Published online Feb 21, 2015. doi: 10.3748/wjg.v21.i7.2089
Peer-review started: July 3, 2014
First decision: August 6, 2014
Revised: August 25, 2014
Accepted: October 15, 2014
Article in press: October 15, 2014
Published online: February 21, 2015
Processing time: 223 Days and 7.2 Hours
AIM: To investigate the prevalence of nature tyrosine-methionine-aspartic acid-aspartic acid motif mutations in chronic hepatitis B (CHB) patients and to evaluate the efficacy of lamivudine.
METHODS: A total of 1268 CHB patients were recruited from the outpatient and inpatient departments of six centers. Tyrosine-methionine-aspartic acid-aspartic acid (YMDD) mutations were analyzed using the hepatitis B virus (HBV) drug resistance line probe assay. Forty voluntary patients were selected from those with positive or negative natural YMDD mutations to undergo treatment with lamivudine.
RESULTS: YMDD mutations were detected in 288 (22.71%) of the 1268 CHB patients. Multivariate analysis revealed that the patients’ HBV DNA level (P = 0.0282) and hepatitis B e antigen status (P = 0.0133) were also associated with natural YMDD mutations. The rates of normalization of alanine aminotransferase levels and HBV DNA nondetection at 6, 24, 36, and 48 wk were compared between the patients with natural YMDD mutations and those without, and the differences were not significant. However, there was a significant difference in the cumulative emergence rates of virological breakthrough at 48 wk in the patients with natural YMDD mutations and those without (32.5% vs 12.5%, P = 0.032).
CONCLUSION: Naturally occurring YMDD mutations are detectable in a large proportion of CHB patients; breakthrough hepatitis tended to occur in patients with natural YMDD mutations.
Core tip: Although tyrosine-methionine-aspartic acid-aspartic acid (YMDD) motif mutation is considered to occur secondary to the use of lamivudine, it has become increasingly apparent that YMDD mutations exist in nature. A total of 1268 chronic hepatitis B (CHB) patients were recruited from six centers. YMDD mutations were detected using Inno-Lipa hepatitis B virus (HBV) drug resistance line probe assay in 288 (22.71%) of the 1268 CHB patients. Our study demonstrated that lamivudine therapy was well tolerated by Chinese CHB patients with natural YMDD mutations and led to reductions in transaminase levels and HBV-DNA loss. However, breakthrough hepatitis tended to occur in patients with natural YMDD mutations.
- Citation: Tan YW, Ye Y, Ge GH, Zhao W, Gan JH, Zhao Y, Niu ZL, Zhang DJ, Chen L, Yu XJ, Yang LJ. Natural YMDD-motif mutants affect clinical course of lamivudine in chronic hepatitis B. World J Gastroenterol 2015; 21(7): 2089-2095
- URL: https://www.wjgnet.com/1007-9327/full/v21/i7/2089.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i7.2089
Chronic hepatitis B is a worldwide public health problem, and the hepatitis B virus (HBV) is one of the main causes of acute and chronic hepatitis infections in humans. Approximately 350 million people are chronically infected with HBV worldwide[1]. Chronic infection with HBV is linked to cirrhosis and hepatocellular carcinoma and accounts for 0.5 to 0.75 million deaths a year[2].
Antiretroviral therapy has been proven to be the most effective means of treating chronic hepatitis B (CHB). Interferon and efficient, low-resistance nucleoside analogues are the first-line choices for anti-HBV treatment. Lamivudine is widely used to treat CHB in China; the treatment exhibits excellent antiviral activity, is relatively low in cost and is useful for treating chronic HBV infection. However, prolonged treatment with lamivudine is associated with a high rate of viral resistance. In particular, mutations conferring resistance to lamivudine occur in the highly conserved YMDD (Y: Tyrosine; M: Methionine; D: Aspartic acid; D: Aspartic acid) motif of the catalytic domain (C domain) of the polymerase. Substitutions of methionine at codon 204 to either isoleucine (rtM204I, YIDD variant) or valine (rtM204V, YVDD variant) are the predominant mutations causing lamivudine resistance[3].
Although YMDD motif mutations are considered secondary to the use of lamivudine, it has become increasingly apparent that YMDD mutations exist in nature among hepatitis B patients who have not received lamivudine treatment[4]. Recently, an increasing number of studies have reported the incidence and characteristics of YMDD motif mutations in lamivudine-naïve patients who are chronically infected with HBV. However, the study areas were different; furthermore, the results were not identical and, in some cases, were even contradictory. The reported incidences showed a wide range (0%-31.58%)[5-12]. Furthermore, few reports describe the efficacy of lamivudine treatment in chronic HBV patients with natural YMDD mutations. Therefore, the aims of this multicenter study were to explore the prevalence of natural YMDD motif mutations and some other related factors among lamivudine-untreated CHB patients and to assess the benefits of lamivudine therapy for patients with natural YMDD mutations.
