Published online Oct 7, 2015. doi: 10.3748/wjg.v21.i37.10662
Peer-review started: February 26, 2015
First decision: April 13, 2015
Revised: April 18, 2015
Accepted: July 8, 2015
Article in press: July 8, 2015
Published online: October 7, 2015
Processing time: 216 Days and 17.7 Hours
AIM: To investigate the plasma levels of betatrophin in patients with cirrhosis.
METHODS: Forty patients diagnosed at the clinic with liver cirrhosis according to biological, ultrasonographic, or histological criteria were included. The severity of cirrhosis was classified according to Pugh’s modification of Child’s classification and MELD score. Insulin resistance (IR) was assessed by the Homeostasis Model Assessment. A total of 20 patients showed a MELD score higher than 14. The control group consisted in 15 sex-and aged-matched subjects. Fasting blood samples were obtained for subsequent analysis. Serum insulin was determined by Liaison automated immune chemiluminiscence assay (DiaSorin S.p.A.) using a sandwich assay. The sensitivity of the assay was 0.2 μU/mL. The intra and interassay variation coefficients were < 4% and < 10%, respectively. The normal values were between 2 and 17 μU/mL. Human active betatrophin was analyzed by specific quantitative sandwich ELISA (Aviscera Bioscience®). The sensitivity of the assay was 0.4 ng/mL, and the intra and interassay reproducibility were < 6% and < 10%, respectively.
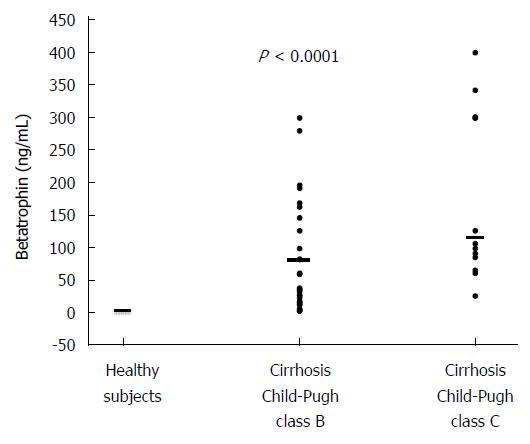
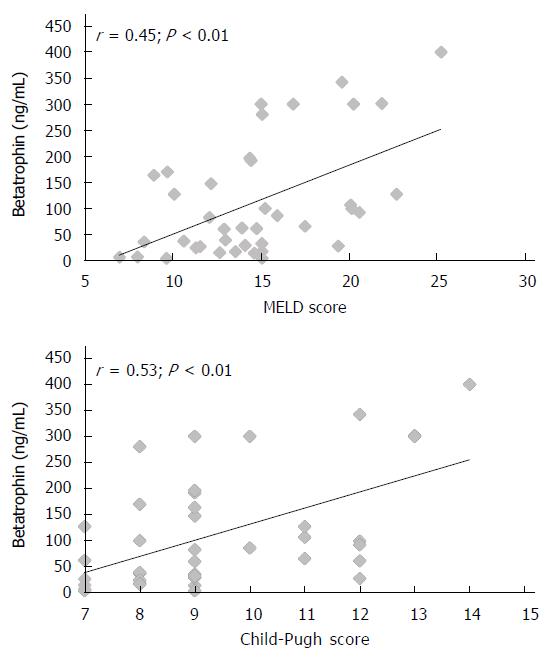
RESULTS: Plasma betatrophin levels were significantly increased in patients with cirrhosis compared with those in healthy subjects (P = 0.0001). Betatrophin levels were also associated with disease severity, being higher in Child-Pugh C patients compared to Child-Pugh B (P < 0.0005) and in patients who displayed a MELD score higher than 14 points compared to patients with lower punctuation (P = 0.01). In addition, we found a positive correlation between plasma betatrophin levels and the severity of cirrhosis according to Child-Pugh classification (r = 0.53; P < 0.01) or MELD score (r = 0.45; P < 0.01). In the overall cohort, a moderate correlation between serum betatrophin and plasmatic bilirrubin (r = 0.39; P < 0.01) has been observed, as well as an inverse correlation between betatrophin and albumin (r = -0.41; P < 0.01) or prothrombin time (r = -0.44; P <0.01). Moreover, insulin resistance was observed in 82.5% of the cirrhotic patients. In this group of patients, betatrophin levels were significantly higher than those in the group of patients without IR (P < 0.05).
