Published online Feb 14, 2015. doi: 10.3748/wjg.v21.i6.1765
Peer-review started: August 6, 2014
First decision: October 14, 2014
Revised: October 25, 2014
Accepted: November 7, 2014
Article in press: November 11, 2014
Published online: February 14, 2015
AIM: To investigate the possible involvement of Sirtuin 1 (SIRT1) in rat orthotopic liver transplantation (OLT), when Institute Georges Lopez 1 (IGL-1) preservation solution is enriched with trimetazidine (TMZ).
METHODS: Male Sprague-Dawley rats were used as donors and recipients. Livers were stored in IGL-1 preservation solution for 8h at 4 °C, and then underwent OLT according to Kamada’s cuff technique without arterialization. In another group, livers were stored in IGL-1 preservation solution supplemented with TMZ, at 10-6 mol/L, for 8 h at 4 °C and then underwent OLT. Rats were sacrificed 24 h after reperfusion, and liver and plasma samples were collected. Liver injury (transaminase levels), mitochondrial damage (glutamate dehydrogenase activity) oxidative stress (malondialdehyde levels), and nicotinamide adenine dinucleotide (NAD+), the co-factor necessary for SIRT1 activity, were determined by biochemical methods. SIRT1 and its substrates (ac-FoxO1, ac-p53), the precursor of NAD+, nicotinamide phosphoribosyltransferase (NAMPT), as well as the phosphorylation of adenosine monophosphate activated protein kinase (AMPK), p-mTOR, p-p70S6K (direct substrate of mTOR), autophagy parameters (beclin-1, LC3B) and MAP kinases (p-p38 and p-ERK) were determined by Western blot.
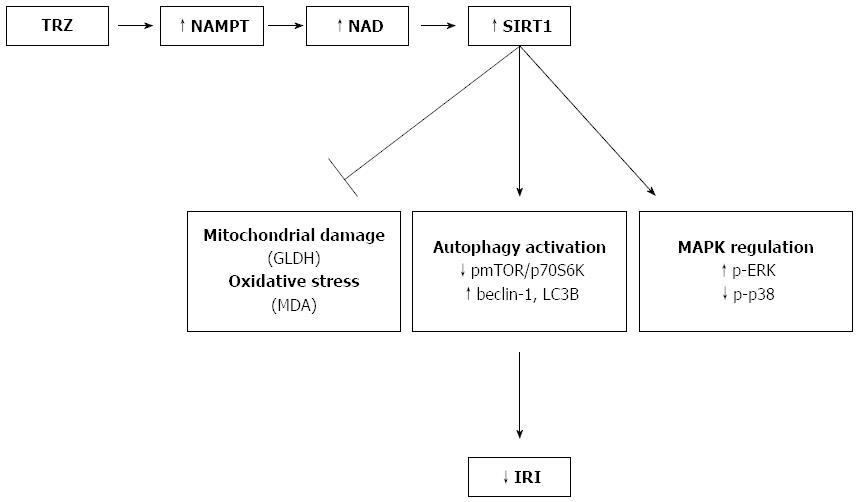
RESULTS: Liver grafts preserved in IGL-1 solution enriched with TMZ presented reduced liver injury and mitochondrial damage compared with those preserved in IGL-1 solution alone. In addition, livers preserved in IGL-1 + TMZ presented reduced levels of oxidative stress. This was consistent with enhanced SIRT1 protein expression and elevated SIRT1 activity, as indicated by decreased acetylation of p53 and FoxO1. The elevated SIRT1 activity in presence of TMZ can be attributed to the enhanced NAMPT protein and NAD+/NADH levels. Up-regulation of SIRT1 was consistent with activation of AMPK and inhibition of phosphorylation of mTOR and its direct substrate (p-p70S6K). As a consequence, autophagy mediators (beclin-1 and LC3B) were over-expressed. Furthermore, MAP kinases were regulated in livers preserved with IGL-1 + TMZ, as they were characterized by enhanced p-ERK and decreased p-p38 protein expression.
CONCLUSION: Our study shows that IGL-1 preservation solution enriched with TMZ protects liver grafts from the IRI associated with OLT, through SIRT1 up-regulation.
Core tip: Sirtuin 1 (SIRT1) has been implicated in pathways associated with ischemia-reperfusion injury (IRI), but its role in rat orthotopic liver transplantation has not yet been established. In our study, SIRT1 protein expression levels and activity increased when Institut Georges Lopez 1 (IGL-1) preservation solution was supplemented with trimetazidine, which was associated with less hepatic injury and mitochondrial damage. The increased deacetylation of FoxO1 by SIRT1 agreed with less oxidative stress and the activation of the autophagy pathway. These findings support the notion that SIRT1 up-regulation may be an effective strategy for reducing IRI and improving liver transplantation outcome.
- Citation: Pantazi E, Zaouali MA, Bejaoui M, Folch-Puy E, Ben Abdennebi H, Varela AT, Rolo AP, Palmeira CM, Roselló-Catafau J. Sirtuin 1 in rat orthotopic liver transplantation: An IGL-1 preservation solution approach. World J Gastroenterol 2015; 21(6): 1765-1774
- URL: https://www.wjgnet.com/1007-9327/full/v21/i6/1765.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i6.1765
Liver ischemia-reperfusion injury (IRI) can cause primary graft non-function and may lead to organ failure[1,2]. The oxygen deprivation during ischemia provokes depletion of cellular energy, whereas the subsequent re-oxygenation during reperfusion initiates a cascade of complex pathways, including the production of reactive oxygen species (ROS), which in part are responsible for the subsequent induction of hepatocellular injury. Given the complexity of IRI pathophysiology, a more profound knowledge of the underlying mechanisms is needed in order to design new therapeutic strategies able to minimize its adverse effects.
SIRT1 is a histone deacetylase that either activates or suppresses the transcription activities of various non-histone proteins through its nicotinamide adenine dinucleotide (NAD+)-dependent activity. SIRT1 has been associated with the pathophysiology of IRI in several organs[3]. In fact, SIRT1 is involved in a wide variety of cellular processes, including apoptosis, cellular stress and autophagy[4-7]. It has been reported that SIRT1 deacetylates p53, thus reducing its transcriptional activity and its ability to induce apoptosis[8]. Forkhead box-containing protein O 1 (FoxO1) is also a target for SIRT1, and its deacetylation has been implicated in the detoxification of ROS and the promotion of autophagy[9]. Furthermore, we have recently shown that SIRT1 activation contributes, in part, to the protective effects of liver ischemic preconditioning against IRI[10].