This study was conducted in accordance with the Declaration of Helsinki (2000) of the World Medical Association and received ethical approval from The Third Hospital of Zhenjiang Affiliated Jiangsu University (No. 200903). All patients provided informed written consent.
A total of 1268 patients were recruited from the outpatient and inpatient departments of six hospitals from December 2008 to June 2011 (854 patients were recruited from the Third Hospital of Zhenjiang Affiliated to Jiangsu University, 215 from the Second Hospital of Nanjin, 89 from the First Affiliated Hospital of Soochow University, 56 from the Affiliated Hospital of Yangzhou University, 29 from the People’s Hospital of Wujiang, and 25 from the People’s Hospital of Danyang). The patients’ demographic data and clinical features are shown in Table 1. CHB was diagnosed according to the diagnostic standard from the Chinese National Program for Prevention and Treatment of Viral Hepatitis. The patients had not received lamivudine treatment or any other antiviral treatment within one year before the detection of serum YMDD. Patients with disorders such as drug-induced liver disease, alcoholic liver disease, other types of viral hepatitis (hepatitis A, hepatitis C, hepatitis D, or hepatitis E), schistosomiasis, autoimmune hepatitis, primary biliary cirrhosis, sclerosing cholangitis, α1-antitrypsin deficiency, hemochromatosis, Wilson’s disease, and biliary obstruction were excluded from the study. Patients who had recently undergone gastrointestinal surgery, were pregnant, suffered from any malignancy, or were taking any type of medication were also excluded. Upon each patient’s inclusion in the study, two blood samples were taken simultaneously. One sample was used for biochemical tests [alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), gamma-glutamyl transpeptidase (GGT), total protein, albumin, total bilirubin and platelet count]. The serum from the second sample was stored at -80 °C and was later used for the HBV drug resistance line probe assay and detection of HBV DNA by polymerase chain reaction (PCR). All of the patients were also evaluated using abdominal ultrasonography. Forty voluntary patients with positive or negative natural YMDD mutations were selected to undergo treatment with lamivudine. All of the patients were treated with lamivudine at a dose of 100 mg/d orally. Clinical and laboratory assessments were performed once every 3 mo. Because ALT, family history, HBV DNA and hepatitis B e antigen (HBeAg) status differed significantly between the YMDD group and non-YMDD group, we selected 40 patients with negative YMDD mutations who were matched to the patients with positive YMDD mutations for ALT, family history, HBV DNA level, and HBeAg status (Table 2). Virological breakthrough was defined as having HBV DNA increase by > 1 log from the nadir level or a redetection of HBV DNA at levels at least 10-fold higher than the assay’s lower limit of detection after having an undetectable result[13]. Hepatitis recurrence was defined as having both virological breakthrough and ALT ≥ 2 times the upper limit of normal. Patients with hepatitis recurrence were switched to adefovir plus lamivudine combination therapy.