CONCLUSION: Plasma betatrophin is increased in patients with cirrhosis. This increase is related to the severity of cirrhosis, as well as with the emergence of insulin resistance.
Core tip: Recently, Douglas A. Melton’s group from Harvard University reported the identification of betatrophin, a circulating protein secreted from the liver under insulin resistant states. Insulin resistance is common in patients with cirrhosis. In our study we confirm that betatrophin is increased in patients with liver cirrhosis, and the increase in plasma betatrophin levels is related to the severity of cirrhosis, and the emergence of insulin resistance. These preliminary results show that betatrophin may contribute to counteract, at least in part, insulin resistance in patients with cirrhosis.
- Citation: Arias-Loste MT, García-Unzueta MT, Llerena S, Iruzubieta P, Puente A, Cabezas J, Alonso C, Cuadrado A, Amado JA, Crespo J, Fábrega E. Plasma betatrophin levels in patients with liver cirrhosis. World J Gastroenterol 2015; 21(37): 10662-10668
- URL: https://www.wjgnet.com/1007-9327/full/v21/i37/10662.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i37.10662
The liver plays a key role in glucose homeostasis, and chronic liver disease leads to chronic disturbances in glucose metabolism. Insulin resistance (IR) is defined as a condition in which a higher insulin concentration is needed to achieve normal glucose metabolism; or when a normal insulin concentration fails to achieve normal glucose metabolism[1-3]. It is common in patients with cirrhosis, and in the 1960s, Megyesi et al[4] found that 57% of cirrhotic patients showed IR. Later, several studies in the 1980s and 1990s[5-12], used either an oral glucose load or the euglycemic-hyperinsulinemic clamp[13], to corroborate that IR is common and holds specific features in cirrhosis, irrespective of etiology. In fact, this process is observed in patients with cirrhosis even before the disturbance of glucose tolerance becomes prominent. In most situations of IR, β-cells compensate for this hormonal resistance for long periods of time by an increase in secretory capacity and in β-cell mass[14]. Recently, Douglas A. Melton’s group from Harvard University reported the identification of betatrophin as a circulating protein secreted from the liver under insulin resistant states. It is sufficient to dramatically and specifically increase the replication of β-cells in mice, resulting in an increased functional β-cell mass over time, with improvements in glucose tolerance[15]. In this regard, there has been increase interest in whether betatrophin is involved in the compensatory response to IR[14]. At present, the role of betatrophin in cirrhosis is unknown. The aim of this study was to investigate the plasma levels of betatrophin in cirrhosis and its relationship with the severity of the disease and IR.
Our study included 40 patients with liver cirrhosis. All patients were diagnosed with cirrhosis at the clinic, based on biological, ultrasonographic, or histological criteria. In all, 38 patients were men and 2 were women. Their ages ranged from 37 to 76 years (mean 56 years). The severity of cirrhosis was classified according to Pugh’s modification of Child’s classification and MELD score[16,17]. IR was assessed by the Homeostasis Model Assessment[18]. There were 27 patients in Child-Pugh’s class B, and 13 in Child-Pugh’s class C. A total of 20 patients showed a MELD score higher than 14. Patients with bacterial infection and or those who had GI bleeding, or taken vasoactive drugs within 14 d before the study were excluded. None of these patients were pregnant, had renal disease, or had specific medical diagnoses (e.g., type 2 diabetes mellitus, type 1 diabetes mellitus, hypothyroidism, Cushing’s disease, or polycystic ovary syndrome). Patients who had undergone previous surgery for portal hypertension were also excluded. No patients received medications known to affect body composition or lipid or glucose metabolism (e.g., the use of thyroid medication, thiazolidinediones, or metformin).