Adequate liver preservation is vital for the success of transplantation, in order to maintain graft quality after cold storage. At present, University of Wisconsin (UW) solution is the most widely used preservation solution. Recent studies have demonstrated that Institute Georges Lopez 1 (IGL-1) preservation solution is a valuable alternative for liver grafts in orthotopic liver transplantation (OLT)[11,12]. Moreover, supplementation of IGL-1 with trimetazidine (TMZ) has been shown to increase the preservation of both steatotic and non-steatotic liver grafts in an isolated and perfused “ex vivo” model[13]. However, the role of SIRT1 in rat OLT when IGL-1 solutions are used has not been assessed to date.
Given that TMZ is a promising additive for increasing liver graft preservation and since SIRT1 exerts a protective role against warm IRI in the liver, the aim of this study is to investigate the potential role of SIRT1 in rat OLT when TMZ-enriched IGL-1 preservation solution is used.
Male Sprague-Dawley rats (200-250 g) were used as donors and recipients. Throughout the study, animals were housed in conventional animal facilities where temperature and humidity were controlled with a 12 h light/dark cycle. All animals had free access to water and a standard laboratory diet. All procedures were performed under isofluorane inhalation anesthesia. The experiments were approved by the Ethics Committees for Animal Experimentation (CEEA, Directive 400/12), University of Barcelona and all procedures complied with European Union regulations for animal experiments (EU guideline 86/609/EEC). Rats were randomly distributed into groups as described below.
The following experimental groups were created: (1) Sham (n = 6): Animals underwent transverse laparotomy and received silk ligatures in the right suprarenal vein, diaphragmatic vein, and hepatic artery; (2) IGL-1 (n = 6): Livers were flushed and stored in IGL-1 preservation solution for 8h at 4 °C, and then underwent OLT according to Kamada’s cuff technique without arterialization. Rats were sacrificed 24 h after reperfusion for liver and plasma sample collection; and (3) IGL-1 + TMZ (n = 6): Same as group 2, but livers were preserved in IGL-1 solution supplemented with trimetazidine (TMZ) at 10-6 mol/L.
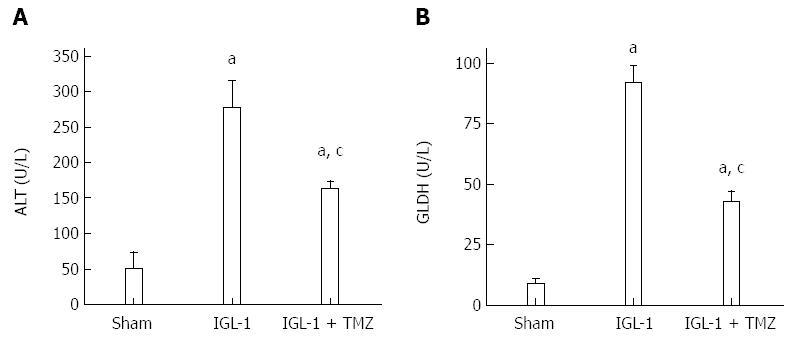
Hepatic injury was assessed in terms of alanine aminotransferase (ALT) levels with commercial kits from RAL (Barcelona, Spain). Briefly, plasma extracts were collected before liver extraction and centrifuged at 4 °C for 10 min at 0.8 g. Then, 200 μL of the supernatant was added to the substrate provided by the commercial kit. ALT levels were determined at 365 nm with a UV spectrometer (DU 800, Beckman Coulter) and calculated following the supplier’s instructions[14].
Glutamate dehydrogenase (GLDH) is a mitochondrial enzyme that catalyses the conversion of glutamate to 2-oxoglutarate. It was used as an indirect marker of mitochondrial damage; it was measured in plasma, as described previously[15].
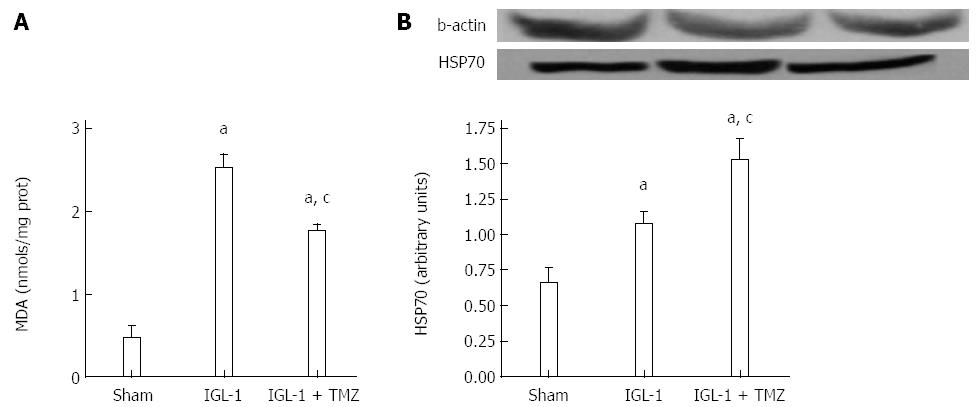
Lipid peroxidation in the liver was used as an indirect measure of the oxidative injury induced by ROS. Lipid peroxidation was determined by measuring the formation of malondialdehyde (MDA) with the thiobarbiturate reaction[16]. Liver samples were homogenized in Tris-HCL pH = 7 and 250 μL of trichloroacetic acid (TCA) were added to 250 μL of liver homogenates. Then, the samples were centrifugated in 3000 rpm at 4 °C for 15 min. Then, 250 μL of thiobarbituric acid (TBA) were added to the supernatant and were heated at 100 °C for 30 min. MDA reacted with TBA to form a pink chromogenic compound whose absorbance at 540 nm was measured. The result was expressed as nmols/mg protein.
NAD+/NADH from liver were quantified with a commercially available kit (MAK037, Sigma Chemical, St. Louis, MO, United States) according to the manufacturer’s instructions.
