCC histologically (Figure 2). There was no evidence of distance metastasis in chest, brain, and Positron emission tomography (PET)-CT scans of whole body. He was treated with sorafenib (Nexavar; Bayer Healthcare Pharmaceuticals, Leverkusen, Germany) 400mg twice a day and tenofovir (Viread; Gilead, CA, United States) 300 mg once a day. After 6 mo of sorafenib therapy, tumor size was decreased to 4.8 cm with 2.7 cm sized arterial enhancing viable portion within tumor mass. The tumor thrombosis in hepatic vein and portal vein disappeared with thin streaky low density lesion in middle hepatic vein. Serum AFP and PIVKA-II level were markedly decreased to 1210 ng/mL and 982 mAU/mL. In contrast to serum PIVKA-II level and tumor size which remained stable, serum AFP level started to increase in 6 mo after sorafenib therapy (Figure 3). In 12 mo after sorafenib therapy, abdominal CT scans revealed a 3 cm sized tumor in liver dome within which a 1.5 cm sized arterial enhancing nodule are observed. After confirming viable tumor by contrast (Sonovue; Bracco, Italy) enhanced ultrasound, percutaneous ultrasound guided RFA was performed with assisting by artificial ascites (Figure 4). Post-RFA abdominal CT scan showed no enhancing lesions in liver with normalization of serum AFP and PIVKA-II levels. Up to 6 mo after RFA, there was no sign of residual viable tumor without complication and serum AFP and PIVKA-II levels were stable.
Efficacy of sorafenib in advanced HCC was confirmed in two large randomized, double-blinded, controlled trials[3,4]. However, there were only limited cases of clinical response in these clinical trials, which is unsatisfactory to clinicians as well as patients in practice. Currently, there are several investigations ongoing for better outcome of sorafenib in patients with unresectable HCC. Strategies to improve the outcomes of sorafenib include combination with transarterial chemoembolization, other chemotherapeutic agents, and radiation therapy[2,7-11]. However the benefits of these treatments are marginal and unsatisfactory and some of studies are still awaited.
The present case shows the possible role of sorafenib as down-staging advanced HCC allowing for curative treatment such as surgical resection or locoregional treatments. There is a case report in which sorafenib allowed surgical resection by down-staging the tumor in patients with advanced HCC[12]. In present case, we performed RFA as a minimally invasive treatment modality for complete treatment of tumor because contrast enhanced ultrasound could help confirming arterial enhancing viable tumor portion by realtime imaging. In addition, tumors in liver dome could be safely visualized and ablated by inducing artificial ascites during RFA procedure[13]. We kept continuing sorafenib therapy supposing that sorafenib showed very good treatment response in present case and tumor markers did not returned to normal values completely, which reflects the possibility of micrometastasis of tumor cells in remnant liver.
There are cases reporting complete remission of advanced HCC after sorafenib therapy[5,12,14-16]. However, these cases are extremely rare in clinical practice and there are no reports on the long-term treatment outcome in these patients. Therefore, in cases of downstaging by sorafenib, it might be more practical and desirable strategy to adopt treatment options in earlier stage which offer better treatment outcome. In this case, good treatment response was predictable by rapid decrease of serum tumor markers, which is consistent with previous studies[5,17]. In present case, rapid drop of serum AFP after RFA explains the surge of serum AFP level after 6 mo originated from viable tumor portion in main tumor mass. The present case also suggests the role of tumor markers in judging and predicting treatment response during sorafenib therapy along with radiologic follow-up imaging studies[17,18].
In conclusion, this report demonstrates the possible role of sorafenib to downstage advanced HCC for locoregional therapy achieving complete remission. Therefore, in a patients who shows treatment response by radiologic imaging studies and serum tumor markers after therapy, active treatment strategies for complete remission should be considered for the chance of long-term disease free survival.
A 59-year-old male with a history of chronic hepatitis B referred for evaluation of hugh liver mass on ultrasound.
Liver was palpable on right upper area of abdomen.
Hepatocellular carcinoma, cholangiocarcinoma.
White blood cell, 3900/mm3, hemoglobin, 11.4 g/dL, platelet, 200000/μL, AST, 54 IU/L, ALT, 77 IU/L, total bilirubin, 0.22 mg/dL, albumin, 3.2 g/dL, PT, 11.8 s; Virologic tests: HBsAg (+), HBeAg(+) and HBV DNA 718742 IU/mL; Tumor marker: alpha-fetoprotein (AFP) 8300 ng/mL, PIVKA-II7651 mAU/mL.
Dynamic multiphasic abdominal computed tomography scans revealed a 12.5 cm sized huge arterial enhancing mass with tumor thrombus in right and middle hepatic vein extending to intrahepatic inferior vena cava.
Ultrasound guided needle biopsy of hepatic mass revealed hepatocellular carcinoma with Edmonson-Steiner’s grade III showing psedoglandular or trabecular pattern.
The patient was treated with radiofrequency ablation following sorafenib therapy.