The control group consisted in 15 sex-and aged-matched subjects. The study was performed in accordance with the principles of the Declaration of Helsinki and its appendices and with local and national laws. Approval was obtained from the hospital’s Internal Review Board and Ethics Committee, and by written informed consent from all patients.
On the day of the study, after 12-h of overnight fasting, an antecubital vein was catheterized. Then, 45 min later, blood samples were taken to measure plasma levels of glucose, and serum insulin concentration. Plasma sodium, potassium, albumin, bilirubin, creatinine concentration, international normalized ratio, and prothrombin time were measured in each patient. In addition, samples for the analysis of betatrophin levels were isolated after centrifugation at 2400 g for 5 min. The samples were stored at -80 °C until analysis.
Serum insulin was determined by Liaison automated immune chemiluminiscence assay (DiaSorin S.p.A., Vercelly, Italy) using two specific monoclonal antibodies (sandwich assay). The sensitivity of the assay was 0.2 μU/mL. The intra and interassay variation coefficients were < 4% and < 10%, respectively. The normal values were between 2 and 17 μU/mL. Human active betatrophin was analyzed by specific quantitative sandwich ELISA (Aviscera Bioscience® AB, Santa Clara, CA, United States). The sensitivity of the assay was 0.4 ng/mL, and the intra and interassay reproducibility were < 6% and < 10%, respectively. These assays do not show significant cross-reactivity with other related hormones.
The data are presented as median and range for continuous variables and as count and percentage for categorical variables. Data distribution was nonparametric, as determined by the Shapiro-Wilk test. Quantitative variables were compared by means of Kruskal- Wallis test. Then, if the test identified a statistically significantly difference in the variable between groups, the Mann- Whitney U probability test was used to compare the different groups. The Bonferroni adjustment was performed according to the number of comparisons carried out. χ2 analysis or Fisher’s exact test was used to compare categorical data. Spearman’s correlation test was used to investigate possible associations between variables. In all analyses, a two-side P value of < 0.05 was considered statistically significant
Table 1 shows the clinical and laboratory characteristics of the three groups of subjects studied. The betatrophin levels were significantly higher in patients with cirrhosis than in healthy subjects (113 ng/mL, range 3-400 ng/mL vs 3.5 ng/mL, range 3-5.1 ng/mL, P < 0.0001). According to Child-Pugh classification, the betatrophin levels in Child-Pugh class B patients (81 ng/mL, range 3-300 ng/mL) were significantly lower than those in class C patients (117 ng/mL; range 27-400 ng/mL; P < 0.0005). Figure 1 shows the individual plasma betatrophin levels in the three groups of patients studied. In addition, the cirrhotic patients with a MELD score higher than 14 showed significantly higher betatrophin levels than the patients with a lower MELD score (152 ng/mL; range 3-400 ng/mL vs 71 ng/mL; range 3-196 ng/mL, P < 0.01). Moreover, IR was observed in 82.5% of the cirrhotic patients and a significant association between the coexistence of this condition and liver function measured according to Child-Pugh’s classification was observed (10 points; range 7-14 vs 8 points; range 7-8, P = 0.001). In these groups of patients, the betatrophin levels were significantly higher than in the group of patients without IR (128 ng/mL; range 6-400 ng/mL vs 38 ng/mL; range 3-170 ng/mL, P < 0.05). In addition, we found a moderate positive correlation between plasma betatrophin levels and severity of cirrhosis according Child-Pugh (r = 0.53; P < 0.01) or MELD score (r = 0.45; P < 0.01) (Figure 2). In the whole population of cirrhotic patients, the circulating levels of betatrophin weakly correlated with bilirubin levels (r = 0.39; P < 0.01), and showed a weak negative correlation with prothrombin time (r = -0.44; P < 0.01) and albumin serum levels (r = -0.41; P < 0.01).