Liver tissue was homogenized in HEPES buffer as previously described[10]. Fifty μg of proteins was separated on 8%-15% SDS-PAGE gels and trans-blotted on PVDF membranes (Bio-Rad). Membranes were then blocked for one hour with 5% (w/v) non-fat milk in T-TBS and incubated overnight at 4 °C with antibody against SIRT1 (#07-131), p-mTOR (Ser2481, #09-343), mTOR (#04-385), all purchased from Merck Millipore, Billerica, MA; ac-p53 (ab37318, abcam, United kingdom); ac-FoxO1 (D-19, sc-49437), BECN1 (H-300, sc-11427), both purchased from Santa Cruz Biotechnology Inc, CA, United States; p-AMPK (Thr172, #2535), p-p38 mitogen activated protein (MAP) kinase (Thr180/Tyr182,#9211), p-70S6K (Thr389, #9205), LC3B (#2775), p-p44/42 MAPK (Erk1/2, Thr202/Tyr204), #9101, all from Cell Signaling, Danvers, MA, NAMPT (AP22021SU, Acris Antibodies GmbH, Germany), HSP70 (610607, Transduction Laboratories, Lexington, KY) and b-actin (A5316, Sigma Chemical, St. Louis, MO, United States). Membranes were then incubated for 1 h at room temperature with the corresponding secondary antibody linked to horseradish peroxidase. Bound complexes were detected using WesternBright ECL-HRP substrate (Advansta, Barcelona, Spain) and were quantified using the Quantity One software for image analysis. Results were expressed as the densitometric ratio between the protein of interest and the loading control (b-actin).
Data are expressed as mean ± SE. Statistical comparison was performed by variance analysis, followed by the Student-Newman-Keuls test (Graft Pad prism software). P < 0.05 was considered significant.
We first determined liver and mitochondrial injury through transaminase and GLDH levels respectively in the different experimental groups. Livers preserved in IGL-1 and subjected to OLT showed the highest ALT and GLDH levels, whereas addition of TMZ to the IGL-1 solution resulted in a significant decrease in liver and mitochondrial injury in comparison with IGL-1 solution alone (Figure 1).
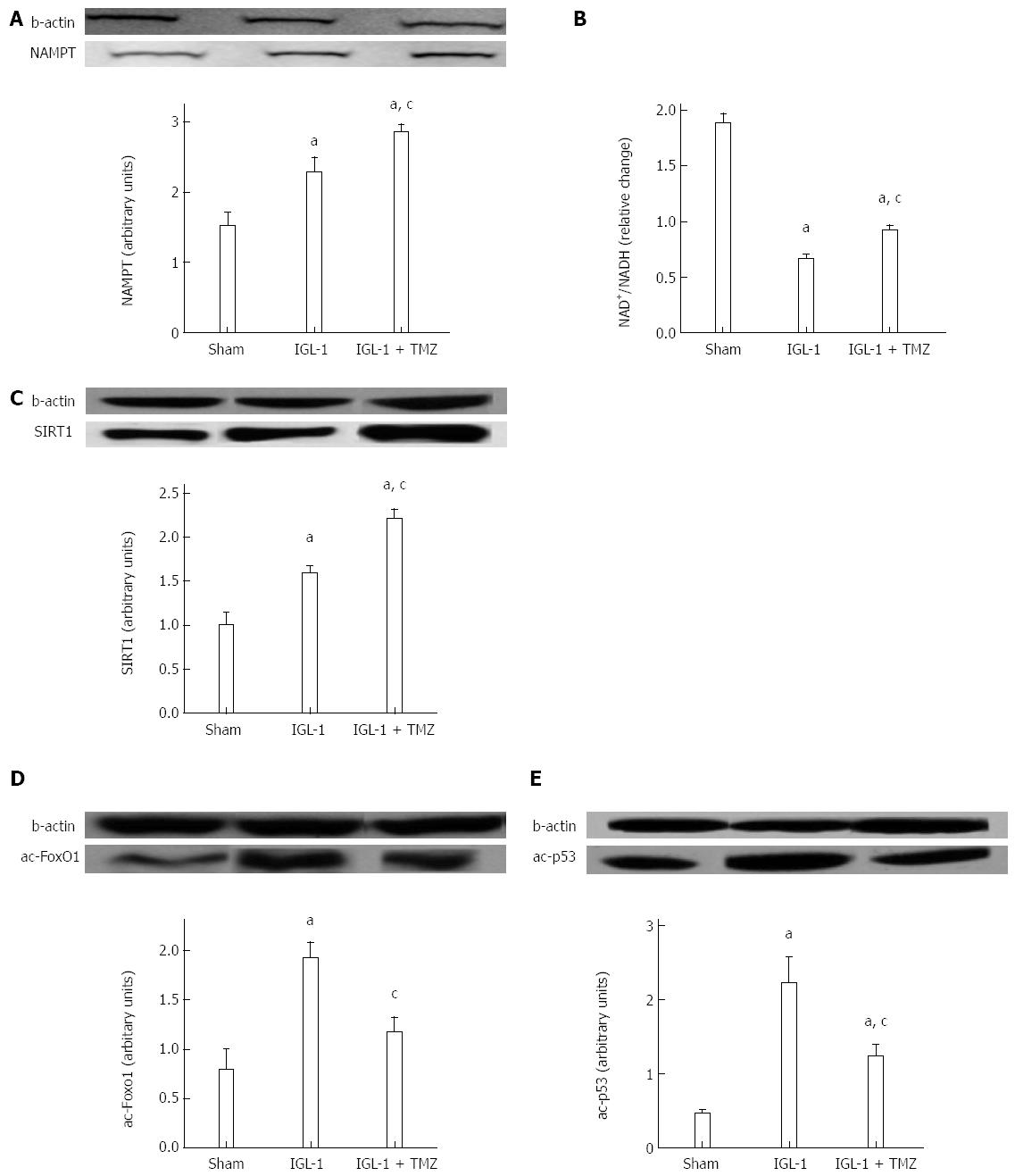
In order to explore the potential involvement of SIRT1 in the protective effects of TMZ on rat OLT, we first determined its protein expression pattern. SIRT1 protein expression was significantly increased when livers were preserved in IGL-1 solution compared with the Sham group (Figure 2C). Interestingly, the addition of TMZ to IGL-1 solution clearly enhanced SIRT1 expression compared with IGL-1 solution alone (Figure 2C). In view of the altered SIRT1 protein levels, we then investigated parameters associated with SIRT1 activity, such as the protein expression of nicotinamide phosphoribosyltransferase (NAMPT), the NAD+/NADH levels, as well as the acetylation state of two direct substrates, p53 and FoxO1. As shown in Figure 2A, liver graft preservation in IGL-1 solution led to high NAMPT protein expression, which was further enhanced in case of IGL-1 supplemented with TMZ. Furthermore, NAD+/NADH levels were significantly reduced in both IGL-1 groups in comparison to non-treated animals (Figure 2B). However, the presence of TMZ in IGL-1 resulted in a better preservation of NAD+/NADH levels than the IGL-1 alone (Figure 2B). This was consistent with decreased acetylated FoxO1 and p53 protein levels in IGL-1 + TMZ group compared with IGL-1 (Figure 2D and Figure 2E respectively). These results suggest an increase in SIRT1 activity in the IGL-1 + TMZ group; therefore, the protective effect of TMZ is exerted, at least in part, through the induction of both SIRT1 expression and activation.
