Published online Feb 28, 2015. doi: 10.3748/wjg.v21.i8.2303
Peer-review started: July 25, 2014
First decision: August 27, 2014
Revised: October 29, 2014
Accepted: December 16, 2014
Article in press: December 16, 2014
Published online: February 28, 2015
Processing time: 219 Days and 5.5 Hours
Most gastrointestinal stromal tumors (GISTs) are characterized by KIT or platelet-derived growth factor alpha (PDGFRA) activating mutations. However, there are still 10%-15% of GISTs lacking KIT and PDGFRA mutations, called wild-type GISTs (WT GISTs). Among these so-called WT GISTs, a small subset is associated with succinate dehydrogenase (SDH) deficiency, known as SDH-deficient GISTs. In addition, GISTs that occur in Carney triad and Carney-Stratakis syndrome represent specific examples of SDH-deficient GISTs. SDH-deficient GISTs locate exclusively in the stomach, showing predilection for children and young adults with female preponderance. The tumor generally pursues an indolent course and exhibits primary resistance to imatinib therapy in most cases. Loss of succinate dehydrogenase subunit B expression and overexpression of insulin-like growth factor 1 receptor (IGF1R) are common features of SDH-deficient GISTs. In WT GISTs without succinate dehydrogenase activity, upregulation of hypoxia-inducible factor 1α may lead to increased growth signaling through IGF1R and vascular endothelial growth factor receptor (VEGFR). As a result, IGF1R and VEGFR are promising to be the novel therapeutic targets of GISTs. This review will update the current knowledge on characteristics of SDH-deficient GISTs and further discuss the possible mechanisms of tumorigenesis and clinical management of SDH-deficient GISTs.
Core tip: Succinate dehydrogenase (SDH) deficiency occurs in about 5%-7.5% of gastrointestinal stromal tumors (GISTs). These so-called SDH-deficient GISTs lack KIT and PDGFRA mutations. Such type of GISTs has its own clinical, morphological and molecular characteristics. The accumulation of hypoxia-inducible factor 1α and the upregulation of its downstream molecules, such as insulin-like growth factor 1 and vascular endothelial growth factor receptor, may play important roles in the tumorigenesis of SDH-deficient GISTs. They are promising to be the novel therapeutic targets of GISTs.
- Citation: Wang YM, Gu ML, Ji F. Succinate dehydrogenase-deficient gastrointestinal stromal tumors. World J Gastroenterol 2015; 21(8): 2303-2314
- URL: https://www.wjgnet.com/1007-9327/full/v21/i8/2303.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i8.2303
Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal tumors of the gastrointestinal tract. They are most common in the stomach (50%-60%) and small intestine (30%-35%) and are less frequent in the colon and rectum (5%) and the esophagus (< 1%)[1]. Furthermore, GISTs are able to grow within the abdominal cavity, usually in the omentum, mesentery or the retroperitoneum (< 5% of all GISTs)[2]. Most GISTs are driven by gain-of-function mutations in KIT (75%-80%) or PDGFRA (10%)[3-6]. In addition, 10%-15% of GISTs do not have detectable KIT or PDGFRA mutations and are called KIT/PDGFRA wild-type GISTs. It has been reported that succinate dehydrogenase subunit (SDHA, SDHB, SDHC and SDHD) mutations, neurofibromatosis 1 (NF1) gene mutations, retrovirus-associated DNA sequences (RAS) family mutations (HRAS, NRAS, KRAS) and BRAF mutations may contribute to these so-called “wild-type” tumors[7-10]. Among these, SDH-deficient GISTs account for between 5% and 7.5% of all unselected apparently sporadic gastric GISTs, including the majority of pediatric GISTs (<18 years old) and a small proportion of young adult GISTs (18-30 years old)[11,12]. The GISTs that occur in Carney triad (the non-familial association of gastric GISTs, pulmonary chondroma and extra-adrenal paraganglioma) and Carney-Stratakis syndrome (the familial association of GISTs and paraganglioma) represent specific examples of SDH-deficient GISTs[11-15].
Succinate dehydrogenase (succinate-coenzyme Q reductase or mitochondrial complex II) consists of four subunit proteins (SDHA, SDHB, SDHC and SDHD) and two succinate dehydrogenase assembly factors (SDHAF1 and SDHAF2)[16]. SDHA and SDHB comprise the catalytic component of the succinate-coenzyme Q reductase, whereas SDHC and SDHD comprise the anchoring component which attaches the complex to the inner mitochondrial membrane. SDHAF1 may be associated with SDHB and may be involved in the insertion or retention of the complex II Fe-S center; SDHAF2 is required for insertion of the FAD co-factor into SDHA[16,17]. The SDH localized in the inner mitochondrial membrane plays an integral role in cellular metabolism. This complex acts at the interphase of the tricarboxylic acid cycle and electron transport chain and catalyzes the oxidation of succinate to fumarate[18,19]. Deficiencies of complex II are responsible for many diseases, including hereditary paragangliomas and pheochromocytomas, a specific group of renal cell carcinomas, a subset of Leigh syndrome, infantile leukoencephalopathy and a certain type of GISTs. In 2006, one study reported a family with multiple paragangliomas (including a patient with GIST) that carried a germline SDHB mutation; the authors proposed the relationship between GISTs and SDH mutations for the first time[20]. A year later, McWhinney et al[21] identified six germline SDHB, SDHC, and SDHD mutations in GIST patients with the Carney-Stratakis syndrome. Then, numerous studies confirmed the SDHA, SDHB, SDHC, and SDHD mutations in GIST patients[22-26].
The insulin-like growth factor (IGF) system, which is composed of IGF ligands (IGF1 and IGF2), receptors (IGFR and the insulin receptor) and six regulatory IGF-binding proteins (IGFBP1-6), plays a critical role in the growth and development of many tissues and regulates overall cell growth[27]. The IGF1 receptor (IGF1R) is a transmembrane receptor that interacts with both IGF1 and IGF2, and has been proven to be necessary for transforming several oncogenes[28-30]. It has also been reported that IGF1 has the potential to stimulate the proliferation of tumor cells in vitro[31]. Genetic manipulations that reduced IGF signaling, such as by IGF1 gene knockout or by growth hormone (GH) antagonist transgene, can lead to decreased tumor growth in mouse models[32,33]. In addition, epidemiological evidence indicates that the IGF1 levels influence cancer risk and/or cancer prognosis[34,35]. We will now summarize the clinical traits and biological events contributing to the tumorigenesis of SDH-deficient GISTs and the rationale for antineoplastic agents.
SDH-deficient GISTs account for 5% to 7.5% of all unselected GISTs[11,12]. These tumors have a tendency to appear in children and young adults. Nearly all gastric GISTs patients < 18 years old and a substantial percentage of patients < 30 years old belonged to this group. However, the older adult patients rarely displayed an SDH-deficient pattern. Additionally, there was a female predominance greater than 2:1, although the gender distribution was equal in age groups > 30 years[36].
SDH-deficient GISTs occur exclusively in the stomach, and although any part of the stomach can be involved, there is some predilection to the distal stomach and antrum. Common clinical manifestations of SDH-deficient GISTs are similar to other gastric tumors, including gastrointestinal bleeding and epigastric discomfort. Occasional patients who were diagnosed with SDH-deficient GISTs showed symptoms related to metastatic tumors in the abdomen or liver[37]. In general, these tumors pursue an indolent course and may sometimes be fatal. Given their lack of oncogenic activating tyrosine kinase mutations, SDH-deficient GISTs show consistent primary resistance to imatinib therapy[11-14,38,39].
