INTRODUCTION
San-Huang-Xie-Xin-Tang (SHXXT), a traditional Chinese medicinal formula, is widely used in Eastern Asia, to ameliorate the symptoms of gastrointestinal (GI) disorders related to gastritis, gastric bleeding, peptic ulcers, and abnormal GI motility[1-3]. SHXXT is composed of Coptidis rhizoma (Coptis chinesis Franch), Rhei rhizoma (Rheum officinale Baill), and Scutellariae radix (Scutellaria baicalensis Georgi). Coptidis Rhizoma has been previously reported to have protective effects against ischemia induced by the renal dysfunction associated with various metabolic conditions, such as hypercholesterolemic diseases[4-6]. Rhei Rhizoma has been reported to regulate hepatic and pancreatic functions, to ameliorate senility and improve cognition[7-10]. Scutellariae Radix has been suggested to have anticonvulsant activity, cytoprotective properties and anti-inflammatory effects[11-13].
In traditional Chinese medicine, SHXXT is thought to reduce heat and the effects of toxins and to promote defecation; thus, it is used to treat constipation with high fever, restlessness, thirst, and insomnia. Recently, SHXXT has been reported to possess a variety of pathophysiological and pharmacological properties, which include anti-inflammatory[14-16], anti-oxidant[17,18], anti-hypertensive[19], immunomodulatory[20], anti-apoptotic[21], and neuroprotective[22,23] effects. In addition, SHXXT is thought to inhibit gastric secretion and promote gastric muscle relaxation[1].
In our previous study, we investigated the effects of SHXXT on the pacemaker potentials of cultured interstitial cells of Cajal (ICCs) derived from the murine small intestine[24]. ICCs are GI pacemaker cells that generate rhythmic oscillations in membrane potentials known as slow waves[25-28]. SHXXT was found to modulate pacemaker potentials in ICCs via 5-HT3 and 5-HT4 receptor-mediated pathways, external Ca2+ influx, and Ca2+ release from internal stores in a mitogen-activated protein kinase dependent manner. In addition, of the ingredients in SHXXT, Coptidis rhizome and Rhei rhizome have been reported to regulate pacemaker potentials[24]. Therefore, we hypothesized that SHXXT could modulate the pacemaking activities of ICCs in the GI tract and thus has potential pharmacological relevance for the treatment of GI motility disorders. However, despite the considerable use of SHXXT to treat GI dysfunction, little is known of its in vivo regulatory effects on GI motility. Therefore, we performed this study to investigate the effects of SHXXT on the mouse GI tract.
MATERIALS AND METHODS
Reagents and plant materials
HPLC grade ethanol, acetonitrile, and water were purchased from Fisher Scientific (Pittsburgh, PA, United States). Baicalein (> 97.2%), wogonin (> 99.8%), and sennoside A (> 97.7%) were obtained from the Korean Food and Drug Association (O-song, South Korea). Baicalin (> 95%), berberine (> 95%), palmatine chloride hydrate (> 97%), aloe-emodin (> 95%) and emodin (> 95%) were from Sigma-Aldrich (St. Louis, MO, United States). SHXXT (Coptidis rhizoma (Coptis chinesis Franch) 0.23 g, Scutellariae radix (Scutellaria baicalensis Georgi) 0.56 g, and Rhei rhizoma (Rheum officinale Baill) 1.28 g: total 2.07 g) was obtained from I-World Pharm Co., Ltd. (Anyang, South Korea). The preparation and analysis of some active fractions have been previously reported[24]. To compare the prokinetic activities of the drugs tested in this study, the aqueous extract of the dried immature fruit of Poncirus trifoliata Raf. (PF) was obtained as previously described[26]. PF, one of the most popular traditional folk medicines in South Korea derived from the Rutaceae fruits, is known to have unique and potent prokinetic activities in normal and GI motility dysfunction (GMD) rodents[26,29].