Next, we evaluated lipid peroxidation as an indicator of oxidative stress in OLT. Livers preserved in IGL-1 solution showed significantly increased MDA compared with Sham (Figure 3A). This increase was prevented by the addition of TMZ in IGL-1 solution. Moreover, livers preserved in IGL-1 solution up-regulated the levels of the cytoprotective heat shock protein 70 (HSP70), which was further enhanced in the presence of TMZ (Figure 3B).
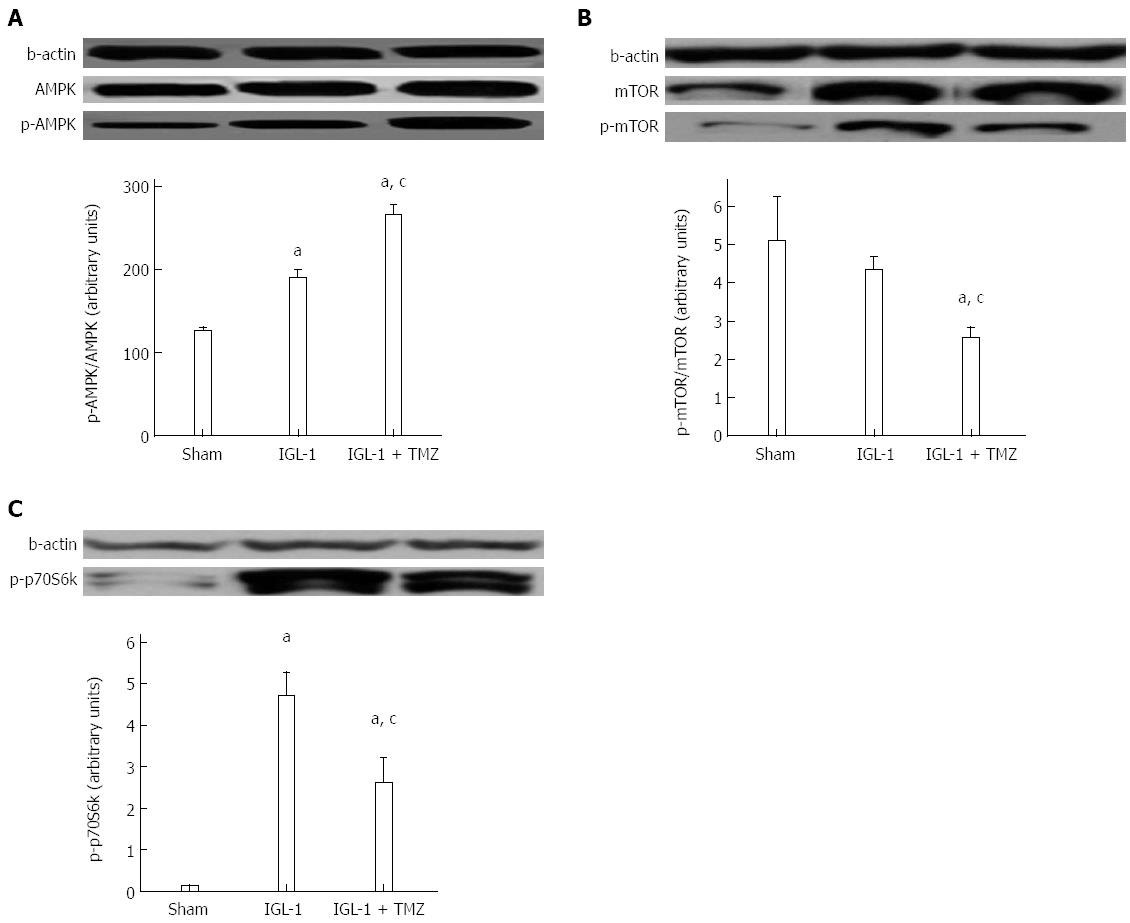
It is known that both AMP-activated protein kinase (AMPK) and SIRT1 regulate each other and share many common target molecules[17]. We therefore assessed the possible involvement of AMPK activation in the protective effects of TMZ. As shown in Figure 4A, livers preserved with IGL-1 solution showed increased phosphorylated AMPK (p-AMPK). However, this AMPK activation was further enhanced in the presence of TMZ. Given that the mammalian target of rapamycin (mTOR) activity is regulated by AMPK, we next evaluated both phosphorylated mTOR (p-mTOR) and mTOR protein levels as well as the phosphorylation levels of its direct substrate, p-p70S6K. As shown in Figure 4B and C, the addition of TMZ to IGL-1 solution significantly reduced p-mTOR/mTOR and p-p70S6k protein levels compared with IGL-1 preservation solution alone.