| Characteristic | Total | YMDD variants | Without YMDD variants | P value |
| (n = 1268) | (n = 288) | (n = 980) | ||
| Age (yr) | 42.12 ± 13.24 | 42.42 ± 14.25 | 43 ± 12.15 | 0.812 |
| Sex | ||||
| Male | 923 (72.79) | 205 (71.18) | 718 (73.26) | 0.485 |
| Female | 345 (27.21) | 83 (28.82) | 262 (26.73) | 0.485 |
| Alcohol use | ||||
| Yes | 143 (11.3) | 36 (12.5) | 107 (10.9) | 0.456 |
| No | 1125 (88.7) | 252 (87.5) | 873 (89.1) | |
| Family history1 | ||||
| Yes | 613 (48.3) | 118 (41) | 495 (50.5) | 0.004 |
| No | 655 (51.7) | 170 (59) | 485 (49.5) | |
| Aspartate aminotransferase (IU/L) | 53 ± 25 | 56 ± 24 | 54 ± 23 | 0.963 |
| Alanine aminotransferase (IU/L) | 67 ± 26 | 63 ± 33 | 69 ± 31 | 0.012 |
| Alkaline phosphatase (IU/L) | 102 ± 45 | 106 ± 46 | 112 ± 54 | 0.064 |
| Gamma-glutamyl transpeptidase (IU/L) | 26 ± 4 | 25 ± 3 | 26 ± 3 | 0.852 |
| HBV genotype | ||||
| A | 5 (0.4) | 2 (0.7) | 3 (0.3) | 0.320 |
| B | 284 (22.4) | 72 (25) | 212 (21.6) | |
| C | 971 (76.6) | 211 (73.3) | 760 (77.6) | |
| Others | 8 (0.6) | 3 (1.0) | 5 (0.5) | |
| HBeAg status | ||||
| Positive | 543 (42.8) | 106 (36.8) | 437 (44.6) | 0.019 |
| Negative | 725 (57.2) | 182 (63.2) | 543 (55.4) | |
| HBV DNA (LGE) | 6.67 ± 2.47 | 7.27 ± 3.16 | 6.37 ± 2.64 | 0.024 |
| Patients with YMDD variants (n = 40) | Patients without YMDD variants (n = 40) | P value | |
| Age (yr) | 42 ± 14 | 43 ± 12 | NS |
| Sex (male/female) | 23/17 | 23/17 | Matched |
| Liver cirrhosis, n (%) | 6 (15.0) | 7 (17.5) | NS |
| Family history1, n (%) | 21(52.5) | 19 (47.5) | NS |
| Median duration of treatment (mo) | 24 ± 6 | 24 ± 5 | NS |
| Aspartate aminotransferase (IU/L) | 81 ± 21 | 61 ± 27 | NS |
| Alanine aminotransferase (IU/L) | 132 ± 12 | 106 ± 16 | NS |
| Serum bilirubin (μmol/L) | 28.7 ± 13.4 | 31.8 ± 15.5 | NS |
| Serum albumin (g/L) | 38 ± 12 | 36 ± 13 | NS |
| HBV-DNA (LGE/mL) | 6.7 ± 2.7 | 6.8 ± 2.8 | Matched |
| HBeAg positive, n (%) | 15 (37.5) | 15 (37.5) | Matched |
| HBV genotype (A/B/C/other) | 1/4/33/2 | 0/4/32/4 | NS |
Total HBVm, anti-hepatitis C virus (HCV), anti-hepatitis D virus (HDV) and anti-hepatitis E virus (HEV) antibodies were determined using enzyme-linked immunosorbent assays (ELISA) (Roche, Shanghai, China).
The experimental method used was described in a previous study[14]. Briefly, HBV DNA was isolated from 200 μL of serum using a High Pure Viral Nucleic Acid Kit (Roche). The Inno-Lipa HBV DR v1 assay (Inno-Lipa; Innogenetics, Beijing, Beijing Pason Pharmaceuticals, Inc.) was performed according to the manufacturer’s instructions using Hot Start Taq DNA polymerase (Invitrogen, Shanghai, China). The assay is based on the amplification of a part of the viral polymerase gene using predesigned primers and reverse hybridization with the probes coated on a strip. The assay detects wild-type HBV polymerase mutations and known drug-induced mutations associated with lamivudine and famciclovir resistance (codons 180, 204, and 207).
Serum HBV DNA were measured quantitatively using real-time polymerase chain reaction (PCR; Model 5700, ABI Company, United States) with a lower detection limit of 1 × 103 HBV DNA copies/mL and 1 × 103 copies/μg total DNA.
HBV genotypes were detected using PCR-microcosmic nucleic acid cross-ELISA.
All data are presented as mean ± SD. Demographic data were analyzed using descriptive statistical tests. The independent samples t-test, the χ2 test and Fisher’s exact test were used for group comparisons. The factors included were patient age, sex, ALT, AST, ALP, GGT level, HBeAg status, HBV DNA level, alcohol consumption, family history and HBV genotype. Initially, univariate analysis was conducted using logistic regression. Next, all factors that were at least marginally associated with the prevalence of YMDD mutation (P < 0.15) were tested by multivariate analysis using a stepwise logistic model. A P-value < 0.05 was considered statistically significant. SPSS software version 21.0 (SPSS Inc., Chicago, IL, United States) was used for statistical analyses.
YMDD mutations were detected in 288 (22.71%) of the 1268 CHB patients. The demographic data and clinical features are summarized in Table 1. Of the 288 patients, 164 (56.94%) also had an accompanying L180M mutation. Two of the patients had a mutation at codon 207. The combination of wild-type and YMDD-variant HBV was present in 251 of the 288 patients. Mixtures of YMDD + YVDD, YMDD + YIDD and YMDD + YVDD + YIDD were found in 49, 82 and 121 patients, respectively. In 12 patients, only YIDD and/or YVDD variants were detected, without the presence of the wild-type YMDD motif; YIDD + YVDD, YIDD and YVDD variants were shown in six, four and three patients, respectively.