Carney’s triad, which is commonly found in girls and young women, is a rare non-heritable syndrome consisting of gastric GIST, paraganglioma, and pulmonary chondroma[39-41]. KIT or PDGFRA mutations were undetectable in GISTs associated with Carney’s triad, although the loss of expression of SDHB can be observed within the tumors[14,42]. Carney-Stratakis syndrome is characterized by the development of gastric GISTs and paragangliomas. Unlike Carney’s triad, it is inherited in an autosomal dominant manner and affects both men and women[43]. These tumors also lack KIT and PDGFRA mutations and have been proven to be SDH-deficient, according to immunohistochemical staining results, which indicates defects in SDHB[13,22]. Moreover, in a series of 11 patients with Carney-Stratakis syndrome, eight were found to harbor germline mutations in SDH genes (5 SDHB, 2 SDHC, and 1 SDHD)[22], whereas GISTs of Carney triad do not harbor SDHA, SDHB, SDHC, or SDHD mutations[42,44]. Pediatric GISTs, which account for 1% to 2% of GISTs, often occur in young girls. The majority of pediatric GISTs, despite showing strong KIT immunoreactivity, lack KIT and PDGFRA mutations and display a characteristic similar to GISTs associated with Carney triad and Carney-Stratakis syndrome patients[45]. Recently, it was recognized that occasional GISTs in adults, so-called “pediatric-type” GISTs, show virtually identical features to those observed in pediatric patients[38,46]. Thus, the clinical features of Carney triad, Carney-Stratakis syndrome, pediatric GISTs, and adult pediatric-type GISTs are strikingly similar. Therefore, we can reason that these GISTs are similar because they are all SDH-deficient.
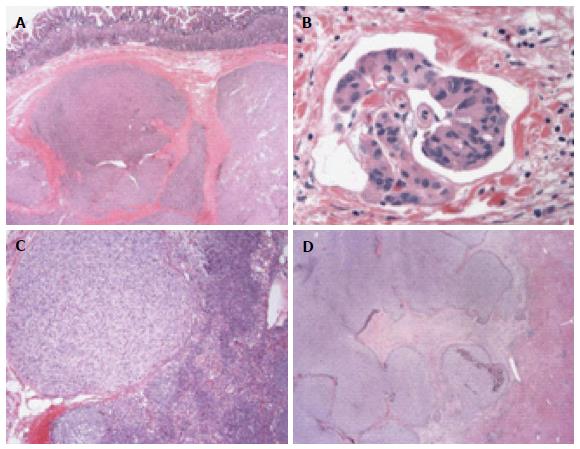
As for morphological traits, SDH-deficient GISTs are typically described as multinodular or, sometimes, bilobed masses, often divided by apparent fibrous septa. Ulceration was frequently found in such GISTs. Multifocal disease, lymphovascular invasion around the tumor nodules and lymph node metastases are also common, whereas these features are extraordinarily rare in conventional KIT-mutant GISTs (Figure 1). In contrast to pure spindle cells in adult mutant GISTs, SDH-deficient GISTs are often composed of epithelioid or mixed epithelioid and spindled cells[12,17,25,47,48] (Figure 2).
Loss of SDHB expression is a consistent feature of SDH-deficient GISTs, whereas SDHB expression is intact in KIT-mutant GISTs[11,12,49]. As mentioned above, most pediatric GISTs, as well as a small proportion of young adult GISTs, and GISTs in patients with Carney triad or Carney-Stratakis syndrome, have been found to be SDHB deficient[11,13,14]. Succinate dehydrogenase consists of four subunit proteins (SDHA, SDHB, SDHC and SDHD). Lack of any component of the mitochondrial complex II will result in the instability of the entire complex and the degradation of the SDHB subunit. Therefore, immunohistochemistry for SDHB becomes negative whenever there is a mutation/inactivation of SDHA, SDHB, SDHC or SDHD; negative staining for SDHB is now validated as a highly sensitive marker for germline mutations of any of the SDH subunits[11,14,48,50,51].
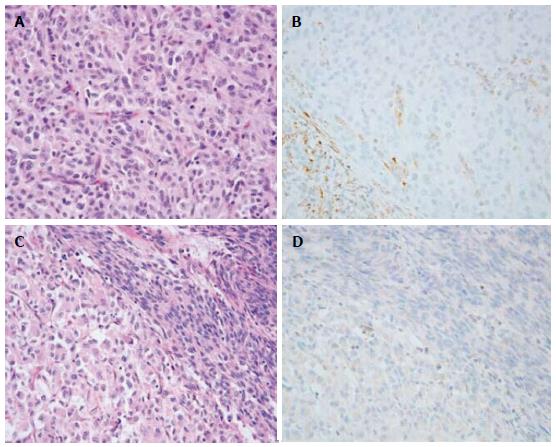
In 2012, Chou et al[52] proposed that IGF1R overexpression is a feature of SDH-deficient GISTs. They assessed SDHB and IGF1R expression by immunohistochemistry in eight confirmed SDH-deficient GISTs, three GISTs arising in the setting of neurofibromatosis type 1 syndrome and 40 unselected GISTs. Selected KIT and PDGFRA exons were amplified and sequenced. All eight SDH-deficient tumors were wild-type for KIT and PDGFRA, SDHB negative and demonstrated IGF1R overexpression. The three neurofibromatosis-related tumors were SDHB positive and IGF1R negative. Of the 40 unselected GISTs, five were wild-type for KIT and PDGFRA in the selected exons. Two of the wild-type GISTs were SDHB negative and showed IGF1R overexpression; three were SDHB positive and IGF1R negative. There are still some shortcomings in their study; for example, the exon 12 and exon 14 of PDGFRA were not sequenced, and their sample size is very small. However, when Corless et al[53] sequenced 1105 GISTs, only 11 (1%) were found to harbor PDGFRA exon 12 mutations, and only 3 (0.3%) were found to harbor exon 14 mutations[53]. Therefore, the results are unlikely to affect their basic conclusion that IGF1R overexpression is a feature of SDH-deficient GISTs. The limited sample size was corrected by a study carried out by Lasota et al[54]; in their study, IGF1R expression was examined immunohistochemically in 1078 well-characterized GISTs representing different clinicogenetic categories and 103 non-GIST gastrointestinal tumors. IGF1R expression was detected in 71/80 (89%) of SDH-deficient GISTs (SDHB-negative GISTs), but only in 9/625 (1%) of the SDHB-positive gastric GISTs. In addition, several studies support this conclusion, showing that IGF1R is highly expressed in wild-type GISTs and pediatric GISTs[55-57]. However, there are still some differences in these studies; the study by Pantaleo MA and colleagues[56] showed that wild-type GISTs have a higher level of amplification of the IGF1R gene compared with mutants and that there are no mutations in the IGF1R gene in wild-type GISTs. However, another study by Janeway et al[57] indicated that IGF1R gene amplification was not detected in pediatric wild-type GISTs. Two immunohistochemical studies by Rios-Moreno et al[58] and Braconi et al[59] are discordant with the former findings. In Rios-Moreno’s study, among the 22 IGF1R-positive samples examined, 82% (18/22) had a KIT mutation, 14% (3/22) had a PDGFRA mutation, and only 4% (1/22) were wild-type KIT/PDGFRA. In Braconi’s study, immunohistochemistry was performed on 13 wild-type GISTs and 81 mutant GISTs to detect the expression of IGF1R. They found that IGF1R was strongly expressed in the cytoplasm of all GISTs. However, both of the studies used a polyclonal anti-IGF1R antibody for immunohistochemistry. This polyclonal anti-IGF1R antibody has now been shown to lack specificity for this receptor. For example, it can produce multiple non-specific bands on Western blot and stain positively in cell lines derived from IGF1R knockout mice[60]. Therefore, it is likely that the non-specific (false-positive) staining of the polyclonal anti-IGF1R antibody used in the previous two studies accounts for the apparent discrepancy. Recently, Nannini et al[61] found that IGF1R was upregulated in all patients harboring SDH mutations or displaying SDH dysfunction, with respect to KIT/PDGFRA wild-type GISTs without SDH mutations. This report confirmed that IGF1R overexpression in KIT/PDGFRA wild-type GISTs could be driven by the loss of function of the SDH mitochondrial complex.