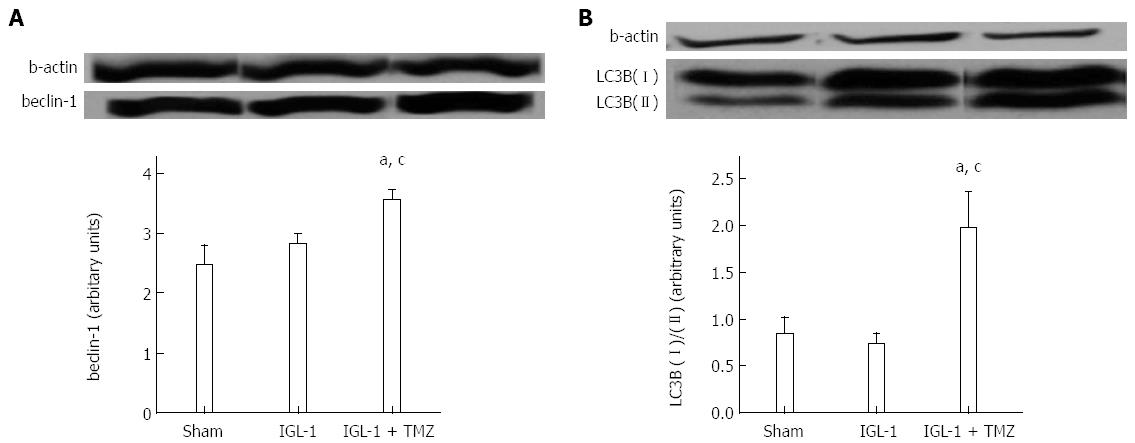
Autophagy is a conserved cellular process that is activated under conditions of nutrient stress to promote cell survival. Given the importance of the mTOR signaling pathway in the suppression of autophagy[18] and the fact that IGL-1 preservation solution enriched with TMZ was characterized by inactivation of both mTOR and p70S6k protein, we sought to test whether TMZ-induced SIRT1 protective effects might be mediated through the activation of autophagy. To do so, we explored the expression of beclin-1 and LC3B, two well-known proteins involved in the autophagic pathway. Both proteins presented significant up-regulation in the IGL-1 + TMZ group compared with both Sham and IGL-1 group (Figure 5A, B).
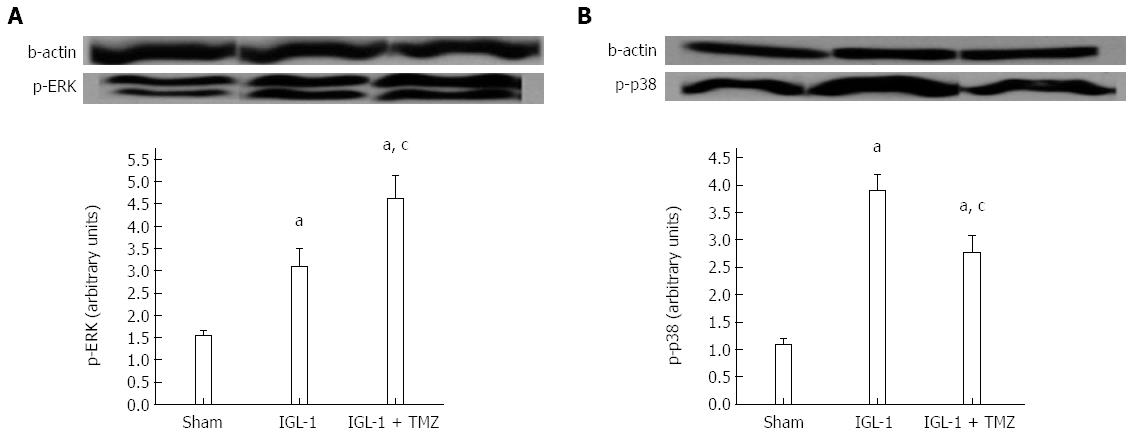
Finally, we explored the modulation of MAP kinases by TMZ. Here we observed increased phosphorylation of extracellular signal-regulated kinase (ERK) in IGL-1 preserved livers, which was further enhanced when TMZ was added to the preservation solution (Figure 6A). Moreover, the presence of TMZ in IGL-1 solution reversed the increased phosphorylation of p38 protein levels detected in the livers preserved in IGL-1 solution (Figures 6B and 7).
The present study provides the first evidence that SIRT1 up-regulation in a rat model of OLT contributes to a better preservation of liver grafts against IRI. SIRT1 over-expression is attributed to the use of a modified IGL-1 preservation solution enriched with TMZ, an anti-ischemic drug administered for the treatment of angina pectoris[19]. In earlier work with an isolated perfused rat liver model, we already showed that TMZ addition to IGL-1 solution promotes cytoprotective markers that are induced during ischemia, such as hypoxia inducible factor-1α through nitric oxide generation, as well as those promoted during reperfusion, such as heme oxygenase-1[13]. Here, we report that the addition of TMZ in IGL-1 preservation solution favors the activation of SIRT1 in rat OLT.
The increases observed in SIRT1 protein levels were consistent with an effective prevention of liver injury and mitochondrial damage. We previously reported the protective effect of TMZ on liver damage after graft preservation, and showed that its addition to UW solution reduces liver injury and improves liver function[20]. Our current data confirm that SIRT1 up-regulation in OLT due to TMZ can be considered as a protective cellular signaling response against IRI. This finding corroborates previous reports of a protective effect for SIRT1 against IRI in different organs such as heart, kidney and brain[21-23].
SIRT1 over-expression in liver grafts preserved in IGL-1 + TMZ was concomitant with increased levels of both NAMPT and NAD+. NAD+ is a cofactor required for SIRT1 enzymatic activity. Furthermore, it has been shown that NAMPT is the rate-limiting enzyme in the NAD+ biosynthetic pathway and directly regulates SIRT1 activity in mammalian cells[24]. NAMPT up-regulation has been shown to be beneficial against cardiac IRI through preservation of NAD+ levels and SIRT1 regulation[25]. Taking this into account, we suggest that in the IGL-1 + TMZ group the increased NAMPT protein levels promote NAD+ production, which in turn contributes to the high SIRT1 deacetylase activity. Indeed, in the same group we observed decreased levels of acetylated p53 and FoxO1, two direct substrates of SIRT1, which have been reported to be SIRT1 activity markers[26,27]. Therefore, the TMZ protective effects observed are mediated, at least in part, by enhanced SIRT1 activity.
SIRT1 plays an important role in cellular stress, including the oxidative stress associated with IRI. FoxO1 deacetylation by SIRT1 has been linked with the capacity of FoxO1 to enhance the transcription of anti-oxidant enzymes, thus contributing to the resistance against oxidative stress[4,5,21]. For these reasons, we decided to explore the potential relationship between SIRT1, ac-FoxO1 and the lipid peroxidation that occurs in OLT. In the present study, we found that the significant SIRT1 induction and FoxO1 deacetylation were accompanied by marked decreases in lipid peroxidation when TMZ was added to IGL-1. Moreover, SIRT1 up-regulation in OLT may contribute to reducing hepatic vulnerability against oxidative stress and to improving the mitochondrial status during oxidative stress conditions. Our results corroborate those of Ou et al[28] who demonstrated that over-expression of SIRT1 under oxidative stress conditions enhanced mitochondrial function in embryonic stem cells. In addition, Hsu et al[21] demonstrated that hearts over-expressing SIRT1 were more resistant to oxidative stress in response to IRI, due, in part, to the effective FoxO1 deacetylation by SIRT1.