Potential risk factors for the emergence of the YMDD motif mutation that were assessed included sex, alcohol use, family history, AST, ALT, ALP, HBV-DNA level, HBeAg, and HBV genotype. Univariate analysis of individual baseline factors showed that family history, HBeAg status and HBV DNA level were predictive factors of natural YMDD mutations. There was no association with the other factors: patient age, sex, alcohol consumption, AST, ALP, GGT and HBV genotype. As Table 3 shows, multivariate analysis revealed that the HBV DNA level (P = 0.0282) and HBeAg status (P = 0.0133) were associated with natural YMDD mutations.
| Univariate | Multivariate | |||
| OR (95%CI) | P value | OR (95%CI) | P value | |
| Age (yr) (< 60, ≥ 60) | 1.39 (0.57, 3.40) | 0.4700 | 2.02 (0.72, 5.67) | 0.1824 |
| Gender (male, female) | 1.41 (0.64, 3.12) | 0.4850 | 1.24 (0.33, 4.59) | 0.7518 |
| Alcohol (yes, no) | 1.75 (0.72, 4.24) | 0.4560 | 1.27 (0.57, 2.85) | 0.5608 |
| Family history1 (yes, no) | 2.45 (1.15, 5.25) | 0.0040 | 1.77 (0.24, 3.84) | 0.3254 |
| Aspartate aminotransferase (IU/L; < 40, ≥ 40) | 2.08 (0.91, 4.81) | 0.9630 | 1.22 (0.28, 5.29) | 0.7876 |
| Alanine aminotransferase (IU/L; < 40, ≥ 40) | 5.44 (1.45, 20.45) | 0.0122 | 2.59 (1.24, 8.44) | 0.0734 |
| Alkaline phosphatase (IU/L; < 120, ≥ 120) | 2.62 (0.67, 10.23) | 0.9520 | 3.42 (0.81, 14.54) | 0.0952 |
| Gamma-glutamyl transpeptidase (IU/L; < 40, ≥ 40) | 1.08 (0.47, 2.50) | 0.8520 | 1.18 (0.35, 3.99) | 0.7957 |
| HBV genotype (A/B/C/other) | 1.17 (0.55, 2.49) | 0.3200 | 1.05 (0.40, 2.77) | 0.5246 |
| HBeAg state (+/-) | 3.01 (1.37, 6.63) | 0.0190 | 3.56 (1.55, 8.18) | 0.0282 |
| HBV DNA (LGE; < 5, ≥ 5) | 2.45 (1.15, 5.25) | 0.0240 | 2.70 (1.23, 5.92) | 0.0133 |
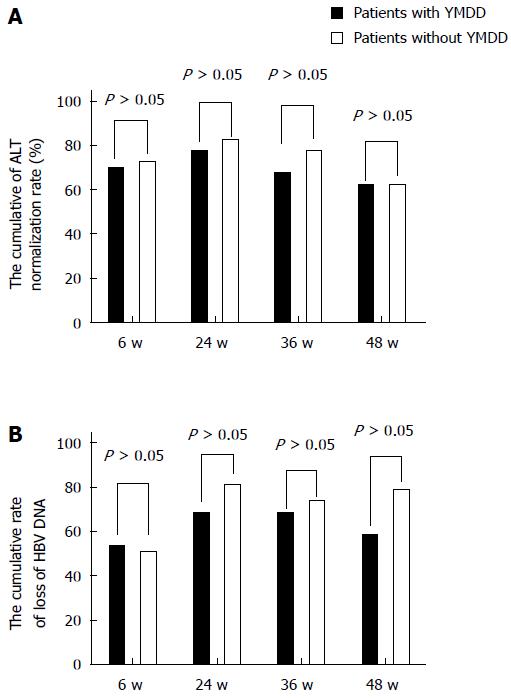
The rates of normalization of the ALT levels and nondetection of HBV DNA at 6, 24, 36, and 48 wk in the patients matched for ALT, family history, HBeAg status, and HBV-DNA level during lamivudine therapy were studied. The ALT normalization rates at 6, 24, 36, and 48 wk showed no significant differences between the patients with natural YMDD mutations and those without (χ2 = 0.061, P= 0.805; χ2 = 0.313, P = 0.576; χ2 = 1.003, P = 0.317; χ2 = 0.22, P = 0.639; Figure 1A).
