Copyright
©The Author(s) 2015.
World J Gastroenterol. Nov 7, 2015; 21(41): 11740-11747
Published online Nov 7, 2015. doi: 10.3748/wjg.v21.i41.11740
Published online Nov 7, 2015. doi: 10.3748/wjg.v21.i41.11740
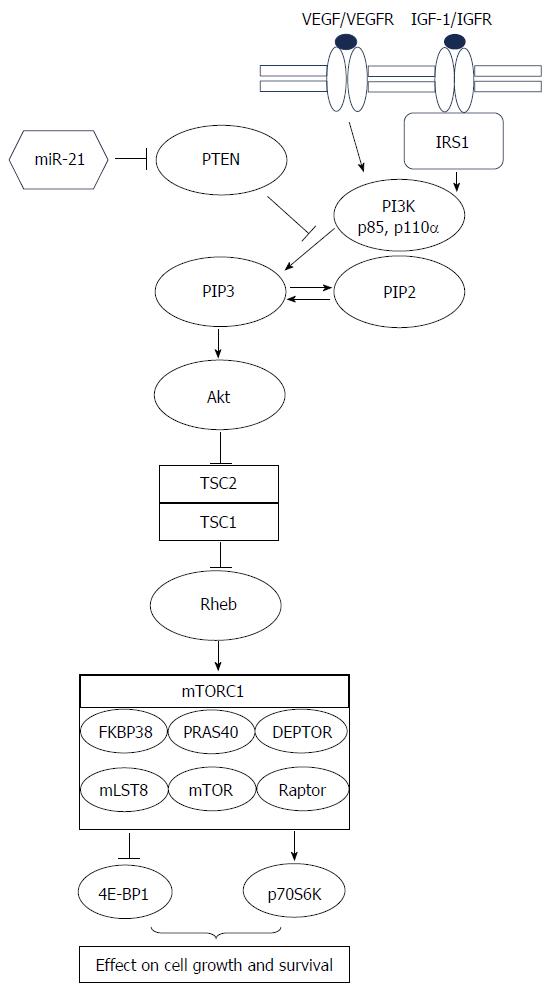
Figure 1 The PI3K/PTEN/Akt/mTOR-cascade (Modified according to Cingarlini et al[29] and McCubrey et al[32]).
Phosphatidylinositol-3-kinase (PI3K) is a heterodimeric protein with an p85-kDA regulatory subunit and a p110α-kDa catalytic subunit (PIK3CA). PI3K phosphorylates membrane phospholipids, thereby forming the second messenger lipids phosphatidylinositol 3,4-biphosphate (PIP2) and phosphatidylinositol 3,4,5-triphosphate (PIP3). Pleckstrin-homology (PH) domain of kinase Akt binds to PIP3, thereby promoting activation of Akt via phosphorylation by phosphotidylinositide-dependent kinase 1 (PDK1, not displayed in the Figure). Akt inhibits tuberous sclerosis 2 (TSC2 or tuberin) function through direct phosphorylation. TSC2 phosphorylation by Akt represses activity of the TSC1/TSC2 complex, allowing the small GTPase Rheb to activate the protein kinase function of mTOR (mammalian target of rapamycin). mTOR forms the catalytic core of the mTORC1 complex, which comprises additionally Raptor (Regulatory associated protein of mTOR) adaptor protein, DEPTOR (DEP domain containing mTOR-interacting protein), mLST8 (member of the Lethal-with-Sec-Thirteen gene family), FKBP38 (FK506 binding protein 38), and PRAS40 (proline-rich Akt substrate 40 kDa protein). Activated mTOR phosphorylates p70S6K, which induces a negative feedback loop uncoupling IRS-1 (insulin receptor substrate-1) from PI3K, thus preventing further signal transduction through this pathway. Negative regulation of the PI3K pathway is primarily accomplished though the PTEN tumor suppressor protein, which dephosphorylates phosphoinositide substrates as PIP3. Expression of PTEN is regulated by microRNA miR-21.
- Citation: Kleist B, Poetsch M. Neuroendocrine differentiation: The mysterious fellow of colorectal cancer. World J Gastroenterol 2015; 21(41): 11740-11747
- URL: https://www.wjgnet.com/1007-9327/full/v21/i41/11740.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i41.11740