It is well known that increases in heat shock protein expression are involved in the protection against oxidative stress[29]. Our results also demonstrate strong HSP70 protein expression in the IGL-1 + TMZ group. This direct connection between SIRT1 and HSP70 during liver IRI was recently reported by our group in liver ischemic preconditioning[10].
Autophagy plays an important role in both sensing oxidative stress and removing oxidative damaged proteins and organelles[30]. This process involves the inhibition of p-mTOR (mammalian TOR) and the subsequent inactivation of its direct substrate, p70S6k[31]. During autophagy, specific proteins such as beclin-1 (in the initial stages of autophagosome formation) and LC3B (during autophagosome expansion) are activated[32,33]. AMPK activation results in mTOR inhibition and subsequent autophagy activation[31]. In addition, SIRT1 deacetylase activity has been associated with activation of AMPK, and SIRT1- mediated deacetylation of FoxO1 has also been implicated in increased autophagy[34-36]. In our study, SIRT1 over-expression and the enhanced activity in the IGL-1 + TMZ group was concomitant with AMPK activation, reduced levels of p-mTOR/p-p70S6k, and increased autophagy (beclin-1 and LC3B). Our findings are in agreement with other investigations carried out in liver[37-39] and kidney[40] which have shown the protective effect of autophagy against IRI. Autophagy activation through a NAMPT/SIRT1-dependent mechanism has also been shown to be protective against cerebral ischemia[41].
However, the role of autophagy in IRI has been controversial, as various studies have evidenced either a beneficial or detrimental role. For example Jiang et al[40] have reported a renoprotective role of autophagy against IRI; during ischemia, autophagy can contribute to the provision of nutrients, whereas during reperfusion can eliminate damaged proteins and organelles. Similarly, Matsui et al[42] in cardiac IRI showed that autophagy was protective during ischemia, but beclin-1 dependent autophagy activation during reperfusion was associated with cell death. Autophagy is stimulated by various factors and it still remains unknown which steps determine the decision for the survival or death. Between them, the time of ischemia seems to influence the autophagy outcome, as a prolonged ischemia time can result in excessive activation of autophagy and subsequently to cell death[43]. In our case, autophagy is associated with decreased graft injury and thus we may suppose that eight hours of cold preservation is not sufficient time for an excessive and detrimental activation of autophagy. More profound investigations are required in order to define the regulatory mechanisms of autophagy.
In a recent publication, we reported that SIRT1 modulates (MAP) kinases, whose activation is a consequence of oxidative stress generation associated to IRI[16]. Here, we show that TMZ addition to IGL-1 enhances p-ERK protein levels and reduces p-p38 protein levels in comparison to IGL-1 alone. As a result, the increased SIRT1 over-expression in OLT coincided with the modulation of the MAP kinases, as reported in other studies[44].
Taken together, our results show that TMZ exerts its protective role against IRI associated with OLT, in part, through the induction of SIRT1 protein expression and activity. We found that SIRT1 up-regulation prevented liver injury and oxidative stress and promoted liver autophagy (see Figure 7). Our findings support the benefits of pharmacological activation of SIRT1, a new therapeutic strategy for improving liver graft preservation.
The authors would like to thank Michael Maudsley at the Language Advisory Service of the University of Barcelona for revising the English text.
Ischemia-reperfusion injury (IRI) is a complex but unavoidable situation during liver transplantation, which contributes to organ failure. Sirtuin 1 (SIRT1) is a NAD+-dependent deacetylase that regulates several cellular pathways associated with IRI, including oxidative stress and autophagy. Institut Georges Lopez 1 (IGL-1) preservation solution has been proposed as a good alternative to UW solution for the preservation of liver grafts. Moreover, the addition of trimetazidine (TMZ), an anti-ischemic drug, to both preservation solutions has been shown to improve liver graft preservation. In this study, the authors demonstrate that TMZ addition in IGL-1 solution reduces IRI associated with rat orthotopic liver transplantation (OLT) through increases in both SIRT1 protein expression and activity.
SIRT1 plays an important role in several processes, including IRI. Here we report for the first time that the presence of TMZ in IGL-1 provoked elevated FoxO1 deacetylation, oxidative stress diminution and augmented autophagy and was associated with SIRT1 activation.
SIRT1 exerts a protective effect against IRI in several organs through a variety of mechanisms. However, SIRT1 involvement in models of transplantation has not been determined to date. The present study evaluated the potential role of SIRT1 in a rat OLT model. SIRT1 was up-regulated when livers were stored in IGL + TMZ preservation solution and helped to improve the protection of liver grafts against IRI, as reflected by decreases in hepatic injury, mitochondrial damage, and oxidative stress.
Pharmacological treatment in order to enhance SIRT1 activity is a promising tool for reducing the detrimental effects of IRI associated with liver transplantation.
This is a relevant manuscript on to date important issue as IRI in organ transplantation as well as in other clinical condition. The manuscript is well written and the study is well conducted. The beneficial effect of adding trimetazidine on preservation solution is documented in several ways ranging from hepatic enzyme dosage to all the biological cellular expression of damage (SIRT1, ac-p53 ac-Fox O1 protein expression, to oxidative stress and HSP70 protein expression.