There are limited reports on the role of sorafenib allowing for curative treatment by down-staging.
Depending on response after sorafenib therapy, active strategy should be needed to offer chance for cure in advanced stage hepatocellular carcinoma (HCC).
Though complete remission was based on radiological diagnosis, response of serum AFP level predict prognosis of patients with HCC.
P- Reviewer: Jin B, Morales-Gonzalez JA, Pan GD S- Editor: Qi Y L- Editor: A E- Editor: Zhang DN
| 1. | Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74-108. [PubMed] |
| 2. | Marrero JA. Multidisciplinary management of hepatocellular carcinoma: where are we today? Semin Liver Dis. 2013;33 Suppl 1:S3-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 3. | Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9016] [Cited by in RCA: 10271] [Article Influence: 604.2] [Reference Citation Analysis (2)] |
| 4. | Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3854] [Cited by in RCA: 4653] [Article Influence: 273.7] [Reference Citation Analysis (0)] |
| 5. | Ahn SY, Lee HS, Kweon YO, Tak WY, Park SY. Sustained remission over 36 months of advanced hepatocellular carcinoma after short-term sorafenib therapy. Dig Dis Sci. 2013;58:1428-1432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Curtit E, Thiery-Vuillemin A, Nguyen T, Heyd B, Pivot X, Di Martino V, Borg C. Complete histologic response induced by sorafenib in advanced hepatocellular carcinoma: a case report. J Clin Oncol. 2011;29:e330-e332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 7. | Pawlik TM, Reyes DK, Cosgrove D, Kamel IR, Bhagat N, Geschwind JF. Phase II trial of sorafenib combined with concurrent transarterial chemoembolization with drug-eluting beads for hepatocellular carcinoma. J Clin Oncol. 2011;29:3960-3967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 246] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 8. | Abou-Alfa GK, Johnson P, Knox JJ, Capanu M, Davidenko I, Lacava J, Leung T, Gansukh B, Saltz LB. Doxorubicin plus sorafenib vs doxorubicin alone in patients with advanced hepatocellular carcinoma: a randomized trial. JAMA. 2010;304:2154-2160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 333] [Cited by in RCA: 340] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 9. | Dal Lago L, D’Hondt V, Awada A. Selected combination therapy with sorafenib: a review of clinical data and perspectives in advanced solid tumors. Oncologist. 2008;13:845-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 84] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 10. | Dawson LA. Overview: Where does radiation therapy fit in the spectrum of liver cancer local-regional therapies? Semin Radiat Oncol. 2011;21:241-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 11. | Zhu AX, Blaszkowsky LS, Ryan DP, Clark JW, Muzikansky A, Horgan K, Sheehan S, Hale KE, Enzinger PC, Bhargava P. Phase II study of gemcitabine and oxaliplatin in combination with bevacizumab in patients with advanced hepatocellular carcinoma. J Clin Oncol. 2006;24:1898-1903. [PubMed] |
| 12. | Irtan S, Chopin-Laly X, Ronot M, Faivre S, Paradis V, Belghiti J. Complete regression of locally advanced hepatocellular carcinoma induced by sorafenib allowing curative resection. Liver Int. 2011;31:740-743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 61] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 13. | Park SY, Tak WY, Jeon SW, Cho CM, Kweon YO, Kim SK, Choi YH. The efficacy of intraperitoneal saline infusion for percutaneous radiofrequency ablation for hepatocellular carcinoma. Eur J Radiol. 2010;74:536-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Kim MS, Jin YJ, Lee JW, Lee JI, Kim YS, Lee SY, Chae MH. Complete remission of advanced hepatocellular carcinoma by sorafenib: A case report. World J Gastrointest Oncol. 2013;5:38-42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 15. | Kim R, Aucejo F. Radiologic complete response with sirolimus and sorafenib in a hepatocellular carcinoma patient who relapsed after orthotopic liver transplantation. J Gastrointest Cancer. 2011;42:50-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Wang SX, Byrnes A, Verma S, Pancoast JR, Rixe O. Complete remission of unresectable hepatocellular carcinoma treated with reduced dose of sorafenib: a case report. Target Oncol. 2010;5:59-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Personeni N, Bozzarelli S, Pressiani T, Rimassa L, Tronconi MC, Sclafani F, Carnaghi C, Pedicini V, Giordano L, Santoro A. Usefulness of alpha-fetoprotein response in patients treated with sorafenib for advanced hepatocellular carcinoma. J Hepatol. 2012;57:101-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 181] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 18. | Riaz A, Ryu RK, Kulik LM, Mulcahy MF, Lewandowski RJ, Minocha J, Ibrahim SM, Sato KT, Baker T, Miller FH. Alpha-fetoprotein response after locoregional therapy for hepatocellular carcinoma: oncologic marker of radiologic response, progression, and survival. J Clin Oncol. 2009;27:5734-5742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 177] [Article Influence: 11.1] [Reference Citation Analysis (0)] |