| Healthy | Cirrhosis | Cirrhosis | P value | |
| Subjects | Child-Pugh's B | Child-Pugh's C | ||
| No. of subjects | 15 | 27 | 13 | NS |
| Sex (male/female) | 14/1 | 25/2 | 13/0 | NS |
| Age (yr) | 55 (30-74) | 57 (37-76) | 53 (44-60) | NS |
| Etiology of cirrhosis (alcohol/viral) | 19/8 | 10/3 | NS | |
| Insulin resistance (yes/no) | 0/15 | 20/7 | 13/0 | < 0.05 |
| Serum albumin (g/L) | 46 (43-49) | 30 (22-39) | 23 (20-29) | < 0.0001 |
| Serum bilirubin (mg/dL) | 0.7 (0.5-1.1) | 1.8 (0.4-3.2) | 5.8 (2.3-13.7) | < 0.0001 |
| Prothrombin time (%) | 97 (88-110) | 62 (37-94) | 46 (35-68) | < 0.0001 |
| Serum creatinine (mg/dL) | 0.8 (0.5-1.3) | 0.8 (0.4-1.4) | 0.7 (0.5-1.3) | NS |
Insulin resistance may play a role in the earlier stages of progression of chronic liver disease, as has already been suggested in patients with nonalcoholic fatty liver disease[19]. There is evidence that insulin stimulates the proliferation of hepatic stellate cells (HSCs) and affects the endothelial synthesis of nitric oxide and endothelin mediated by activated HSCs, which play key roles in the pathogenesis of hepatic fibrosis[20-24]. Hence, insulin can contribute to liver fibrosis and the deterioration of hepatic function by modulating the production and deposition of extracellular matrix and the regulation of vascular structure[22,23]. The present study confirms that IR is a very frequent phenomenon in patients with cirrhosis. These findings are in agreement with previous reports of IR in liver cirrhosis[4-13], even though our cohort included patients with more severe liver disease. Specifically, IR was found in 82% of our patients. Several mechanisms have been proposed to account for the IR in cirrhosis, including the desensitization and down regulation of insulin receptors (downstream of the receptor in the insulin signaling cascade)[25-27], insufficient hepatic insulin clearance due to reduced hepatocellular function, porto-systemic shunting of insulin from the splanchnic circulation to systemic circulation, and insufficient enhanced insulin secretion by pancreatic β-cells to maintain a normal glucose tolerance[8,28-31]. In fact, at the early stages of liver cirrhosis, patients do not lack insulin secretion or synthesis, and the pancreatic β-cells can secrete enough insulin to compensate for the IR. With the development of advanced liver cirrhosis, eventually, the pancreatic β-cells cannot continue to increase the secretion of insulin to compensate for the IR. This manifests as relatively insufficient insulin secretion and impaired glucose tolerance. Furthermore, hyperinsulinemia in these cirrhotic patients is also potentiated by the increase in counter-insulin hormones (e.g., glucagon, growth hormone, and insulin-like growth factor, free fatty acids, and cytokines)[7,8,32]. However, whether the hyperinsulinaemia in cirrhosis is a consequence of increased pancreatic insulin secretion, decreased hepatic insulin removal, or impaired feedback regulation of insulin secretion is still doubtful. In a recent study conducted by Greco et al[33], it was suggested that hyperinsulinemia, at least in Child-Pugh class B cirrhotic patients, is the consequence of increased β-cell sensitivity to glucose, whereas hepatic insulin extraction does not seem to play a significant role in this condition. The authors postulate that in euglycemic cirrhosis with advancement of liver disease, there is a compensatory increase in pancreatic β-cell insulin secretion to overcome the IR[34]. The factors contributing to β-cell hyperplasia in insulin-resistant states remain poorly understood, although there has been evidence that there is a circulating factor contributing to the increase secretory capacity, β-cell mass, or both, in insulin-resistant states[35,36].