P- Reviewer: Fourtounas C, Fulop T, Salvadori M S- Editor: Qi Y L- Editor: A E- Editor: Liu XM
| 1. | Casillas-Ramírez A, Mosbah IB, Ramalho F, Roselló-Catafau J, Peralta C. Past and future approaches to ischemia-reperfusion lesion associated with liver transplantation. Life Sci. 2006;79:1881-1894. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 139] [Cited by in F6Publishing: 151] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 2. | Schemmer P, Lemasters JJ, Clavien PA. Ischemia/Reperfusion injury in liver surgery and transplantation. HPB Surg. 2012;2012:453295. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 3. | Pantazi E, Zaouali MA, Bejaoui M, Folch-Puy E, Ben Abdennebi H, Roselló-Catafau J. Role of sirtuins in ischemia-reperfusion injury. World J Gastroenterol. 2013;19:7594-7602. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 52] [Cited by in F6Publishing: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 4. | Nogueiras R, Habegger KM, Chaudhary N, Finan B, Banks AS, Dietrich MO, Horvath TL, Sinclair DA, Pfluger PT, Tschöp MH. Sirtuin 1 and sirtuin 3: physiological modulators of metabolism. Physiol Rev. 2012;92:1479-1514. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 426] [Cited by in F6Publishing: 486] [Article Influence: 40.5] [Reference Citation Analysis (0)] |
| 5. | Hori YS, Kuno A, Hosoda R, Horio Y. Regulation of FOXOs and p53 by SIRT1 modulators under oxidative stress. PLoS One. 2013;8:e73875. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 222] [Cited by in F6Publishing: 241] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 6. | Anekonda TS, Adamus G. Resveratrol prevents antibody-induced apoptotic death of retinal cells through upregulation of Sirt1 and Ku70. BMC Res Notes. 2008;1:122. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 54] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 7. | Stein S, Schäfer N, Breitenstein A, Besler C, Winnik S, Lohmann C, Heinrich K, Brokopp CE, Handschin C, Landmesser U. SIRT1 reduces endothelial activation without affecting vascular function in ApoE-/- mice. Aging (Albany NY). 2010;2:353-360. [PubMed] [Cited in This Article: ] |
| 8. | Kim DH, Jung YJ, Lee JE, Lee AS, Kang KP, Lee S, Park SK, Han MK, Lee SY, Ramkumar KM. SIRT1 activation by resveratrol ameliorates cisplatin-induced renal injury through deacetylation of p53. Am J Physiol Renal Physiol. 2011;301:F427-F435. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 121] [Cited by in F6Publishing: 132] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 9. | Calnan DR, Brunet A. The FoxO code. Oncogene. 2008;27:2276-2288. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 867] [Cited by in F6Publishing: 913] [Article Influence: 57.1] [Reference Citation Analysis (0)] |
| 10. | Pantazi E, Zaouali MA, Bejaoui M, Serafin A, Folch-Puy E, Petegnief V, De Vera N, Ben Abdennebi H, Rimola A, Roselló-Catafau J. Silent information regulator 1 protects the liver against ischemia-reperfusion injury: implications in steatotic liver ischemic preconditioning. Transpl Int. 2014;27:493-503. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 11. | Mosbah IB, Zaouali MA, Martel C, Bjaoui M, Abdennebi HB, Hotter G, Brenner C, Roselló-Catafau J. IGL-1 solution reduces endoplasmic reticulum stress and apoptosis in rat liver transplantation. Cell Death Dis. 2012;3:e279. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 12. | Ben Abdennebi H, Elrassi Z, Scoazec JY, Steghens JP, Ramella-Virieux S, Boillot O. Evaluation of IGL-1 preservation solution using an orthotopic liver transplantation model. World J Gastroenterol. 2006;12:5326-5330. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 23] [Cited by in F6Publishing: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Zaouali MA, Ben Mosbah I, Boncompagni E, Ben Abdennebi H, Mitjavila MT, Bartrons R, Freitas I, Rimola A, Roselló-Catafau J. Hypoxia inducible factor-1alpha accumulation in steatotic liver preservation: role of nitric oxide. World J Gastroenterol. 2010;16:3499-3509. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 41] [Cited by in F6Publishing: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 14. | Zaouali MA, Padrissa-Altés S, Ben Mosbah I, Ben Abdennebi H, Boillot O, Rimola A, Saidane-Mosbahi D, Roselló-Catafau J. Insulin like growth factor-1 increases fatty liver preservation in IGL-1 solution. World J Gastroenterol. 2010;16:5693-5700. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 19] [Cited by in F6Publishing: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 15. | Bejaoui M, Zaouali MA, Folch-Puy E, Pantazi E, Bardag-Gorce F, Carbonell T, Oliva J, Rimola A, Abdennebi HB, Roselló-Catafau J. Bortezomib enhances fatty liver preservation in Institut George Lopez-1 solution through adenosine monophosphate activated protein kinase and Akt/mTOR pathways. J Pharm Pharmacol. 2014;66:62-72. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 16. | Zaoualí MA, Reiter RJ, Padrissa-Altés S, Boncompagni E, García JJ, Ben Abnennebi H, Freitas I, García-Gil FA, Rosello-Catafau J. Melatonin protects steatotic and nonsteatotic liver grafts against cold ischemia and reperfusion injury. J Pineal Res. 2011;50:213-221. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | Ruderman NB, Xu XJ, Nelson L, Cacicedo JM, Saha AK, Lan F, Ido Y. AMPK and SIRT1: a long-standing partnership? Am J Physiol Endocrinol Metab. 2010;298:E751-E760. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 600] [Cited by in F6Publishing: 640] [Article Influence: 45.7] [Reference Citation Analysis (0)] |
| 18. | Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27-42. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4916] [Cited by in F6Publishing: 5437] [Article Influence: 339.8] [Reference Citation Analysis (0)] |
| 19. | Gupta R, Sawhney JP, Narain VS. Treatment of stable angina pectoris with trimetazidine modified release in Indian primary-care practice. Am J Cardiovasc Drugs. 2005;5:325-329. [PubMed] [Cited in This Article: ] |
| 20. | Ben Mosbah I, Casillas-Ramírez A, Xaus C, Serafín A, Roselló-Catafau J, Peralta C. Trimetazidine: is it a promising drug for use in steatotic grafts? World J Gastroenterol. 2006;12:908-914. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 21] [Cited by in F6Publishing: 23] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Hsu CP, Zhai P, Yamamoto T, Maejima Y, Matsushima S, Hariharan N, Shao D, Takagi H, Oka S, Sadoshima J. Silent information regulator 1 protects the heart from ischemia/reperfusion. Circulation. 2010;122:2170-2182. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 391] [Cited by in F6Publishing: 418] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 22. | Fan H, Yang HC, You L, Wang YY, He WJ, Hao CM. The histone deacetylase, SIRT1, contributes to the resistance of young mice to ischemia/reperfusion-induced acute kidney injury. Kidney Int. 2013;83:404-413. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 105] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 23. | Hernández-Jiménez M, Hurtado O, Cuartero MI, Ballesteros I, Moraga A, Pradillo JM, McBurney MW, Lizasoain I, Moro MA. Silent information regulator 1 protects the brain against cerebral ischemic damage. Stroke. 2013;44:2333-2337. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 162] [Cited by in F6Publishing: 172] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 24. | Revollo JR, Grimm AA, Imai S. The NAD biosynthesis pathway mediated by nicotinamide phosphoribosyltransferase regulates Sir2 activity in mammalian cells. J Biol Chem. 2004;279:50754-50763. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 684] [Cited by in F6Publishing: 737] [Article Influence: 36.9] [Reference Citation Analysis (0)] |