Preparation of standard and sample solutions
To prepare stock solutions of standard compounds, we accurately weighed out 10 mg of each of the compounds, except for aloe-emodin and sennoside A, and dissolved them in 10 mL of methanol. Aloe-emodin was dissolved in methanol containing 1% of DMSO, and sennoside A was dissolved in an NaHCO3 solution as described in The Korean Pharmacopoeia. Stock solutions were then diluted (10-fold) and filtered through a 0.2 μm syringe filter before injection. The final concentration of the standard solutions injected was 100 μg/mL. Sample solutions were made by dissolving 1.000 g of SHXXT in 10 mL of 60% methanol, diluted 10-fold, and filtered through a 0.2 μm syringe filter prior to injection. The final concentration of the sample solution injected was 10 mg/mL.
Chromatographic conditions
A smart LC system comprised of an LC800 (GL sciences, Japan) equipped with a solvent delivery unit, an autosampler, a column oven, and a UV-visible detector was used. Chromatographic separation was performed on an Inertsil ODS-4 column (2.1 mm x 150 mm, i.d. 3 μm; GL Sciences, Japan) at 40 °C. The mobile phase consisted of water (A) and acetonitrile (B), and the gradient program used was as follows: 25% (B) for 2 min, 25%-68% (B) from 2 to 18.5 min, 68%-25% (B) from 18.5 to 20 min, and 25% (B) for 2 min. The flow rate was set at 0.3 mL/min and the injection volume was 1 μL. The detection wavelength used for standard compounds was set at 280 nm. Acquired data were processed using EZChrom Elite software (Ver. 3.3.2 SP1, Pleasanton, CA, United States).
Animals
All animals were obtained from Samtako Bio Korea Co., Ltd. Male ICR mice weighing 25-30 g were used in the study. Animals were maintained under controlled conditions (22 ± 3 °C, humidity 52% ± 6%, and illumination from 6 a.m. to 6 p.m.). Animal care and experiments were conducted in accordance with the principles issued by the ethics committee of Pusan National University (South Korea). Animals were allowed free access to a commercial diet and tap water, but were deprived of food for 24 h before experiments. All experiments were conducted between 10 a.m. and 6 p.m.
Single oral dose toxicity of SHXXT extract in mice
SHXXT extract was administered intragastrically to mice through an orogastric tube at doses of 0, 0.5, 1, 2 or 5 g/kg delivered at 10 mL/kg. Seventy mice were tested for each dose and gender combination, and thus, a total of 60 mice were used. Each group was carefully observed for overt clinical signs and mortality at hourly intervals for 5 h after administration, and then on a daily basis for 14 d. Individual body weights were measured before dosing and on days 1, 3, 7 and 14 after administration. On day 14, the last day of observation, all animals were euthanized under ether anesthesia and necropsied.
Measurement of evans blue intestinal transit rates
The effect of SHXXT extract on intestinal propulsion was assessed by measuring the intestinal transit distance of Evans blue solution (5%, w/v, in DW). At 30 min after the intragastric administration of SHXXT extract in normal mice, Evans blue solution was intragastrically administered through an orogastric tube at a volume of 0.1 mL/kg of body weight. Animals were sacrificed 30 min after administration, and intestinal transit distance was determined by measuring the distance the Evans blue had migrated in the intestine from the pylorus. Intestinal transit rate (ITR) (%) was defined as the distance traveled by Evans blue expressed as a percentage of total small intestine length (from the pylorus to the ileal extremity). In order to minimize inter-day variations, ITRs were determined in normal and GDM mice on the same day.
Mouse model of peritoneal irritation induced by acetic acid
Peritoneal irritation was induced using acetic acid (AA) in mice 30 min after the intragastric administration of SC extract (or DW as vehicle). Peritoneal irritation was induced by an intraperitoneal injection of acetic acid (0.6%, w/v, in saline) at a dose of 10 mL/kg, as previously described[29,30]. After injecting acetic acid, mice were placed in individual cages and allowed to recover for 30 min.