Braconi et al[59] investigated the immunohistochemical expression of IGF1 and IGF2 in 94 samples of GISTs and found that strong IGF expression significantly correlated with a higher mitotic index, larger tumor size, a higher risk for metastases and relapsed GISTs. Another study by Gu et al[62] showed similar results. Although the samples of these two studies were unselected GISTs rather than SDH-deficient GISTs, they are able to clarify the important role of IGF in GIST development. Consequently, the IGF family (IGF and its receptors) provides valuable clues for the treatment of GISTs.
As early as 2004, Antonescu et al[63] determined the variation of gene expression in 28 GIST samples from 24 patients and showed that gene expression was different between wild-type and mutant GISTs. Strong gene expression of vascular endothelial growth factor (VEGF), macrophage colony stimulating factor, and BCL2 was found in the wild-type group while overexpression of these genes was not observed in mutant GISTs. Variations in gene expression between spindle and epithelioid GIST cells also follow a similar pattern. Most of the gastric epithelioid GISTs lack KIT mutations, whereas genes associated with the epithelial cell phenotype (TP73L and Keratin1) were detectable. Compared with the spindle cells, genes involved in apoptosis (BCL2 and Caspase 10), angiogenesis (VEGF) and proliferation (PDGF1) were up-regulated in the epithelioid GIST cells. In addition, there was a remarkable difference in gene expression between stomach and small bowel GISTs. A number of genes involved in muscle contraction and development were found to be differentially expressed in these two anatomical sites. Because SDH-deficient GISTs were not well recognized at that time, we can speculate that these differences are most likely caused by SDH dysfunction.
Additionally, positive KIT, DOG1/Ano1 and CD34 were observed in the majority of SDH-deficient GISTs by immunohistochemistry while muscle markers (SMA and desmin) were rarely detected. None of the tumors were S100 protein positive[12].
Although the etiology of SDH deficiency in these tumors remains unclear, the relationship between SDH deficiency and SDH gene mutation is well known. Many studies have identified the germline mutations of SDHB (IVS1+1 G→T or c.72+1G>T; IVS4+1G>C or c.423+1G>C; c.423+1 G→C; c.45_46insCC), SDHC (c.43+1 C→T; IVS5+1 G→A), and SDHD (c.57delG) in patients with Carney-Stratakis syndrome[21,22]. It is worth noting that these patients did not have KIT or PDGFRA mutations. Recently, despite the complexity of its locus (15 exons) and the presence of three pseudogenes, SDHA was analyzed and the nonsense or missense mutations in exon 2 (c.91C>T), exon 5 (c.553C>T), exon 8 (c.1043-1055del; c.688delG; c.985C4T), exon 9 (c.1151C>G), exon 11 (c.1534C4T) and exon 13 (c.1765C>T) were found in KIT/PDGFRA WT GIST patients[23,25].
Celestino et al[64] studied a series of 25 apparently sporadic primary KIT/PDGFRA/BRAF wild-type GISTs occurring in patients without personal or familial history of paragangliomas (PGLs), finding that SDHB expression was absent in 20% of wild-type GISTs while SDHB germline mutations were detected in 12% of wild-type GISTs. The type of SDHB germline mutations includes promoter region or exon 1 deletion and point mutation. However, there are still many SDH-deficient GISTs that have no mutations in the gene coding for SDH subunits. To explore additional pathogenetic mechanisms in these GISTs, Kelly et al[65] investigated the post-transcriptional regulation of these tumors by conducting microRNA (miRNA) profiling of a mixed cohort of 73 cases, which included 18 gastric pediatric wild-type GISTs, 25 (20 gastric, 4 small bowel and 1 retroperitoneal) adult wild-type GISTs and 30 gastric adult mutant GISTs. Using this approach, they identified a cluster of miRNAs on 14q32 that show significantly different expression patterns among GISTs, which appears to be explained at least in part by differential allelic methylation of this imprinted region. Interestingly, some wild-type GISTs lack SDH gene mutations but show either a marked reduction or an absence of SDHB protein expression by immunohistochemistry and a corresponding loss of respiratory chain complex II enzymatic activity. Furthermore, SDHB, SDHC and SDHD mRNA levels in these GISTs are comparable with those in KIT-mutant GISTs, which suggests that SDHB downregulation occurs at the level of protein translation[4,62]. In addition, other possible mechanisms, including deficiency or alterations in proteins involved in stabilization of the SDH complex, should be considered. For example, loss of function mutations in SDHAF2 can also result in the destabilization of the SDH complex and a loss of complex II activity[66,67].
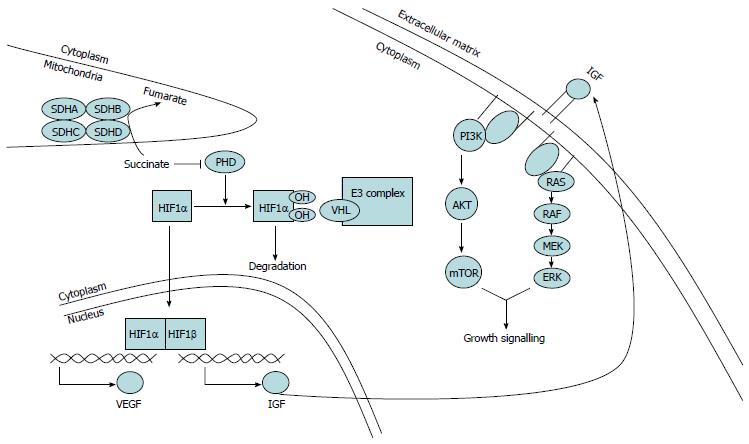
Succinate dehydrogenase is localized in the inner mitochondrial membrane and consists of four subunit proteins (SDHA, SDHB, SDHC and SDHD)[16]. The function of SDH is to act at the interphase of the tricarboxylic acid cycle and electron transport chain and catalyze the oxidation of succinate to fumarate[18,19]. This complex is an important regulator of hypoxia-inducible factor 1α (HIF1α), a subunit of HIF1. HIF1, a heterodimer of HIF1α and HIF1β, is a transcriptional activator of IGF2 and VEGF[68,69]. In normoxic conditions, HIF1α undergoes proteasomal degradation after ubiquitination by a ubiquitin ligase complex targeted at HIF1α by the VHL protein[70]. Binding of VHL to HIF1α requires hydroxylation of proline residues in HIF1α, which is mediated by the oxygen-dependent activity of a prolyl hydroxylase-domain (PHD) protein[71], an enzyme that also converts a ketoglutarate to succinate[68]. Because the activity of PHD is oxygen dependent, hypoxia leads to decreased activity of PHD, which, in turn, results in reducing the hydroxylation of HIF1α, a lack of VHL-mediated ubiquitination of HIF1α, and ultimately stabilization (and hence accumulation) of HIF1α[72]. When HIF1α accumulates, it migrates to the nucleus where it dimerizes with HIF1β to form an active transcription factor that induces expression of genes involved in glycolysis and angiogenesis (including IGF and VEGF)[73,74], thereby promoting the adaptation of cells to low oxygen by inducing neovascularization and glycolysis. The dysfunction of SDH leads to the accumulation of succinate, which in turn inhibits PHD activity, induces a pseudohypoxia phenomenon and promotes downstream gene expression (Figure 3). Consistent with the previous findings, HIF1α and VEGF expression is higher in wild-type GISTs and SDH-deficient tumors than in KIT-mutant GISTs[75-77]. Therefore, the upregulation of HIF1α may lead to enhanced growth signaling by increasing the combination of IGF and VEGF with their receptors (IGF1R and VEGFR) in SDH-deficient GISTs[2,68,78]. In addition, other factors may contribute to the development of SDH-deficient tumors. For example, SDH mutations may result in redox stress due to the increased production of reactive oxygen species, which is considered the oncogenic trigger of many tumors[76,79,80].