Figure 1B shows the cumulative HBV DNA loss rates for both groups. At 6, 24, 36, and 48 wk in the patients matched for ALT, family history, HBeAg status, and HBV DNA level during lamivudine therapy, the HBV-DNA loss rate in the patients with natural YMDD mutations was almost stable at all time points. In contrast, the rates among the patients without natural YMDD mutations tended to be higher than those in the patients with natural YMDD mutations, although the differences were not significant. The cumulative HBV DNA loss rates were 52.5% vs 50% at 6 wk (χ2 = 0.05, P = 0.823), 67.5% vs 80% at 24 wk (χ2 = 1.614, P = 0.204), 67.5% vs 72.5% at 36 wk (χ2 = 0.549, P = 0.459), and 62.5% vs 77.5% at 48 wk (χ2 = 2.143, P = 0.143).
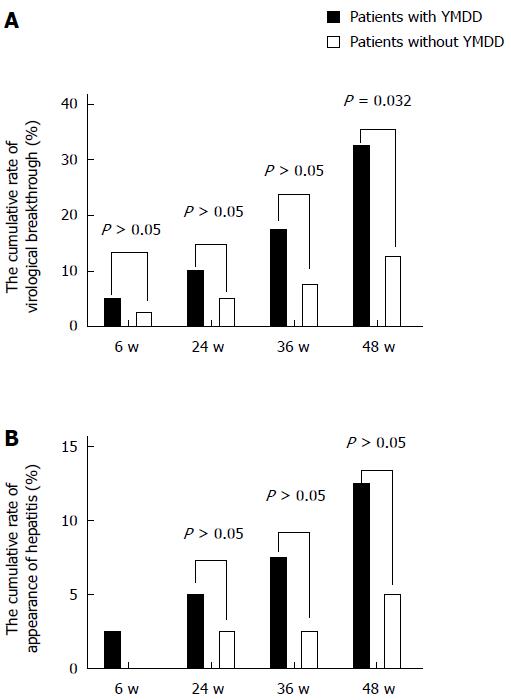
The cumulative emergence rates of virological breakthrough in patients with and without natural YMDD mutations were 5% (2/40) and 2.5% (1/40) at 6 wk (χ2 = 0.346, P = 0.556), 10% (4/40) and 5% (2/40) at 24 wk (χ2 = 0.721, P = 0.396), 17.5% (7/40) and 7.5% (3/40) at 36 wk (χ2 = 1.829, P = 0.176), and 32.5% (13/40) and 12.5% (5/40) at 48 wk (Figure 2A). There was a significant difference at 48 wk (χ2 = 4.588, P = 0.032). The emergence rates of virological breakthrough in the patients with natural YMDD mutations were similar to those in the patients without at the other three time intervals (Figure 2A).
The cumulative breakthrough hepatitis rates in patients with and without natural YMDD mutations were 1 2.5% (1/40) and 0% (0/40) at 6 wk (χ2 = 0, P = 1), 5% (2/40) and 2.5% (1/40) at 24 wk (χ2 = 0.346, P = 0.556), 7.5% (3/40) and 5% (2/40) at 36 wk (χ2 = 0.213, P = 0.664), and 12.5% (5/40) and 5% (2/40) at 48 wk (χ2 = 1.409, P = 0.432; Figure 2B). The rates of breakthrough hepatitis in patients without natural YMDD mutations tended to be lower than those in patients with natural YMDD mutations, although the differences were not statistically significant (Figure 2B).
Although the YMDD motif mutation increased during lamivudine treatment[15,16], many studies have reported that this mutation can also be spontaneous[3,4,12,17-19]. Previous reports have shown that the incidence of YMDD mutations was 0% to 27.7% for asymptomatic HBV carriers and 0% to 31.58% for CHB patients. Our previous study demonstrated that the pooled incidence of natural YMDD mutations was as high as 12.21% (95%CI: 9.69-14.95)[19]. This result primarily represented the current situation in East Asia, especially China. In this study, our results showed that YMDD mutations naturally existed in 22.71% of untreated CHB patients, most of whom had wild-type HBV. The discrepancy may have occurred because a well-characterized commercial assay was used that can detect mutations that make up as little as 10% of the viral population[20]. A recent report showed that the INNO-LiPA HBV DR assay had convincing diagnostic sensitivity and accuracy for monitoring HBV-infected patients undergoing NA treatment[21]. Moreover, in this study, a large number of subjects were recruited, which allowed us to more accurately determine the prevalence of natural YMDD mutations in Chinese patients.