Streptozotocin-induced diabetic mouse model
Male ICR mice (aged 5 wk) were used in this experiment. Mice were randomly allocated to either a control group or a diabetic group. To produce diabetes, mice were fasted overnight and, on the following day, streptozotocin (STZ) (Sigma-Aldrich, St. Louis, MO) solution was administered intraperitoneally. STZ was prepared freshly in 0.1 mol/L ice-cold citrate buffer (pH = 4.0) and administered at a dose of 200 mg/kg body weight. Control mice were intraperitoneally administered the same volume of 0.1 mol/L citrate buffer. Animals had free access to food and water and were maintained under standard housing conditions (24-27 °C, RH: 60%-65%) under a 12 h light/dark cycle. After two months, blood was withdrawn via the tail vein after an 8 h fast and blood glucose concentrations were measured using a one-touch blood glucose monitoring system (Johnson and Johnson). Diabetes was defined as a blood glucose level > 16 mmol/L.
Statistical analysis
Results are expressed as means ± SE. The Student’s t-test for unpaired data was used to compare the control and experimental groups. Statistical significance was accepted for P-values < 0.05.
RESULTS
Acute toxicity of SHXXT extract in normal mice
To examine the effect of SHXXT extract on GI motility, we used ICR normal mice. Of the 70 mice tested, no fatality occurred during 2 wk post-administration even at a dose of 5 g/kg, indicating that the minimal lethal dose of SHTTX extract in normal mice exceeds 5 g/kg. Furthermore, no abnormal clinical signs were observed and near identical increases in body weight were observed in treated and untreated mice. In addition, no abnormal findings were evident at the 14 d necropsy. Accordingly, SHXXT extract appeared to be safe, which is consistent with its widespread use in traditional Korean medicine at single doses of 9-12 g in humans[31].
Effects of SHXXT extract on ITR in normal mice
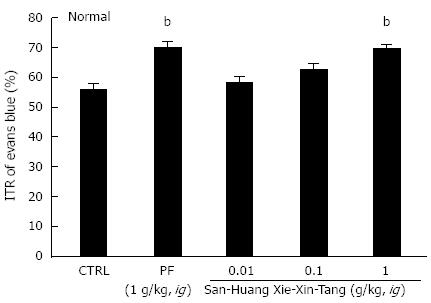
Mean ITR (%) values for Evans blue after 30 min in normal mice are shown in Figure 1. Mean ITR for non-treated normal mice (control) was 55.8% ± 1.7%. PF significantly accelerated ITR at an intragastric dose of 1 g/kg (69.5% ± 2.3%, P < 0.01), which is consistent with previous reports[32,33]. On the other hand, the ITR values for SHXXT extract at 0.01, 0.1 and 1 g/kg were 58.0% ± 1.5%, 62.3% ± 2.1% and 69.3% ± 1.6%, respectively (P < 0.01; Figure 1).
Figure 1 Effect of San-Huang-Xie-Xin-Tang extract on intestinal transit rate (%) in normal mice.
Mice (n = 7 for each bar, except for control) were treated with San-Huang-Xie-Xin-Tang extract and then intragastrically administered Evans blue 30 min later. Intestinal transit rate (ITR) (%) values were determined 30 min after Evans blue administration. Bars represent mean ± SE. bP < 0.01 vs control group. CTRL: Control; PF: Poncirus trifoliata Raf.