Previous studies have demonstrated that wild-type GISTs are characterized by IGF1R overexpression at both the mRNA and protein levels without genomic amplification[55-57]. It has been reported that SDH dysfunction may be related to tumorigenesis in GISTs via induction of a pseudohypoxic pathway involving HIF1α, which exerts its proliferative effects by activating key receptor tyrosine kinases such as IGF1R[68,81-84]. Therefore, it would be reasonable to speculate that the overexpression of IGF1R in SDH-deficient tumors occurs as a consequence of HIF1α activation induced by SDH dysfunction[61].
IGF1R is a transmembrane receptor that is activated through autophosphorylation once ligands (IGF1 or IGF2) bind, thereby leading to the activation of the phosphatidylinositol 3-kinase (PI3K) and mitogen-activated protein kinase (MAPK) cascades. In the PI3K pathway, AKT is phosphorylated by PI3K indirectly, which can promote cell survival via multiple effectors. AKT effectors that regulate growth include mammalian target of rapamycin (mTOR) within a complex, which enhances the translation of proteins involved in proliferation. Meanwhile, in a parallel pathway, the sequential activation of RAS, RAF, and MAPK isoforms ERKs, results in the transcription of genes that drive growth[85,86] (Figure 3). The overexpression of IGF1R has been identified in several tumor types and because of its role in cancer cell metabolism and the potential relevance to the survival of malignant cells, IGF1R has become a target for anticancer therapy[87-89]. In addition to IGF1R overexpression, IGF2 can exert its mitogenic and anti-apoptotic effects by binding to the IGF1R and the insulin receptor (IR) isoform A. In addition, there is evidence that IGF2 can lead to an autocrine stimulation loop in several tumor types[90-93]. Moreover, Rikhof et al[94] proved that IGF2 is commonly expressed in GISTs, suggesting that secreted IGF2 is involved in the pathogenesis of GISTs by providing a pro-survival signal in an autocrine manner.
GISTs are resistant to traditional chemotherapy but are responsive to the tyrosine kinase inhibitor imatinib[95,96]. However, GISTs that are deficient in succinate dehydrogenase generally respond poorly to imatinib because of a lack of activating tyrosine kinase mutations[7,95]. Consequently, it is of great importance to develop new therapeutic targets and novel specific drugs.
Because IGF1R overexpression is a feature of SDH-deficient GISTs, it seems to be a promising target for anticancer therapy. Currently, it is increasingly acknowledged that IGF1R is able to serve as a potential target for the treatment of various tumors. There are three target strategies: anti-receptor antibodies, anti-ligand antibodies and small-molecule receptor kinase inhibitors. All of these strategies have been used to target the IGF1R family, and certain drug candidates from each of classes were considered promising enough in preclinical models to be taken forwards to clinical trials[97,98].
All of the anti-receptor antibodies were designed to spare the insulin receptors because they all interfere with ligand binding to the IGFR. Although they lack interference with insulin binding, the use of these antibodies is associated with dangerous side effects, such as hyperglycaemia and hyperinsulinaemia[97]. Furthermore, because the pituitary attempts to compensate for the perceived lack of IGF biological activity, the use of these agents results in a remarkable increase in growth hormone (GH) secretion. This leads not only to increases in circulating IGF levels but also to insulin resistance induced by GH, which is responsible for the observed hyperglycaemia and hyperinsulinaemia in treated patients[97]. In addition, IGF (both IGF1 and IGF2) has been shown to increase islet growth[99]. When IGF1R is blocked, this effect is enhanced by the increased free IGF. It is also reported that IGF1 and GH could induce β-cell proliferation[100]. Similarly, the blockade of IGF1R is likely to promote β-cell proliferation. Thus, these mechanisms may further contribute to the side effects when using anti-receptor antibodies.
Anti-ligand antibodies have a high affinity against both IGF1 and IGF2. Consequently, ligand-antibody complexes would replace ligand-binding protein complexes in circulation (IGFBPs normally bind greater than 90% of circulating IGFs), resulting in high levels of free IGFBPs. There is evidence that free IGFBPs have antiproliferative activity that is independent of their IGF-binding capacity[101]. In addition, the autocrine loop of IGF2 cannot be interrupted by IGFIR-specific antibodies but could be inhibited by ligand-specific antibodies, leading to the reduction of the secretion of IGF. The small-molecule tyrosine kinase inhibitors tend to inhibit all members of the IGF1R family in vivo. However, such agents do not cause severe metabolic toxicity. At the dosages used, insulin receptor signaling is incompletely inactivated. Additionally, it seems that the drug concentrations in muscle are fairly low, which is a major insulin-stimulated glucose disposition site. Therefore, the function of the insulin receptor is fairly intact, leading to a modest, rather than a severe effect of these small-molecule kinase inhibitors on systemic glucose metabolism.
However, several studies suggest that these antineoplastic strategies did not work in all GISTs patients. Although several early phase clinical trials of the use of IGF1R-specific antibodies for cancer treatment raised enthusiasm, the results of the initial phase III in unselected patients have proven disappointing[102]. Therefore, comprehensive clinical trials and reasonable therapeutic targets for IGF1R remain to be carried out in vivo in the future.
Additionally, there is evidence that the PI3K-mTOR signaling pathway is one of the most important pathways in the growth of GIST cells[103]. Multiple medications targeting this pathway, such as Perifosine and Everolimus, are in clinical development[104]. However, as described in previous reviews, these targets are downstream not only of the IGF receptor but also of other receptor tyrosine kinases[102]. Thus, targeting these signaling nodes has the potential to not only inactivate certain important parts of the signaling networks downstream of the IGF family but also to decrease pivotal, proliferative survival signals that are initiated by other receptor tyrosine kinases. However, no drug candidates are expected to be tumor-specific, and all of them tend to inhibit downstream signaling of IGF family members in normal tissues. PI3K or AKT inhibition would be expected to lead to initial hyperglycemia and, secondarily, to compensatory hyperinsulinaemia. Metformin, a biguanide that is commonly used in the treatment of type 2 diabetes, can lower blood glucose by inhibiting gluconeogenesis and, secondarily, can reduce hyperinsulinaemia. In addition to the treatment of hyperglycemia and hyperinsulinaemia that are associated with IGF1R-targeting agents or PI3K inhibitors, metformin could also exert antineoplastic effects toward some tumors. Several experimental studies have demonstrated that metformin can reduce insulin-stimulated tumor growth in vivo. On one hand, it can reduce insulin levels and insulin receptor activation; on the other hand, it can decrease the effect of a high-energy diet on phosphorylation of AKT and the expression of fatty acid synthase[105,106]. Consequently, combining PI3K pathway inactivators or IGF family specific inhibitors with metformin seems to be a promising strategy for antineoplastic therapy, resulting in better treatment and fewer adverse effects.
Moreover, VEGFR targeted drugs, such as sunitinib, motesanib, sorafenib, regorafenib, vatalanib, pazopanib and the monoclonal antibody bevacizumab, have the potential to decrease tumor growth by inhibition of angiogenesis[4]. Sunitinib maleate (sunitinib malate; SU11248; SUTENT) is a small-molecule inhibitor of multiple receptor tyrosine kinases involved in cancer, including VEGFR, PDGFR and the KIT receptor[107]. It was approved by the US Food and Drug Administration for the treatment of GISTs after disease progression or intolerant to imatinib mesylate and advanced renal-cell carcinoma in January 2006. In addition, many studies have shown that for patients with imatinib-resistant/intolerant GIST, continuous daily sunitinib dosing appears to be an active alternative dosing strategy with acceptable safety[108].