Da Silva et al[22] and Ye et al[15] found that anti-HBe was positive in most patients with YMDD mutations and considered that YMDD mutations might be more likely to occur if mutations take place in the pre-c-zone. In this study, our findings revealed that the prevalence of YMDD mutations was 14.35% in patients who were negative for HBeAg and 8.35% in patients who were positive for HBeAg, a difference that was significant. Moreover, multivariate analysis revealed that the HBeAg status was associated with YMDD mutations. These findings were in accordance with those of Da Silva et al[22] and Ye et al[15].
Some reports[23,24] have demonstrated a close correlation between serum HBV DNA level and the incidence of YMDD mutations. Our results were consistent with these studies in that, in terms of the prevalence of YMDD mutations, a significant difference was found among patients with different serum HBV DNA levels, suggesting that HBV DNA level might have a positive correlation with YMDD mutations.
Lamivudine is an oral cytosine nucleoside analog that potently inhibits HBV replication by interfering with HBV reverse transcriptase activity[25]. Many studies have reported the effectiveness of lamivudine for suppressing HBV replication and improving transaminase levels and liver histology[3,26-28]. Our study demonstrated that lamivudine therapy was well tolerated by Chinese CHB patients with natural YMDD mutations and led to reductions in transaminase levels and HBV-DNA loss.
The prevalence of pre-existing natural YMDD mutants is well known, but their clinical significance during LAM therapy is unknown. Lee et al[12] investigated whether pre-existing YMDD mutants were selected during LAM therapy. Their study included 14 treatment-naïve patients who were treated with LAM for at least 9 mo. A virological response was observed at 3 mo in all patients with pre-existing YMDD mutants. All mutations disappeared after 3 mo of LAM therapy, and during the follow-up period, no re-emergence was detected by any of the three detection methods. Furthermore, the viral load was suppressed optimally.
In our study, patients with natural YMDD mutations showed a weekly increase in the normalization rate of transaminases. However, compared with patients without natural YMDD mutations, the high normalization rate of transaminases was similar. The cumulative HBV DNA loss rates in both groups were almost stable at all time points during lamivudine therapy. However, the cumulative emergence rates of virological breakthrough in patients with natural YMDD mutations were higher than those in patients without YMDD mutations. Moreover, the rate of breakthrough hepatitis tended to be lower in patients without natural YMDD mutations than in those with natural YMDD mutations. The low frequency of breakthrough hepatitis in patients without natural YMDD mutations was attributed to the cumulative emergence rates of virological breakthrough.
In conclusion, naturally occurring YMDD mutations are found in a large proportion of CHB patients who have not undergone anti-viral therapy. The incidence of YMDD mutations may be correlated with the HBeAg status and the HBV DNA level. Our results also suggest that lamivudine therapy improved the clinical course in HBV patients with natural YMDD mutations. Lamivudine therapy for patients with natural YMDD mutations was well tolerated and resulted in reduced ALT and HBV-DNA levels. The cumulative emergence rates of virological breakthrough in patients with natural YMDD mutations were higher than those in patients without natural YMDD mutations. Moreover, breakthrough hepatitis tended to occur in patients with natural YMDD mutations more often than in patients without. Further studies with a longer follow-up and more patients are needed to confirm our findings.
Although tyrosine-methionine-aspartic acid-aspartic acid (YMDD) motif mutations are considered secondary to the use of lamivudine, the pre-existing prevalence of natural YMDD mutants is well known; however, its clinical significance during lamivudine therapy is unknown.
Many studies have reported that YMDD mutations can occur spontaneously. Previous reports have shown that the incidence of YMDD mutations was 0% to 27.7% for asymptomatic hepatitis B virus (HBV) carriers and 0% to 31.58% for chronic hepatitis B (CHB) patients.
To the authors’ knowledge, few reports describe the efficacy of lamivudine treatment in chronic HBV patients with natural YMDD mutations. Therefore, the aims of this multicenter study were to explore the prevalence of natural YMDD motif mutations and some other related factors among lamivudine-untreated CHB patients and to assess the benefits of lamivudine therapy for patients with natural YMDD mutations.
This study provided evidence for the antiviral treatment of chronic hepatitis B.
This work aimed to study the prevalence of YMDD motif mutants in the HBV polymerase in CHB patients and the relationship to the clinical course of the disease. This could be an interesting original work, and the sample size is large. The study demonstrated that lamivudine therapy was well tolerated by Chinese CHB patients with natural YMDD mutations and led to reductions in transaminase levels and HBV-DNA loss. However, breakthrough hepatitis tended to occur in patients with natural YMDD mutations.