Effects of SHXXT extract on ITR in mice with GMD
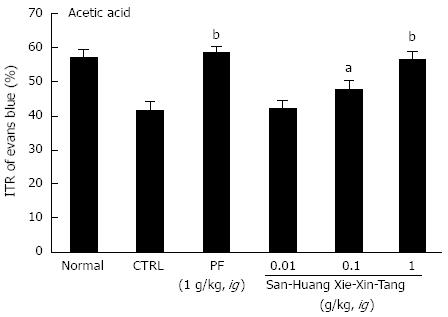
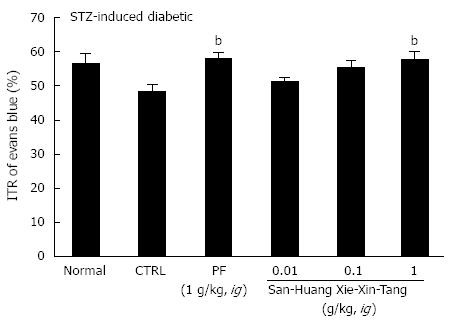
To examine the effect of SHXXT extract on GI motility, we used AA mouse and STZ-induced diabetic mouse models with experimental GMD. As expected, a significant retardation in ITR (%) was observed in the AA mouse model (41.5% ± 2.6%; Figure 2). Conversely, significant inhibition of this retardation was observed when SHXXT extract was intragastrically administered at 0.01, 0.1, or 1 g/kg [42.3% ± 2.2%, 47.8% ± 2.1% (P < 0.05) and 56.7% ± 2.4% (P < 0.01), respectively; Figure 2]. Additionally, PF (an intragastric dose of 1 g/kg) significantly accelerated ITR in a similar manner to SHXXT in the AA mouse model (58.8% ± 1.5%, P < 0.01; Figure 2). No abnormal clinical signs or changes were observed in AA mice after the intragastric administration of SHXXT. A significant retardation in ITR (%) was also observed in STZ-induced diabetic mice (48.5 ± 2.0%; Figure 3), and significant inhibition of this retardation was observed after intragastric administration of SHXXT extract at 0.01, 0.1 or 1 g/kg [51.2% ± 1.3%, 55.5% ± 1.9% and 57.8% ± 2.4% (P < 0.01), respectively; Figure 3]. PF (an intragastric dose of 1 g/kg) significantly accelerated ITR in a similar manner to SHXXT in STZ-induced diabetic mice (58.1% ± 1.8%, P < 0.01; Figure 3). No abnormal clinical signs or changes were observed in STZ-induced diabetic mice after administering SHXXT extract at any intragastric dose. These results suggest that SHXXT extract increased ITR in mice with GMD.
Figure 2 Effect of San-Huang-Xie-Xin-Tang extract on intestinal transit rate in acetic acid treated mice.
Mice (n = 6 for each bar) were treated with San-Huang-Xie-Xin-Tang extract and then intragastrically administered Evans blue 30 min later. Intestinal transit rate (ITR) (%) values were determined 30 min after Evans blue administration. Bars represent mean ± SE. aP < 0.05 vs control group; bP < 0.01 vs control group. CTRL: Control; PF: Poncirus trifoliata Raf.