| 25. | Yamamoto T, Byun J, Zhai P, Ikeda Y, Oka S, Sadoshima J. Nicotinamide mononucleotide, an intermediate of NAD+ synthesis, protects the heart from ischemia and reperfusion. PLoS One. 2014;9:e98972. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 174] [Cited by in F6Publishing: 204] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 26. | Rebollo A, Roglans N, Baena M, Padrosa A, Sánchez RM, Merlos M, Alegret M, Laguna JC. Liquid fructose down-regulates liver insulin receptor substrate 2 and gluconeogenic enzymes by modifying nutrient sensing factors in rats. J Nutr Biochem. 2014;25:250-258. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 27. | Tajbakhsh N, Sokoya EM. Sirtuin 1 is upregulated in young obese Zucker rat cerebral arteries. Eur J Pharmacol. 2013;721:43-48. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 28. | Ou X, Lee MR, Huang X, Messina-Graham S, Broxmeyer HE. SIRT1 positively regulates autophagy and mitochondria function in embryonic stem cells under oxidative stress. Stem Cells. 2014;32:1183-1194. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 217] [Cited by in F6Publishing: 249] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 29. | Chien CY, Chien CT, Wang SS. Progressive thermopreconditioning attenuates rat cardiac ischemia/reperfusion injury by mitochondria-mediated antioxidant and antiapoptotic mechanisms. J Thorac Cardiovasc Surg. 2014;148:705-713. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 30. | Lee J, Giordano S, Zhang J. Autophagy, mitochondria and oxidative stress: cross-talk and redox signalling. Biochem J. 2012;441:523-540. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1031] [Cited by in F6Publishing: 1059] [Article Influence: 88.3] [Reference Citation Analysis (0)] |
| 31. | Inoki K, Kim J, Guan KL. AMPK and mTOR in cellular energy homeostasis and drug targets. Annu Rev Pharmacol Toxicol. 2012;52:381-400. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 540] [Cited by in F6Publishing: 564] [Article Influence: 43.4] [Reference Citation Analysis (0)] |
| 32. | Kroemer G, Mariño G, Levine B. Autophagy and the integrated stress response. Mol Cell. 2010;40:280-293. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2401] [Cited by in F6Publishing: 2580] [Article Influence: 184.3] [Reference Citation Analysis (0)] |
| 33. | Codogno P, Meijer AJ. Autophagy in the liver. J Hepatol. 2013;59:389-391. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 34. | Hou X, Xu S, Maitland-Toolan KA, Sato K, Jiang B, Ido Y, Lan F, Walsh K, Wierzbicki M, Verbeuren TJ. SIRT1 regulates hepatocyte lipid metabolism through activating AMP-activated protein kinase. J Biol Chem. 2008;283:20015-20026. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 590] [Cited by in F6Publishing: 623] [Article Influence: 38.9] [Reference Citation Analysis (0)] |
| 35. | Ng F, Tang BL. Sirtuins’ modulation of autophagy. J Cell Physiol. 2013;228:2262-2270. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 145] [Cited by in F6Publishing: 159] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 36. | Hariharan N, Maejima Y, Nakae J, Paik J, Depinho RA, Sadoshima J. Deacetylation of FoxO by Sirt1 Plays an Essential Role in Mediating Starvation-Induced Autophagy in Cardiac Myocytes. Circ Res. 2010;107:1470-1482. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 487] [Cited by in F6Publishing: 529] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 37. | Cardinal J, Pan P, Dhupar R, Ross M, Nakao A, Lotze M, Billiar T, Geller D, Tsung A. Cisplatin prevents high mobility group box 1 release and is protective in a murine model of hepatic ischemia/reperfusion injury. Hepatology. 2009;50:565-574. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 65] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 38. | Kim JS, Nitta T, Mohuczy D, O’Malley KA, Moldawer LL, Dunn WA, Behrns KE. Impaired autophagy: A mechanism of mitochondrial dysfunction in anoxic rat hepatocytes. Hepatology. 2008;47:1725-1736. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 155] [Cited by in F6Publishing: 166] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 39. | Lu Z, Dono K, Gotoh K, Shibata M, Koike M, Marubashi S, Miyamoto A, Takeda Y, Nagano H, Umeshita K. Participation of autophagy in the degeneration process of rat hepatocytes after transplantation following prolonged cold preservation. Arch Histol Cytol. 2005;68:71-80. [PubMed] [Cited in This Article: ] |
| 40. | Jiang M, Liu K, Luo J, Dong Z. Autophagy is a renoprotective mechanism during in vitro hypoxia and in vivo ischemia-reperfusion injury. Am J Pathol. 2010;176:1181-1192. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 286] [Cited by in F6Publishing: 305] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 41. | Wang P, Guan YF, Du H, Zhai QW, Su DF, Miao CY. Induction of autophagy contributes to the neuroprotection of nicotinamide phosphoribosyltransferase in cerebral ischemia. Autophagy. 2012;8:77-87. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 243] [Cited by in F6Publishing: 266] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 42. | Matsui Y, Takagi H, Qu X, Abdellatif M, Sakoda H, Asano T, Levine B, Sadoshima J. Distinct roles of autophagy in the heart during ischemia and reperfusion: roles of AMP-activated protein kinase and Beclin 1 in mediating autophagy. Circ Res. 2007;100:914-922. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1118] [Cited by in F6Publishing: 1176] [Article Influence: 69.2] [Reference Citation Analysis (0)] |
| 43. | Decuypere JP, Pirenne J, Jochmans I. Autophagy in renal ischemia-reperfusion injury: friend or foe? Am J Transplant. 2014;14:1464-1465. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 44. | Becatti M, Taddei N, Cecchi C, Nassi N, Nassi PA, Fiorillo C. SIRT1 modulates MAPK pathways in ischemic-reperfused cardiomyocytes. Cell Mol Life Sci. 2012;69:2245-2260. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 112] [Article Influence: 9.3] [Reference Citation Analysis (0)] |