SDH-deficient GISTs have gained increasing attention in recent years, but minimal molecular insight into its tumorigenesis and target therapy exists. In this review, we identified the features of SDH-deficient GISTs and further illustrated the possible mechanisms of tumorigenesis and clinical treatment of such GISTs. In SDH-deficient GISTs, SDH inactivation leads to the accumulation of HIF-1α, which dimerizes with HIF-1β in cell nuclei to form an intact HIF. HIF, acting as an active transcription factor, induces the expression of downstream genes, including IGF and VEGF. When these ligands combined with their receptors, a number of signaling pathways were activated and, finally, resulted in growth promotion and apoptosis inhibition of the tumor cells. Because of the important roles of IGF and VEGF in SDH-deficient GISTs, these molecules and their receptors have become potential therapeutic targets. However, many unknowns remain about such GISTs, including the reason why patients with SDH-deficient GISTs show differences in age, sex, position, phenotype, metastasis and pathological pattern compared with those with mutant GISTs. Moreover, the expression of IGF in SDH-deficient GISTs needs to be further studied. In addition, effective drugs and targeted therapy with better efficiency and fewer side effects need to be introduced via clinical trials. Finally, there certainly remains much to look forward to learning about this fascinating, newly recognized family of tumors.
P- Reviewer: Chen YC S- Editor: Yu J L- Editor: Wang TQ E- Editor: Zhang DN
| 1. | Joensuu H, Vehtari A, Riihimäki J, Nishida T, Steigen SE, Brabec P, Plank L, Nilsson B, Cirilli C, Braconi C. Risk of recurrence of gastrointestinal stromal tumour after surgery: an analysis of pooled population-based cohorts. Lancet Oncol. 2012;13:265-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 576] [Cited by in RCA: 669] [Article Influence: 47.8] [Reference Citation Analysis (0)] |
| 2. | Kim KH, Nelson SD, Kim DH, Choi KU, Kim SJ, Min KW, Jang KS, Paik SS, Oh YH, Chae SW. Diagnostic relevance of overexpressions of PKC-theta; and DOG-1 and KIT/PDGFRA gene mutations in extragastrointestinal stromal tumors: a Korean six-centers study of 28 cases. Anticancer Res. 2012;32:923-937. [PubMed] |
| 3. | Hirota S, Isozaki K, Moriyama Y, Hashimoto K, Nishida T, Ishiguro S, Kawano K, Hanada M, Kurata A, Takeda M. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279:577-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3215] [Cited by in RCA: 3104] [Article Influence: 115.0] [Reference Citation Analysis (0)] |
| 4. | Corless CL, Barnett CM, Heinrich MC. Gastrointestinal stromal tumours: origin and molecular oncology. Nat Rev Cancer. 2011;11:865-878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 635] [Cited by in RCA: 618] [Article Influence: 44.1] [Reference Citation Analysis (0)] |
| 5. | Heinrich MC, Corless CL, Duensing A, McGreevey L, Chen CJ, Joseph N, Singer S, Griffith DJ, Haley A, Town A. PDGFRA activating mutations in gastrointestinal stromal tumors. Science. 2003;299:708-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1712] [Cited by in RCA: 1717] [Article Influence: 78.0] [Reference Citation Analysis (0)] |
| 6. | Hirota S, Ohashi A, Nishida T, Isozaki K, Kinoshita K, Shinomura Y, Kitamura Y. Gain-of-function mutations of platelet-derived growth factor receptor alpha gene in gastrointestinal stromal tumors. Gastroenterology. 2003;125:660-667. [PubMed] |
| 7. | Yantiss RK, Rosenberg AE, Sarran L, Besmer P, Antonescu CR. Multiple gastrointestinal stromal tumors in type I neurofibromatosis: a pathologic and molecular study. Mod Pathol. 2005;18:475-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 90] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 8. | Kinoshita K, Hirota S, Isozaki K, Ohashi A, Nishida T, Kitamura Y, Shinomura Y, Matsuzawa Y. Absence of c-kit gene mutations in gastrointestinal stromal tumours from neurofibromatosis type 1 patients. J Pathol. 2004;202:80-85. [PubMed] |
| 9. | Agaram NP, Wong GC, Guo T, Maki RG, Singer S, Dematteo RP, Besmer P, Antonescu CR. Novel V600E BRAF mutations in imatinib-naive and imatinib-resistant gastrointestinal stromal tumors. Genes Chromosomes Cancer. 2008;47:853-859. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 313] [Cited by in RCA: 277] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 10. | Miranda C, Nucifora M, Molinari F, Conca E, Anania MC, Bordoni A, Saletti P, Mazzucchelli L, Pilotti S, Pierotti MA. KRAS and BRAF mutations predict primary resistance to imatinib in gastrointestinal stromal tumors. Clin Cancer Res. 2012;18:1769-1776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 443] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 11. | Gill AJ, Chou A, Vilain R, Clarkson A, Lui M, Jin R, Tobias V, Samra J, Goldstein D, Smith C. Immunohistochemistry for SDHB divides gastrointestinal stromal tumors (GISTs) into 2 distinct types. Am J Surg Pathol. 2010;34:636-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 153] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 12. | Miettinen M, Wang ZF, Sarlomo-Rikala M, Osuch C, Rutkowski P, Lasota J. Succinate dehydrogenase-deficient GISTs: a clinicopathologic, immunohistochemical, and molecular genetic study of 66 gastric GISTs with predilection to young age. Am J Surg Pathol. 2011;35:1712-1721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 255] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 13. | Gaal J, Stratakis CA, Carney JA, Ball ER, Korpershoek E, Lodish MB, Levy I, Xekouki P, van Nederveen FH, den Bakker MA. SDHB immunohistochemistry: a useful tool in the diagnosis of Carney-Stratakis and Carney triad gastrointestinal stromal tumors. Mod Pathol. 2011;24:147-151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 166] [Cited by in RCA: 142] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 14. | Janeway KA, Kim SY, Lodish M, Nosé V, Rustin P, Gaal J, Dahia PL, Liegl B, Ball ER, Raygada M. Defects in succinate dehydrogenase in gastrointestinal stromal tumors lacking KIT and PDGFRA mutations. Proc Natl Acad Sci USA. 2011;108:314-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 436] [Cited by in RCA: 462] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 15. | Gill AJ, Chou A, Vilain RE, Clifton-Bligh RJ. “Pediatric-type” gastrointestinal stromal tumors are SDHB negative (“type 2”) GISTs. Am J Surg Pathol. 2011;35:1245-127; author reply 1245-127;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 16. | Hoekstra AS, Bayley JP. The role of complex II in disease. Biochim Biophys Acta. 2013;1827:543-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 95] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 17. | Gill AJ. Succinate dehydrogenase (SDH) and mitochondrial driven neoplasia. Pathology. 2012;44:285-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 140] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 18. | Rutter J, Winge DR, Schiffman JD. Succinate dehydrogenase - Assembly, regulation and role in human disease. Mitochondrion. 2010;10:393-401. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 334] [Cited by in RCA: 291] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 19. | Gottlieb E, Tomlinson IP. Mitochondrial tumour suppressors: a genetic and biochemical update. Nat Rev Cancer. 2005;5:857-866. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 334] [Cited by in RCA: 291] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 20. | Bolland M, Benn D, Croxson M, McCall J, Shaw JF, Baillie T, Robinson B. Gastrointestinal stromal tumour in succinate dehydrogenase subunit B mutation-associated familial phaeochromocytoma/paraganglioma. ANZ J Surg. 2006;76:763-764. [PubMed] |
| 21. | McWhinney SR, Pasini B, Stratakis CA. Familial gastrointestinal stromal tumors and germ-line mutations. N Engl J Med. 2007;357:1054-1056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 165] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 22. | Pasini B, McWhinney SR, Bei T, Matyakhina L, Stergiopoulos S, Muchow M, Boikos SA, Ferrando B, Pacak K, Assie G. Clinical and molecular genetics of patients with the Carney-Stratakis syndrome and germline mutations of the genes coding for the succinate dehydrogenase subunits SDHB, SDHC, and SDHD. Eur J Hum Genet. 2008;16:79-88. [PubMed] |