Figure 3 Effect of San-Huang-Xie-Xin-Tang extract on intestinal transit rate in streptozotocin-induced diabetic mice.
Two months after administering streptozotocin (STZ), mice (n = 7 for each bar) were treated with San-Huang-Xie-Xin-Tang extract and then intragastrically administered Evans blue 30 min later. Intestinal transit rate (ITR) (%) values were determined 30 min after Evans blue administration. Bars represent mean ± SE. bP < 0.01 vs control group. CTRL: Control; PF: Poncirus trifoliata Raf.
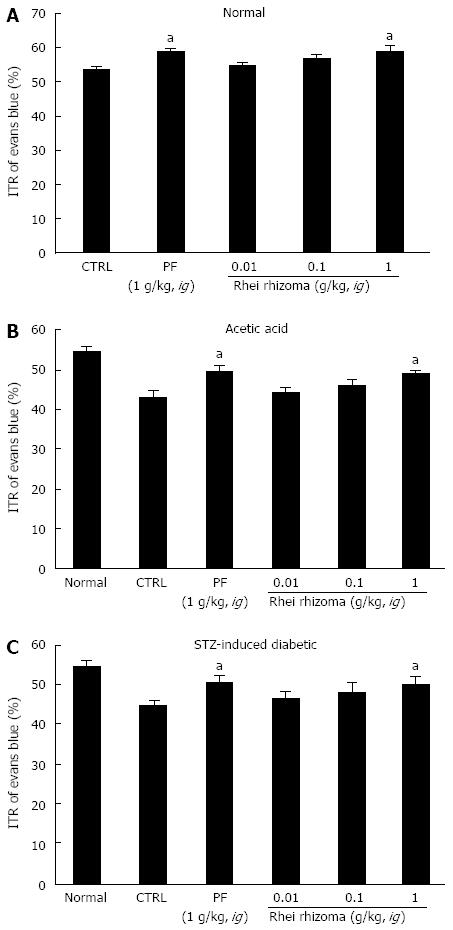
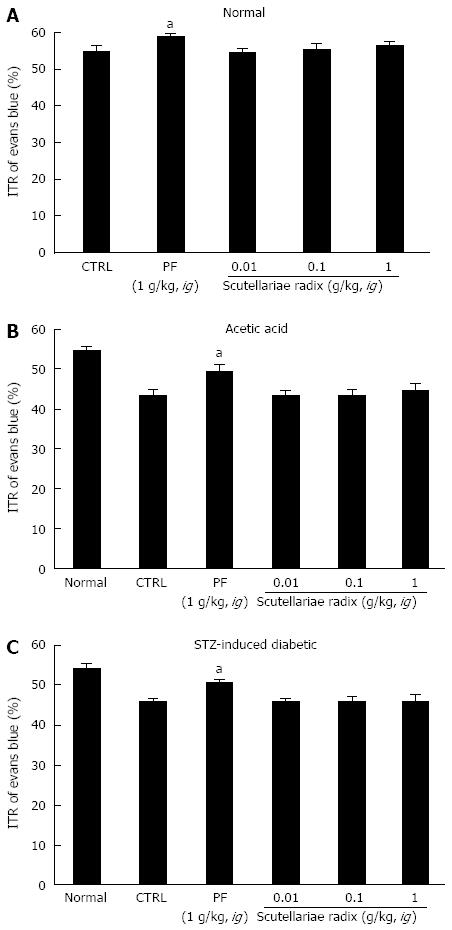
Effects of coptidis rhizoma, rhei rhizoma, and scutellariae radix extract on ITR in GI motility
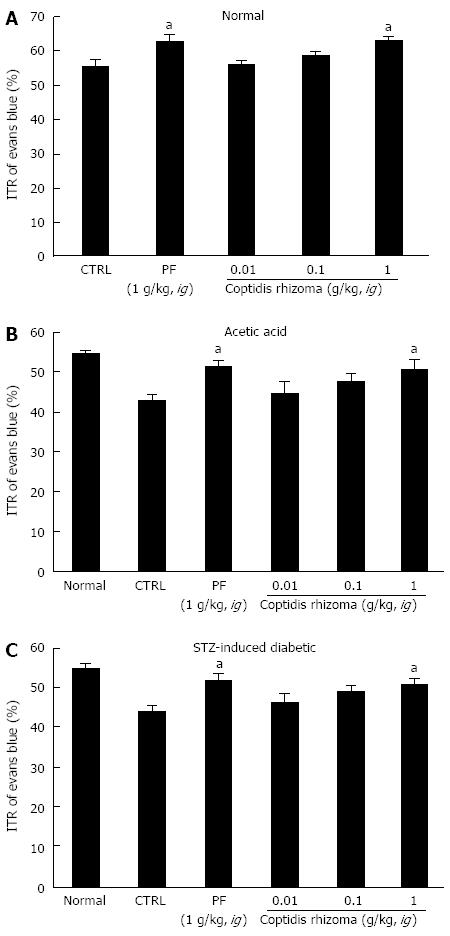
SHXXT is composed of Coptidis rhizoma (CR, Coptis chinesis Franch), Rhei rhizoma (RR, Rheum officinale Baill) and Scutellariae radix (SR, Scutellaria baicalensis Georgi). Therefore, we investigated the effects of these components in normal mice and in mouse GMD models. Mean ITR values for Evans blue during 30 min in normal mice treated with CR and RR are shown in Figures 4 and 5. CR and RR significantly accelerated ITR at intragastric doses of 1 g/kg [63.1% ± 1.4% (P < 0.05) and 59.2% ± 1.8% (P < 0.05), respectively; Figures 4A and 5A]. In addition, as expected, a significant retardation in ITR was observed in the AA and STZ-induced diabetic mouse models, and significant inhibition of this retardation was observed when CR or RR extract was intragastrically administered at 1 g/kg in the AA mouse model [51.1% ± 2.1% (P < 0.05) and 49.1% ± 0.8% (P < 0.05), respectively; Figures 4B and 5B] and in the STZ-induced diabetic mouse model [51.1% ± 1.6% (P < 0.05) and 50.1% ± 2.1% (P < 0.05), respectively; Figures 4C and 5C]. No abnormal clinical signs or changes were observed in the AA and STZ-induced diabetic mouse models after CR or RR administration. PF (an intragastric dose of 1 g/kg) significantly accelerated ITR in a similar manner to CR and RR (Figures 4 and 5). However, when SR was administered, no ITR changes were observed in normal or GDM mouse models (Figure 6). These results suggest that CR and RR increased ITR in mice.
