Copyright
©The Author(s) 2015.
World J Gastroenterol. Nov 7, 2015; 21(41): 11502-11521
Published online Nov 7, 2015. doi: 10.3748/wjg.v21.i41.11502
Published online Nov 7, 2015. doi: 10.3748/wjg.v21.i41.11502
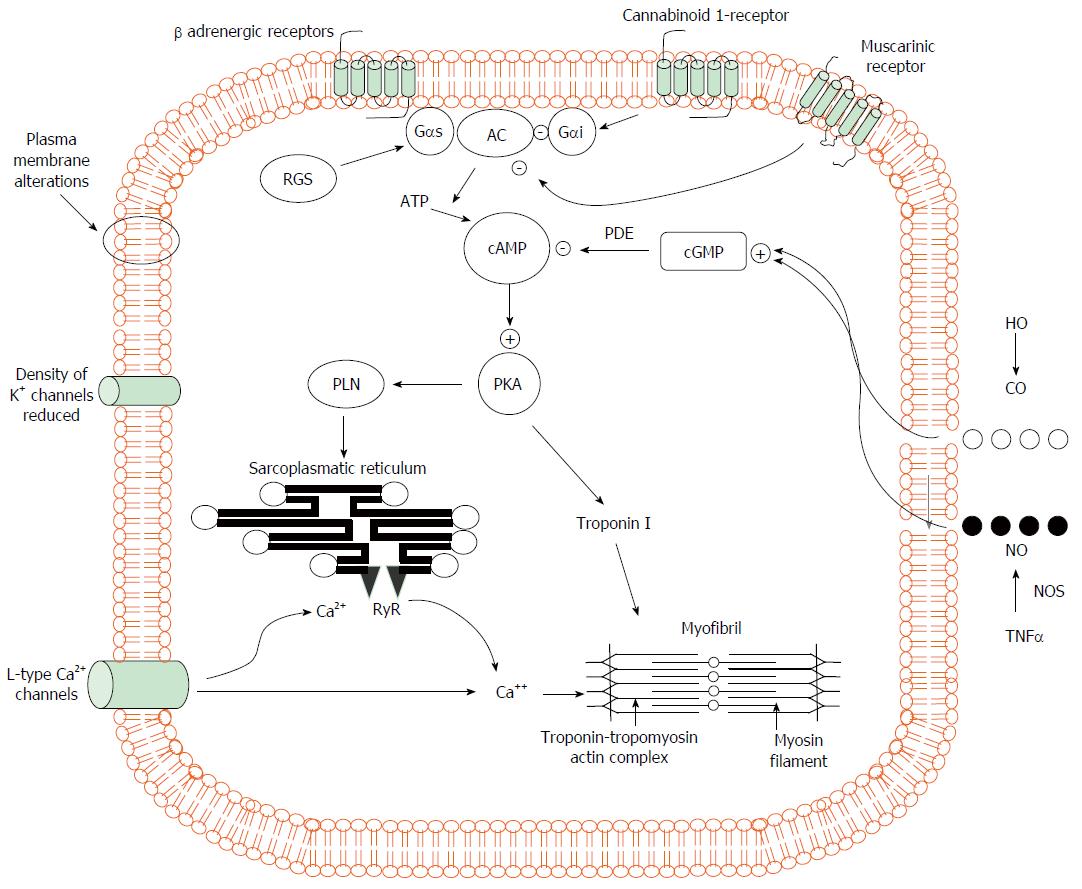
Figure 1 Pathogenic mechanisms of cardiomyocyte contraction in experimental cirrhosis.
Cardiomyocytes of cirrhotic animals showed reduced contractility via impairment of the βAR signalling (down-regulation, desensitization) which in turn leads to a reduction in βAR density, Gs proteins, and AC activity with resultant decreased cAMP generation. This second messenger cAMP, phosphorylates several proteins leading to intracellular calcium fluxes, calcium release and myocyte contraction. Postreceptor signaling through cAMP is severely impaired. In addition, the decreased fluidity of cardiomyocyte membrane interferes with the function of βAR, L-type calcium and potassium channels and impair stimulation of muscarinic acetylcholine receptors. The activation of CB1 inhibiting L-type calcium channels and AC together with increased NO and CO levels via a cGMP-dependent mechanism also contribute to decreased calcium ion influx or release from the sarcoplasmic reticulum with a related fall of calcium ion content and contractility. +: stimulatory influence; -: inhibitory influence. βAR: β-adrenergic receptors; CB1: Cannabinoid 1-receptor; AC: Adenylcyclase; cAMP: Cyclic adenosine monophosphate; Gαi: Inhibitory G protein; Gαs: Stimulatory G protein; RGS: Regulator of G-protein signaling; PLN: Phosphoslamban; PKA: Protein kinase A; cGMP: Cyclic guanosine monophosphate; PDE: Phosphosdiesterase; RyR: Ryanodine receptor; HO: Haemoxygenase; CO: Carbon monoxide; NO: Nitric oxide; NOS: Nitric oxide synthase; TNFα: Tumour necrosis factor α.
- Citation: Ruiz-del-Árbol L, Serradilla R. Cirrhotic cardiomyopathy. World J Gastroenterol 2015; 21(41): 11502-11521
- URL: https://www.wjgnet.com/1007-9327/full/v21/i41/11502.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i41.11502