| 23. | Pantaleo MA, Astolfi A, Indio V, Moore R, Thiessen N, Heinrich MC, Gnocchi C, Santini D, Catena F, Formica S. SDHA loss-of-function mutations in KIT-PDGFRA wild-type gastrointestinal stromal tumors identified by massively parallel sequencing. J Natl Cancer Inst. 2011;103:983-987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 109] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 24. | Pantaleo MA, Nannini M, Astolfi A, Biasco G. A distinct pediatric-type gastrointestinal stromal tumor in adults: potential role of succinate dehydrogenase subunit A mutations. Am J Surg Pathol. 2011;35:1750-1752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 25. | Wagner AJ, Remillard SP, Zhang YX, Doyle LA, George S, Hornick JL. Loss of expression of SDHA predicts SDHA mutations in gastrointestinal stromal tumors. Mod Pathol. 2013;26:289-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 93] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 26. | Miettinen M, Killian JK, Wang ZF, Lasota J, Lau C, Jones L, Walker R, Pineda M, Zhu YJ, Kim SY. Immunohistochemical loss of succinate dehydrogenase subunit A (SDHA) in gastrointestinal stromal tumors (GISTs) signals SDHA germline mutation. Am J Surg Pathol. 2013;37:234-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 133] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 27. | LeRoith D, Roberts CT. The insulin-like growth factor system and cancer. Cancer Lett. 2003;195:127-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 778] [Cited by in RCA: 788] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 28. | Sell C, Rubini M, Rubin R, Liu JP, Efstratiadis A, Baserga R. Simian virus 40 large tumor antigen is unable to transform mouse embryonic fibroblasts lacking type 1 insulin-like growth factor receptor. Proc Natl Acad Sci USA. 1993;90:11217-11221. [PubMed] |
| 29. | Sell C, Dumenil G, Deveaud C, Miura M, Coppola D, DeAngelis T, Rubin R, Efstratiadis A, Baserga R. Effect of a null mutation of the insulin-like growth factor I receptor gene on growth and transformation of mouse embryo fibroblasts. Mol Cell Biol. 1994;14:3604-3612. [PubMed] |
| 30. | Toretsky JA, Kalebic T, Blakesley V, LeRoith D, Helman LJ. The insulin-like growth factor-I receptor is required for EWS/FLI-1 transformation of fibroblasts. J Biol Chem. 1997;272:30822-30827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 189] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 31. | Osborne CK, Bolan G, Monaco ME, Lippman ME. Hormone responsive human breast cancer in long-term tissue culture: effect of insulin. Proc Natl Acad Sci USA. 1976;73:4536-4540. [PubMed] |
| 32. | Wu Y, Cui K, Miyoshi K, Hennighausen L, Green JE, Setser J, LeRoith D, Yakar S. Reduced circulating insulin-like growth factor I levels delay the onset of chemically and genetically induced mammary tumors. Cancer Res. 2003;63:4384-4388. [PubMed] |
| 33. | Pollak M, Blouin MJ, Zhang JC, Kopchick JJ. Reduced mammary gland carcinogenesis in transgenic mice expressing a growth hormone antagonist. Br J Cancer. 2001;85:428-430. [PubMed] |
| 34. | Pollak M. Insulin and insulin-like growth factor signalling in neoplasia. Nat Rev Cancer. 2008;8:915-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1484] [Cited by in RCA: 1562] [Article Influence: 91.9] [Reference Citation Analysis (0)] |
| 35. | Gallagher EJ, LeRoith D. Minireview: IGF, Insulin, and Cancer. Endocrinology. 2011;152:2546-2551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 264] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 36. | Ondrej D, Monika S, Magdalena D, Michal M. KIT mutations and sequence changes in genes encoding SDH complex possibly need not be mutually exclusive in gastrointestinal stromal tumors. Appl Immunohistochem Mol Morphol. 2012;20:523-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 37. | Miettinen M, Lasota J. Succinate dehydrogenase deficient gastrointestinal stromal tumors (GISTs) - a review. Int J Biochem Cell Biol. 2014;53:514-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 113] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 38. | Rege TA, Wagner AJ, Corless CL, Heinrich MC, Hornick JL. “Pediatric-type” gastrointestinal stromal tumors in adults: distinctive histology predicts genotype and clinical behavior. Am J Surg Pathol. 2011;35:495-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 88] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 39. | Zhang L, Smyrk TC, Young WF, Stratakis CA, Carney JA. Gastric stromal tumors in Carney triad are different clinically, pathologically, and behaviorally from sporadic gastric gastrointestinal stromal tumors: findings in 104 cases. Am J Surg Pathol. 2010;34:53-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 145] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 40. | Carney JA, Sheps SG, Go VL, Gordon H. The triad of gastric leiomyosarcoma, functioning extra-adrenal paraganglioma and pulmonary chondroma. N Engl J Med. 1977;296:1517-1518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 259] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 41. | Carney JA. Gastric stromal sarcoma, pulmonary chondroma, and extra-adrenal paraganglioma (Carney Triad): natural history, adrenocortical component, and possible familial occurrence. Mayo Clin Proc. 1999;74:543-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 275] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 42. | Matyakhina L, Bei TA, McWhinney SR, Pasini B, Cameron S, Gunawan B, Stergiopoulos SG, Boikos S, Muchow M, Dutra A. Genetics of carney triad: recurrent losses at chromosome 1 but lack of germline mutations in genes associated with paragangliomas and gastrointestinal stromal tumors. J Clin Endocrinol Metab. 2007;92:2938-2943. [PubMed] |
| 43. | Carney JA, Stratakis CA. Familial paraganglioma and gastric stromal sarcoma: a new syndrome distinct from the Carney triad. Am J Med Genet. 2002;108:132-139. [PubMed] |
| 44. | Stratakis CA, Carney JA. The triad of paragangliomas, gastric stromal tumours and pulmonary chondromas (Carney triad), and the dyad of paragangliomas and gastric stromal sarcomas (Carney-Stratakis syndrome): molecular genetics and clinical implications. J Intern Med. 2009;266:43-52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 249] [Cited by in RCA: 198] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 45. | Agaram NP, Laquaglia MP, Ustun B, Guo T, Wong GC, Socci ND, Maki RG, DeMatteo RP, Besmer P, Antonescu CR. Molecular characterization of pediatric gastrointestinal stromal tumors. Clin Cancer Res. 2008;14:3204-3215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 177] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 46. | Agaimy A, Wünsch PH. Lymph node metastasis in gastrointestinal stromal tumours (GIST) occurs preferentially in young patients & lt; or = 40 years: an overview based on our case material and the literature. Langenbecks Arch Surg. 2009;394:375-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 55] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 47. | Kang G, Park YS, Jung ES, Joo M, Kang MS, Ahn S, Kang GH, Kim KM. Gastrointestinal stromal tumors in children and young adults: a clinicopathologic and molecular genetic study of 22 Korean cases. APMIS. 2013;121:938-944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 48. | Barletta JA, Hornick JL. Succinate dehydrogenase-deficient tumors: diagnostic advances and clinical implications. Adv Anat Pathol. 2012;19:193-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 49. | Doyle LA, Nelson D, Heinrich MC, Corless CL, Hornick JL. Loss of succinate dehydrogenase subunit B (SDHB) expression is limited to a distinctive subset of gastric wild-type gastrointestinal stromal tumours: a comprehensive genotype-phenotype correlation study. Histopathology. 2012;61:801-809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 72] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 50. | Gimenez-Roqueplo AP, Favier J, Rustin P, Mourad JJ, Plouin PF, Corvol P, Rötig A, Jeunemaitre X. The R22X mutation of the SDHD gene in hereditary paraganglioma abolishes the enzymatic activity of complex II in the mitochondrial respiratory chain and activates the hypoxia pathway. Am J Hum Genet. 2001;69:1186-1197. [PubMed] |
