Copyright
©The Author(s) 2015.
World J Gastroenterol. Feb 14, 2015; 21(6): 1972-1981
Published online Feb 14, 2015. doi: 10.3748/wjg.v21.i6.1972
Published online Feb 14, 2015. doi: 10.3748/wjg.v21.i6.1972
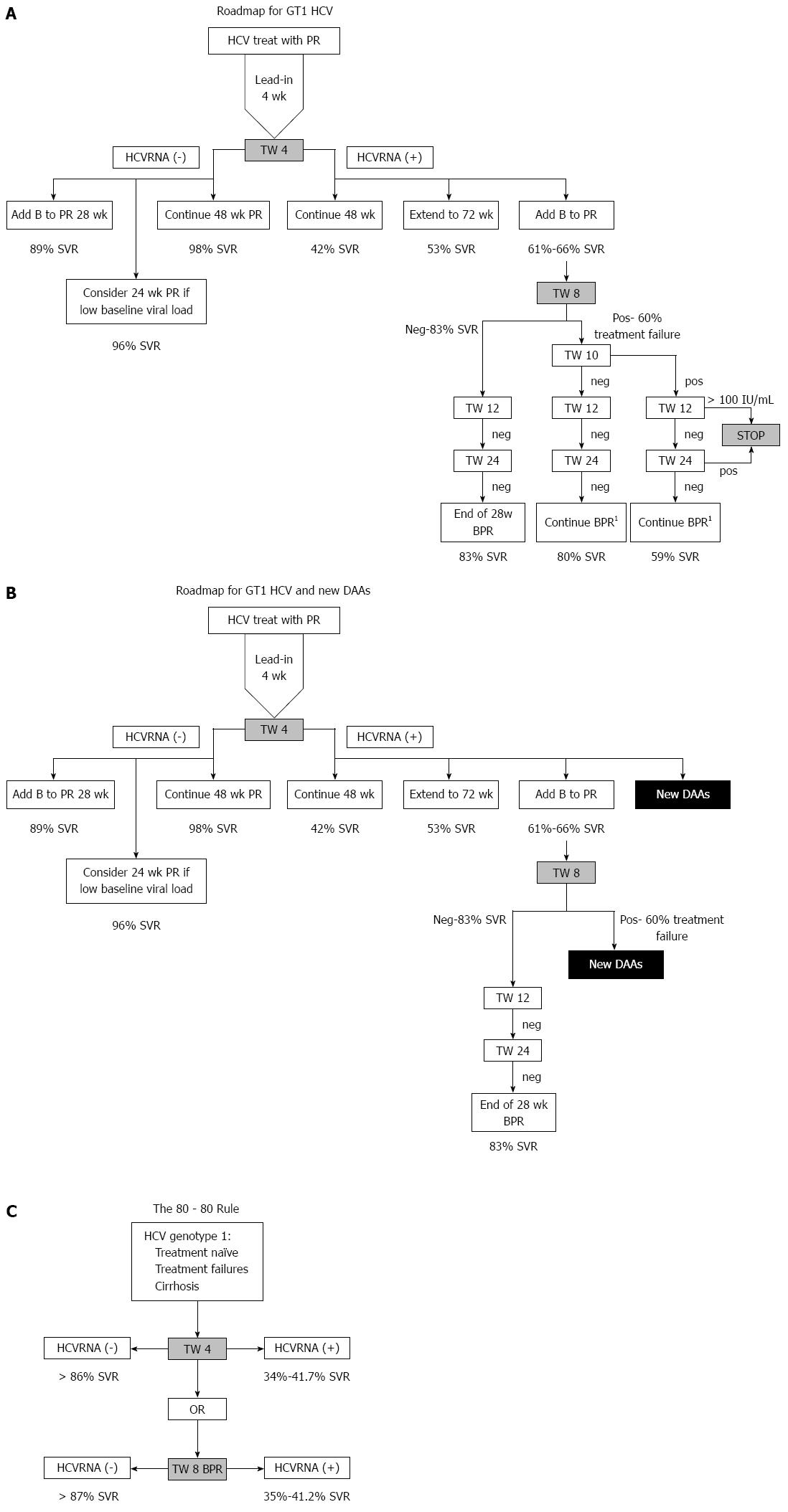
Figure 3 Roadmap for GT1 hepatitis C virus (A), Roadmap for GT1 hepatitis C virus and new direct acting antivirals (B) and the “80-80” Rule (C).
A: Full roadmap showing timepoints, predicted SVR based on undetectable HCVRNA at timepoints, and alternative choices at each timepoint. 1Not eligible for shortened therapy - should complete 32 wk BPR ± 12 wk PR or complete 44 wk BPR; B: Modification of the roadmap showing that new DAAs can be considered at Treatment week (TW)4 or TW8 if HCVRNA is positive; C: Main points of the roadmap showing that if HCVRNA is negative at TW4 (on PR) or 8 (on BPR) then there is > 80% SVR. If HCVRNA is positive at week 4, then there is 41.7% SVR if 48 wk PR is continued, but at week 8, if HCVRNA is positive while on BPR then the possibility of SVR is 35%-41.2%. HCV: Hepatitis C virus; PR: Pegylated interferon and ribavirin; SVR: Sustained virological response.
- Citation: Lim SG. Chronic hepatitis C genotype 1 treatment roadmap for resource constrained settings. World J Gastroenterol 2015; 21(6): 1972-1981
- URL: https://www.wjgnet.com/1007-9327/full/v21/i6/1972.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i6.1972