P- Reviewer: Baran B, Cunha C, Komatsu H, Silva LD, Yang YF, Zhang XY S- Editor: Gou SX L- Editor: Wang TQ E- Editor: Liu XM
| 1. | Evans AA, London WT, Gish RG, Cohen C, Block TM. Chronic HBV infection outside treatment guidelines: is treatment needed? Antivir Ther. 2013;18:229-235. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 2. | Hösel M, Lucifora J, Michler T, Holz G, Gruffaz M, Stahnke S, Zoulim F, Durantel D, Heikenwalder M, Nierhoff D. Hepatitis B virus infection enhances susceptibility toward adeno-associated viral vector transduction in vitro and in vivo. Hepatology. 2014;59:2110-2120. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 3. | Tipples GA, Ma MM, Fischer KP, Bain VG, Kneteman NM, Tyrrell DL. Mutation in HBV RNA-dependent DNA polymerase confers resistance to lamivudine in vivo. Hepatology. 1996;24:714-717. [PubMed] [Cited in This Article: ] |
| 4. | Tsubota A. How do naturally occurring YMDD-motif mutants influence the clinical course of lamivudine-naïve patients with chronic hepatitis B virus infection? J Gastroenterol Hepatol. 2006;21:1769-1771. [PubMed] [Cited in This Article: ] |
| 5. | Yan MH, Zhang C, Ling Q, Zhou RF. [Detection of YMDD motif mutations in lamivudine-untreated patients with chronic hepatitis B]. Zhonghua Ganzangbing Zazhi. 2003;11:430-431. [PubMed] [Cited in This Article: ] |
| 6. | Kirishima T, Okanoue T, Daimon Y, Itoh Y, Nakamura H, Morita A, Toyama T, Minami M. Detection of YMDD mutant using a novel sensitive method in chronic liver disease type B patients before and during lamivudine treatment. J Hepatol. 2002;37:259-265. [PubMed] [Cited in This Article: ] |
| 7. | Shin YM, Heo J, Kim GH, Kang DH, Song GA, Cho M, Yang US, Kim CM, Park HK, Jang HJ. [Natural YMDD motif mutations of HBV polymerase in the chronic hepatitis B virus infected patients]. Taehan Kan Hakhoe Chi. 2003;9:1-9. [PubMed] [Cited in This Article: ] |
| 8. | Kobayashi S. Clinical characteristics of asymptomatic hepatitis B virus carriers with YMDD mutant not treated with lamivudine. Kurume Med J. 2003;50:87-90. [PubMed] [Cited in This Article: ] |
| 9. | Heo J, Cho M, Kim HH, Shin YM, Jang HJ, Park HK, Kim CM, Kim GH, Kang DH, Song GA, Yang US. Detection of YMDD motif mutants by oligonucleotide chips in lamivudine-untreated patients with chronic hepatitis B virus infection. J Korean Med Sci. 2004;19:541-546. [PubMed] [Cited in This Article: ] |
| 10. | Horgan M, Brannigan E, Crowley B, Levis J, Fanning LJ. Hepatitis B genotype and YMDD profiles in an untreated Irish population. J Clin Virol. 2006;35:203-204. [PubMed] [Cited in This Article: ] |
| 11. | Min XC, Miao XH, Zhao SM, Zhao KK, Yang DG. [The spontaneous YMDD mutation rate in chronic hepatitis B patients]. Zhonghua Ganzangbing Zazhi. 2009;17:887-890. [PubMed] [Cited in This Article: ] |
| 12. | Lee SH, Kim HS, Byun IS, Jeong SW, Kim SG, Jang JY, Kim YS, Kim BS. Pre-existing YMDD mutants in treatment-naïve patients with chronic hepatitis B are not selected during lamivudine therapy. J Med Virol. 2012;84:217-222. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Chen EQ, Zhou TY, Bai L, Wang JR, Yan LB, Liang LB, Tang H. Lamivudine plus adefovir or telbivudine plus adefovir for chronic hepatitis B patients with suboptimal response to adefovir. Antivir Ther. 2012;17:973-979. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Tan YW, Ge GH, Zhao W, Gan JH, Zhao Y, Niu ZL, Zhang DJ, Chen L, Yu XJ, Yang LJ. YMDD motif mutations in chronic hepatitis B antiviral treatment naïve patients: a multi-center study. Braz J Infect Dis. 2012;16:250-255. [PubMed] [Cited in This Article: ] |