| 51. | van Nederveen FH, Gaal J, Favier J, Korpershoek E, Oldenburg RA, de Bruyn EM, Sleddens HF, Derkx P, Rivière J, Dannenberg H. An immunohistochemical procedure to detect patients with paraganglioma and phaeochromocytoma with germline SDHB, SDHC, or SDHD gene mutations: a retrospective and prospective analysis. Lancet Oncol. 2009;10:764-771. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 434] [Cited by in RCA: 386] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 52. | Chou A, Chen J, Clarkson A, Samra JS, Clifton-Bligh RJ, Hugh TJ, Gill AJ. Succinate dehydrogenase-deficient GISTs are characterized by IGF1R overexpression. Mod Pathol. 2012;25:1307-1313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 53. | Corless CL, Schroeder A, Griffith D, Town A, McGreevey L, Harrell P, Shiraga S, Bainbridge T, Morich J, Heinrich MC. PDGFRA mutations in gastrointestinal stromal tumors: frequency, spectrum and in vitro sensitivity to imatinib. J Clin Oncol. 2005;23:5357-5364. [PubMed] |
| 54. | Lasota J, Wang Z, Kim SY, Helman L, Miettinen M. Expression of the receptor for type i insulin-like growth factor (IGF1R) in gastrointestinal stromal tumors: an immunohistochemical study of 1078 cases with diagnostic and therapeutic implications. Am J Surg Pathol. 2013;37:114-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 55. | Tarn C, Rink L, Merkel E, Flieder D, Pathak H, Koumbi D, Testa JR, Eisenberg B, von Mehren M, Godwin AK. Insulin-like growth factor 1 receptor is a potential therapeutic target for gastrointestinal stromal tumors. Proc Natl Acad Sci USA. 2008;105:8387-8392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 185] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 56. | Pantaleo MA, Astolfi A, Di Battista M, Heinrich MC, Paterini P, Scotlandi K, Santini D, Catena F, Manara MC, Nannini M. Insulin-like growth factor 1 receptor expression in wild-type GISTs: a potential novel therapeutic target. Int J Cancer. 2009;125:2991-2994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 63] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 57. | Janeway KA, Zhu MJ, Barretina J, Perez-Atayde A, Demetri GD, Fletcher JA. Strong expression of IGF1R in pediatric gastrointestinal stromal tumors without IGF1R genomic amplification. Int J Cancer. 2010;127:2718-2722. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 54] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 58. | Ríos-Moreno MJ, Jaramillo S, Díaz-Delgado M, Sánchez-León M, Trigo-Sánchez I, Padillo JP, Amérigo J, González-Cámpora R. Differential activation of MAPK and PI3K/AKT/mTOR pathways and IGF1R expression in gastrointestinal stromal tumors. Anticancer Res. 2011;31:3019-3025. [PubMed] |
| 59. | Braconi C, Bracci R, Bearzi I, Bianchi F, Sabato S, Mandolesi A, Belvederesi L, Cascinu S, Valeri N, Cellerino R. Insulin-like growth factor (IGF) 1 and 2 help to predict disease outcome in GIST patients. Ann Oncol. 2008;19:1293-1298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 64] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 60. | Turney BW, Turner GD, Brewster SF, Macaulay VM. Serial analysis of resected prostate cancer suggests up-regulation of type 1 IGF receptor with disease progression. BJU Int. 2011;107:1488-1499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 61. | Nannini M, Astolfi A, Paterini P, Urbini M, Santini D, Catena F, Indio V, Casadio R, Pinna AD, Biasco G. Expression of IGF-1 receptor in KIT/PDGF receptor-α wild-type gastrointestinal stromal tumors with succinate dehydrogenase complex dysfunction. Future Oncol. 2013;9:121-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 62. | Gu MJ, Bae YK, Choi JH. Clinical significance of Insulin-Growth Factor 1 and Insulin-Growth Factor 1 Receptor expression in Gastrointestinal Stromal Tumors. Hepatogastroenterology. 2013;60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 63. | Antonescu CR, Viale A, Sarran L, Tschernyavsky SJ, Gonen M, Segal NH, Maki RG, Socci ND, DeMatteo RP, Besmer P. Gene expression in gastrointestinal stromal tumors is distinguished by KIT genotype and anatomic site. Clin Cancer Res. 2004;10:3282-3290. [PubMed] |
| 64. | Celestino R, Lima J, Faustino A, Vinagre J, Máximo V, Gouveia A, Soares P, Lopes JM. Molecular alterations and expression of succinate dehydrogenase complex in wild-type KIT/PDGFRA/BRAF gastrointestinal stromal tumors. Eur J Hum Genet. 2013;21:503-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 65. | Kelly L, Bryan K, Kim SY, Janeway KA, Killian JK, Schildhaus HU, Miettinen M, Helman L, Meltzer PS, van de Rijn M. Post-transcriptional dysregulation by miRNAs is implicated in the pathogenesis of gastrointestinal stromal tumor [GIST]. PLoS One. 2013;8:e64102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 66. | Hao HX, Khalimonchuk O, Schraders M, Dephoure N, Bayley JP, Kunst H, Devilee P, Cremers CW, Schiffman JD, Bentz BG. SDH5, a gene required for flavination of succinate dehydrogenase, is mutated in paraganglioma. Science. 2009;325:1139-1142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 553] [Cited by in RCA: 539] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 67. | Schmoldt A, Benthe HF, Haberland G. Digitoxin metabolism by rat liver microsomes. Biochem Pharmacol. 1975;24:1639-1641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 68. | Selak MA, Armour SM, MacKenzie ED, Boulahbel H, Watson DG, Mansfield KD, Pan Y, Simon MC, Thompson CB, Gottlieb E. Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-alpha prolyl hydroxylase. Cancer Cell. 2005;7:77-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1460] [Cited by in RCA: 1575] [Article Influence: 78.8] [Reference Citation Analysis (0)] |
| 69. | Semenza G. Signal transduction to hypoxia-inducible factor 1. Biochem Pharmacol. 2002;64:993-998. [PubMed] |
| 70. | Kim WY, Kaelin WG. Role of VHL gene mutation in human cancer. J Clin Oncol. 2004;22:4991-5004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 699] [Cited by in RCA: 701] [Article Influence: 35.1] [Reference Citation Analysis (0)] |
| 71. | Safran M, Kaelin WG. HIF hydroxylation and the mammalian oxygen-sensing pathway. J Clin Invest. 2003;111:779-783. [PubMed] |
| 72. | Kaelin WG. The von Hippel-Lindau tumour suppressor protein: O2 sensing and cancer. Nat Rev Cancer. 2008;8:865-873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 519] [Cited by in RCA: 541] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 73. | Covello KL, Simon MC. HIFs, hypoxia, and vascular development. Curr Top Dev Biol. 2004;62:37-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 85] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 74. | Pugh CW, Ratcliffe PJ. Regulation of angiogenesis by hypoxia: role of the HIF system. Nat Med. 2003;9:677-684. [PubMed] |
| 75. | Pollard PJ, Brière JJ, Alam NA, Barwell J, Barclay E, Wortham NC, Hunt T, Mitchell M, Olpin S, Moat SJ. Accumulation of Krebs cycle intermediates and over-expression of HIF1alpha in tumours which result from germline FH and SDH mutations. Hum Mol Genet. 2005;14:2231-2239. [PubMed] |
| 76. | Burnichon N, Brière JJ, Libé R, Vescovo L, Rivière J, Tissier F, Jouanno E, Jeunemaitre X, Bénit P, Tzagoloff A. SDHA is a tumor suppressor gene causing paraganglioma. Hum Mol Genet. 2010;19:3011-3020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 489] [Cited by in RCA: 503] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 77. | Gimenez-Roqueplo AP, Favier J, Rustin P, Rieubland C, Crespin M, Nau V, Khau Van Kien P, Corvol P, Plouin PF, Jeunemaitre X. Mutations in the SDHB gene are associated with extra-adrenal and/or malignant phaeochromocytomas. Cancer Res. 2003;63:5615-5621. [PubMed] |
| 78. | Joensuu H, Hohenberger P, Corless CL. Gastrointestinal stromal tumour. Lancet. 2013;382:973-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 396] [Cited by in RCA: 476] [Article Influence: 39.7] [Reference Citation Analysis (0)] |
| 79. | Dahia PL, Ross KN, Wright ME, Hayashida CY, Santagata S, Barontini M, Kung AL, Sanso G, Powers JF, Tischler AS. A HIF1alpha regulatory loop links hypoxia and mitochondrial signals in pheochromocytomas. PLoS Genet. 2005;1:72-80. [PubMed] |