Figure 4 Effect of Coptidis rhizoma extract on intestinal transit rate in normal mice and mice with gastrointestinal motility dysfunction.
Intestinal transit rate (ITR) values were determined 30 min following the intragastric administration of an Evans blue solution to normal mice (A), to mice 30 min after the induction of acetic acid (B), and to mice 2 mo after the induction of streptozotocin (STZ) (C) with Coptidis rhizoma extract. Bars represent mean ± SE. aP < 0.05 vs control group. CTRL: Control; PF: Poncirus trifoliata Raf.
Figure 5 Effect of Rhei rhizoma extract on intestinal transit rate in normal mice and mice with gastrointestinal motility dysfunction.
Intestinal transit rate (ITR) values were determined 30 min following the intragastric administration of an Evans blue solution to normal mice (A), to mice 30 min after the induction of acetic acid (B) and to mice 2 mo after the induction of streptozotocin (STZ) (C) with Rhei rhizoma extract. Bars represent mean ± SE. aP < 0.05 vs control group. CTRL: Control. PF: Poncirus trifoliata Raf.
Figure 6 Effect of Scutellariae radix extract on intestinal transit rate in normal mice and mice with gastrointestinal motility dysfunction.
Intestinal transit rate (ITR) values were determined 30 min following intragastric administration of an Evans blue solution to normal mice (A), mice 30 min after the induction of acetic acid (B) and to mice 2 mo after the induction of streptozotocin (STZ) (C) with Scutellariae radix extract. Bars represent mean ± SE. aP < 0.05 vs control group. CTRL: Control. PF: Poncirus trifoliata Raf.
DISCUSSION
The purpose of the present study was to investigate the effects of SHXXT on GI motor function. The findings of this study indicate for the first time that SHXXT has potent prokinetic activity in normal and GMD mice. Although traditional oriental medicines have been widely used for the treatment of a variety of digestive dysfunctions, few studies have been conducted on their effects on GI motor functions. Fructus Aurantii, the unripe fruit of Citrus aurantium Linn (Rutaceae), is a Qi-regulating drug that is used in traditional Chinese medicine to improve GI function. Fructus Aurantii has been reported to enhance GI motility by altering 5-HT and vasoactive intestinal peptide expression levels in the rat GI tract[34]. Aqueous extracts from the dried mature fruits of Aurantii nobilis Pericarpium have been used in traditional folk medicine to treat GI motility disorders in South Korea, and have been suggested to be potential prokinetic agents for the prevention or alleviation of GMD in humans[29]. On the other hand, Crotonis Fructus is the mature fruit of Croton tiglium L. (Euphorbiaceae), and has been used for thousands of years in traditional Chinese medicine to treat GI diseases, such as constipation, abdominal pain, peptic ulcer, and intestinal inflammation. Crotonis Fructus has been reported to regulate GI motility via the activation of M3 muscarinic receptors and Ca2+ influx through L-type Ca2+ channels[35]. Atractylodes Japonica Koidz (Compositae) is commonly used to treat GI disorders in Korean traditional medicine. It specifically acts on the distal colon longitudinal muscles within the GI smooth muscles via the activation of acetylcholinergic muscarinic receptors[36]. Daikenchuto (TU-100) is a traditional Japanese (Kampo) medicine used to treat postoperative ileus, and it has been reported to increase GI motility by modulating cholinergic and serotonergic mechanisms. Furthermore, it was found to have beneficial effects on functional constipation and irritable bowel syndrome[37]. SHXXT was widely used to ameliorate the symptoms of GI disorders related to gastritis, gastric bleeding, peptic ulcers, and abnormal GI motility. In addition, SHXXT has gastric protective mechanisms and promotes gastric muscle relaxation. SHXXT has been reported to decrease phosphodiesterase activity and smooth muscle tone in the GI tract, and the extents of phosphodiesterase inhibition and smooth muscle relaxation were found to be more prominent in the lower GI tract[2,3].