| 15. | Ye XG, Su QM. Effects of entecavir and lamivudine for hepatitis B decompensated cirrhosis: meta-analysis. World J Gastroenterol. 2013;19:6665-6678. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 29] [Cited by in F6Publishing: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 16. | Lian JS, Zeng LY, Chen JY, Jia HY, Zhang YM, Xiang DR, Yu L, Hu JH, Lu YF, Zheng L. De novo combined lamivudine and adefovir dipivoxil therapy vs entecavir monotherapy for hepatitis B virus-related decompensated cirrhosis. World J Gastroenterol. 2013;19:6278-6283. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 8] [Cited by in F6Publishing: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 17. | Yang J, Chen X, Zhang H, Chen G. HBV genotype C strains with spontaneous YMDD mutations may be a risk factor for hepatocellular carcinoma. J Med Virol. 2014;86:913-917. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 18. | Yang JH, Zhang H, Chen XB, Chen G, Wang X. Relationship between hepatocellular carcinoma and hepatitis B virus genotype with spontaneous YMDD mutations. World J Gastroenterol. 2013;19:3861-3865. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 16] [Cited by in F6Publishing: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 19. | Tan Y, Ding K, Su J, Trinh X, Peng Z, Gong Y, Chen L, Cui Q, Lei N, Chen X. The naturally occurring YMDD mutation among patients chronically infected HBV and untreated with lamivudine: a systematic review and meta-analysis. PLoS One. 2012;7:e32789. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 20. | Aberle SW, Kletzmayr J, Watschinger B, Schmied B, Vetter N, Puchhammer-Stöckl E. Comparison of sequence analysis and the INNO-LiPA HBV DR line probe assay for detection of lamivudine-resistant hepatitis B virus strains in patients under various clinical conditions. J Clin Microbiol. 2001;39:1972-1974. [PubMed] [Cited in This Article: ] |
| 21. | Ducancelle A, Servant-Delmas A, Beuvelet T, Balan V, Pivert A, Maniez M, Laperche S, Lunel-Fabiani F. [Results of a novel real-time PCR, sequence analysis, Inno-LiPA line probe assays in the detection of hepatitis B virus G1896A precore mutation in French blood donors]. Pathol Biol (Paris). 2011;59:e21-e27. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 22. | Da Silva LC, da Fonseca LE, Carrilho FJ, Alves VA, Sitnik R, Pinho JR. Predictive factors for response to lamivudine in chronic hepatitis B. Rev Inst Med Trop Sao Paulo. 2000;42:189-196. [PubMed] [Cited in This Article: ] |
| 23. | Ağca H, Sayıner AA, Sengönül A, Simşek I, Akarsu M. [A real-time PCR assay for the quantification of hepatitis B virus DNA and concurrent detection of YMDD motif mutations]. Mikrobiyol Bul. 2011;45:664-676. [PubMed] [Cited in This Article: ] |
| 24. | Tassopoulos NC, Volpes R, Pastore G, Heathcote J, Buti M, Goldin RD, Hawley S, Barber J, Condreay L, Gray DF. Efficacy of lamivudine in patients with hepatitis B e antigen-negative/hepatitis B virus DNA-positive (precore mutant) chronic hepatitis B. Lamivudine Precore Mutant Study Group. Hepatology. 1999;29:889-896. [PubMed] [Cited in This Article: ] |
| 25. | Honkoop P, de Man RA, Heijtink RA, Schalm SW. Hepatitis B reactivation after lamivudine. Lancet. 1995;346:1156-1157. [PubMed] [Cited in This Article: ] |
| 26. | Dienstag JL, Perrillo RP, Schiff ER, Bartholomew M, Vicary C, Rubin M. A preliminary trial of lamivudine for chronic hepatitis B infection. N Engl J Med. 1995;333:1657-1661. [PubMed] [Cited in This Article: ] |
| 27. | Benhamou Y, Dohin E, Lunel-Fabiani F, Poynard T, Huraux JM, Katlama C, Opolon P, Gentilini M. Efficacy of lamivudine on replication of hepatitis B virus in HIV-infected patients. Lancet. 1995;345:396-397. [PubMed] [Cited in This Article: ] |
| 28. | Jung MC, Zachoval R, Sackmann M. [Therapy of chronic hepatitis B virus infection with the nucleoside analog lamivudine]. Z Gastroenterol. 1996;34:827-828. [PubMed] [Cited in This Article: ] |