| 80. | López-Jiménez E, Gómez-López G, Leandro-García LJ, Muñoz I, Schiavi F, Montero-Conde C, de Cubas AA, Ramires R, Landa I, Leskelä S. Research resource: Transcriptional profiling reveals different pseudohypoxic signatures in SDHB and VHL-related pheochromocytomas. Mol Endocrinol. 2010;24:2382-2391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 163] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 81. | Harris AL. Hypoxia--a key regulatory factor in tumour growth. Nat Rev Cancer. 2002;2:38-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3808] [Cited by in RCA: 3949] [Article Influence: 171.7] [Reference Citation Analysis (0)] |
| 82. | Franovic A, Holterman CE, Payette J, Lee S. Human cancers converge at the HIF-2alpha oncogenic axis. Proc Natl Acad Sci USA. 2009;106:21306-21311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 108] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 83. | Franovic A, Gunaratnam L, Smith K, Robert I, Patten D, Lee S. Translational up-regulation of the EGFR by tumor hypoxia provides a nonmutational explanation for its overexpression in human cancer. Proc Natl Acad Sci USA. 2007;104:13092-13097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 223] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 84. | Gordan JD, Bertout JA, Hu CJ, Diehl JA, Simon MC. HIF-2alpha promotes hypoxic cell proliferation by enhancing c-myc transcriptional activity. Cancer Cell. 2007;11:335-347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 644] [Cited by in RCA: 622] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 85. | Garrouste F, Remacle-Bonnet M, Fauriat C, Marvaldi J, Luis J, Pommier G. Prevention of cytokine-induced apoptosis by insulin-like growth factor-I is independent of cell adhesion molecules in HT29-D4 colon carcinoma cells-evidence for a NF-kappaB-dependent survival mechanism. Cell Death Differ. 2002;9:768-779. [PubMed] |
| 86. | Chitnis MM, Yuen JS, Protheroe AS, Pollak M, Macaulay VM. The type 1 insulin-like growth factor receptor pathway. Clin Cancer Res. 2008;14:6364-6370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 358] [Cited by in RCA: 344] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 87. | Gotlieb WH, Bruchim I, Gu J, Shi Y, Camirand A, Blouin MJ, Zhao Y, Pollak MN. Insulin-like growth factor receptor I targeting in epithelial ovarian cancer. Gynecol Oncol. 2006;100:389-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 57] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 88. | Sarfstein R, Maor S, Reizner N, Abramovitch S, Werner H. Transcriptional regulation of the insulin-like growth factor-I receptor gene in breast cancer. Mol Cell Endocrinol. 2006;252:241-246. [PubMed] |
| 89. | Wu JD, Haugk K, Woodke L, Nelson P, Coleman I, Plymate SR. Interaction of IGF signaling and the androgen receptor in prostate cancer progression. J Cell Biochem. 2006;99:392-401. [PubMed] |
| 90. | Sun Y, Gao D, Liu Y, Huang J, Lessnick S, Tanaka S. IGF2 is critical for tumorigenesis by synovial sarcoma oncoprotein SYT-SSX1. Oncogene. 2006;25:1042-1052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 65] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 91. | El-Badry OM, Minniti C, Kohn EC, Houghton PJ, Daughaday WH, Helman LJ. Insulin-like growth factor II acts as an autocrine growth and motility factor in human rhabdomyosarcoma tumors. Cell Growth Differ. 1990;1:325-331. [PubMed] |
| 92. | Scotlandi K, Picci P. Targeting insulin-like growth factor 1 receptor in sarcomas. Curr Opin Oncol. 2008;20:419-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 85] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 93. | Avnet S, Sciacca L, Salerno M, Gancitano G, Cassarino MF, Longhi A, Zakikhani M, Carboni JM, Gottardis M, Giunti A. Insulin receptor isoform A and insulin-like growth factor II as additional treatment targets in human osteosarcoma. Cancer Res. 2009;69:2443-2452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 86] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 94. | Rikhof B, van der Graaf WT, Suurmeijer AJ, van Doorn J, Meersma GJ, Groenen PJ, Schuuring EM, Meijer C, de Jong S. ‘Big’-insulin-like growth factor-II signaling is an autocrine survival pathway in gastrointestinal stromal tumors. Am J Pathol. 2012;181:303-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 95. | Janeway KA, Albritton KH, Van Den Abbeele AD, D’Amato GZ, Pedrazzoli P, Siena S, Picus J, Butrynski JE, Schlemmer M, Heinrich MC. Sunitinib treatment in pediatric patients with advanced GIST following failure of imatinib. Pediatr Blood Cancer. 2009;52:767-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 110] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 96. | Liegl-Atzwanger B, Fletcher JA, Fletcher CD. Gastrointestinal stromal tumors. Virchows Arch. 2010;456:111-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 155] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 97. | Gualberto A, Pollak M. Emerging role of insulin-like growth factor receptor inhibitors in oncology: early clinical trial results and future directions. Oncogene. 2009;28:3009-3021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 222] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 98. | Olmos D, Basu B, de Bono JS. Targeting insulin-like growth factor signaling: rational combination strategies. Mol Cancer Ther. 2010;9:2447-2449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 99. | Kulkarni RN, Holzenberger M, Shih DQ, Ozcan U, Stoffel M, Magnuson MA, Kahn CR. beta-cell-specific deletion of the Igf1 receptor leads to hyperinsulinemia and glucose intolerance but does not alter beta-cell mass. Nat Genet. 2002;31:111-115. [PubMed] |
| 100. | Rhodes CJ. IGF-I and GH post-receptor signaling mechanisms for pancreatic beta-cell replication. J Mol Endocrinol. 2000;24:303-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 68] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 101. | Firth SM, Baxter RC. Cellular actions of the insulin-like growth factor binding proteins. Endocr Rev. 2002;23:824-854. [PubMed] |
| 102. | Pollak M. The insulin and insulin-like growth factor receptor family in neoplasia: an update. Nat Rev Cancer. 2012;12:159-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 771] [Cited by in RCA: 836] [Article Influence: 64.3] [Reference Citation Analysis (0)] |
| 103. | Bauer S, Duensing A, Demetri GD, Fletcher JA. KIT oncogenic signaling mechanisms in imatinib-resistant gastrointestinal stromal tumor: PI3-kinase/AKT is a crucial survival pathway. Oncogene. 2007;26:7560-7568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 200] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 104. | Kim EJ, Zalupski MM. Systemic therapy for advanced gastrointestinal stromal tumors: beyond imatinib. J Surg Oncol. 2011;104:901-906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 105. | Algire C, Amrein L, Zakikhani M, Panasci L, Pollak M. Metformin blocks the stimulative effect of a high-energy diet on colon carcinoma growth in vivo and is associated with reduced expression of fatty acid synthase. Endocr Relat Cancer. 2010;17:351-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 181] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 106. | Algire C, Amrein L, Bazile M, David S, Zakikhani M, Pollak M. Diet and tumor LKB1 expression interact to determine sensitivity to anti-neoplastic effects of metformin in vivo. Oncogene. 2011;30:1174-1182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 146] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 107. | Atkins M, Jones CA, Kirkpatrick P. Sunitinib maleate. Nat Rev Drug Discov. 2006;5:279-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 120] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 108. | George S, Blay JY, Casali PG, Le Cesne A, Stephenson P, Deprimo SE, Harmon CS, Law CN, Morgan JA, Ray-Coquard I. Clinical evaluation of continuous daily dosing of sunitinib malate in patients with advanced gastrointestinal stromal tumour after imatinib failure. Eur J Cancer. 2009;45:1959-1968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 273] [Article Influence: 17.1] [Reference Citation Analysis (0)] |