In a previous study, SHXXT was found to regulate ICC pacemaker activity. Furthermore, Coptidis rhizome and Rhei rhizoma modulated ICC pacemaking activity, whereas Scutellariae radix had no effect[24]. In the present study, ITR was significantly and dose-dependently increased by SHXXT (0.1-1 g/kg) in normal mice; in GMD mice, ITR was significantly retarded vs normal mice. However, this retardation was significantly and dose-dependently inhibited by SHXXT (0.1-1 g/kg), demonstrating that SHXXT has a modulatory effect on GI motility.
The GI tract consists of enteric neurons, interstitial cells, immune cells and smooth muscle cells. Interstitial cells include ICCs and are platelet-derived growth factor receptor alpha-positive (PDGFRα+). They generate pacemaker activity throughout the GI tract and transduce enteric nerve signals to adjacent smooth muscle cells[38]. Though we experimented with the effects of SHXXT on only ICCs, SHXXT depolarized the pacemaker activity of ICCs and increased the ITR of GI motility. Therefore, we think that SHXXT may have a potential role in the regulation of GI motility through the modulation of ICCs. In the future, we will experiment with the effects of SHXXT on enteric neurons and smooth muscle cells. In addition, it has been shown that the pacemaker activities of ICCs in the murine small intestine are primarily due to periodic activations of nonselective cation channels[25,39] or Cl- channels[40]. Kim et al[25] (2005) suggested that transient receptor potential melastatin (TRPM) 7 is required for ICC pacemaker activity in the murine small intestine, while Hwang et al[41] (2009) suggested that a Ca2+-activated Cl- channel (CaCC, Tmen16A, anoctamin 1 (ANO1)) is involved in ICC slow wave generation. Therefore, it has been proposed that TRPM7 and ANO1 may be important targets for the pharmacological treatment of GI motility disorders. In the future, because the TRPM7 or ANO1 channels are involved in the pacemaker activity of ICC, we will investigate which ion channel is involved in SHXXT action.
Taken together, our results suggest that SHXXT is a good candidate for the development of a gastroprokinetic agent. In addition, we suggest that the active mechanism of SHXXT on GI motility should be explored in vivo.
COMMENTS
Background
San-Huang-Xie-Xin-Tang (SHXXT) (composed of Coptidis Rhizoma (Coptis chinesis Franch), Rhei Rhizoma (Rheum officinale Baill), and Scutellariae Radix (Scutellaria baicalensis Georgi), a traditional Chinese medicinal formula, is widely used in Eastern Asia, to ameliorate the symptoms of gastrointestinal (GI) disorders. However, despite the considerable use of SHXXT to treat GI dysfunction, little is known of its in vivo regulatory effects on GI motility.
Research frontiers
SHXXT is a good candidate for the development of a gastroprokinetic agent.
Innovations and breakthroughs
In normal mice, intestinal transit rates (ITRs) were significantly and dose-dependently increased by SHXXT (0.1-1 g/kg). GI motility dysfunction (GMD) was induced by injecting acetic acid or streptozotocin intraperitoneally. ITRs of GMD mice were significantly reduced compared to normal mice, and these decreases were significantly and dose-dependently inhibited by SHXXT (0.1-1 g/kg).
Applications
SHXXT may be a new target or a novel candidate prokinetic agent for pharmacological treatment of GI motility disorders.
Terminology
GI motility: The movements of the digestive system, and the transit of the contents within it; ITRs: Passage of food (sometimes in the form of a test meal) through the GI tract as measured in minutes or hours; Interstitial cells of Cajal: The pacemaker cells of the GI tract.
Peer review
This study clarified the effect of SHXXT on GI motility. This is an interesting study, especially for those who reside in the Western Hemisphere and do not come in frequent contact with this substance.