Betatrophin, a novel secreted protein, can promote β-cell proliferation in mouse models of IR and improve glucose tolerance or metabolic control[15]. To better understand the roles of betatrophin in human disease, there has been a surge in interest in examining circulating betatrophin levels in patients[37-42]. These studies show that betatrophin levels are altered in various physiologic conditions, such as the postprandial state[38], and pathological conditions, such as type 2 diabetes[37-39,41,42] and, type 1 diabetes[40], and were associated with indexes of IR[39,41]. Yi et al[15] showed that the liver expressed the highest levels of betatrophin in humans. To the best of our knowledge, this is the first study to investigate the changes in betatrophin levels in cirrhosis. In our study, we observed that betatrophin circulates in normal human plasma. These findings are in agreement with previous reports of betatrophin measurements in adult healthy subjects[37-42]. Moreover, this study demonstrates, for the first time, that the plasma levels of betatrophin are increased in patients with liver cirrhosis compared to those of controls. These increases were also more pronounced in patients with advanced liver disease, particularly Child-Pugh class C or those with a MELD score greater than 14. Furthermore, it is interesting to note that betatrophin levels were significantly higher in patients with IR. The fact that these patients with IR had high levels of betatrophin may be interpreted as an acquired insensitivity to the effects of insulin, as occurs with betatrophin in other circumstances of IR, such as type 2 diabetes mellitus[37-39,41,42].
The causes of high plasma levels of betatrophin in cirrhotic patients were not specifically investigated in this study. The findings of a positive correlation between plasma betatrophin levels and severity of cirrhosis according to Child-Pugh or MELD score, an inverse relationship between betatrophin and protrombin time, or albumin levels, and a positive correlation between betatrophin and bilirubin levels may suggest that an impaired clearance of betatrophin could contribute to increased plasma betatrophin levels. However, this hypothesis is unlikely, because betatrophin is cleared from circulation by proteolytic regulation in vivo[43]. Because induced IR is a known potent stimulator of betatrophin expression in liver and fat tissue[15,44], it is possible, that the increased betatrophin concentrations in cirrhotic patients could be explained by IR[39]. However, it is not clear whether increased betatrophin expression is a compensatory response or only a marker of IR.
The present study is limited because we cannot determine a causal relationship between betatrophin and IR, and only an association between both variables can be inferred.
In summary, the present study demonstrates that circulating betatrophin is increased in patients with liver cirrhosis. The increase in plasma betatrophin levels is related to the severity of cirrhosis, and the emergence of IR. Thus, these preliminary results show that betatrophin may contribute to counteract, at least in part, IR in patients with cirrhosis. More studies are needed to confirm this possibility.
Betatrophin, a circulating protein secreted from the liver under insulin resistant states, has been recently described. In animal models it has been shown how betatrophin improves glucose tolerance. In this regard, there has been increase interest in whether betatrophin is involved in the compensatory response to insulin resistance.
Since insulin resistance is a common feature in patients with liver cirrhosis, unraveling the pathophysiological mechanisms underlying this condition is of great interest, and in this sense, the role of betatrophin in the setting of liver cirrhosis has not been previously addressed.
For the first time, the present study described an association between increased levels of plasmatic betatrophin and cirrhosis, with increasing levels according to disease severity. Moreover, in cirrhotic patients who display insulin resistance, betatrophin is significantly increased.
The results of this study may be of interest in the study of the different mechanisms that attempt to counteract insulin resistance in patients with cirrhosis
Maria Teresa and her colleagues highlighted the important relation between Betatrophin and cirrhosis. This study is so important, especially because many scientists and clinicians world wide want/ would like to support MELD score with supportive lab such Na and proteins such as Betatrophin prior to Liver Transplantation, or even in follow patients up. The study is normal extension of Melton DA novel work about Betatrophin, however this extension is vitally important, additionally it will open/support the future publication competition related- cirrhosis and circulating harming proteins.
P- Reviewer: Ali AEM, Morales-Gonzalez J, Toshikuni N S- Editor: Wang JL L- Editor: A E- Editor: Liu XM
| 1. | Kirwan JP, Solomon TP, Wojta DM, Staten MA, Holloszy JO. Effects of 7 days of exercise training on insulin sensitivity and responsiveness in type 2 diabetes mellitus. Am J Physiol Endocrinol Metab. 2009;297:E151-E156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 155] [Cited by in RCA: 142] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 2. | Cefalu WT. Insulin resistance: cellular and clinical concepts. Exp Biol Med (Maywood). 2001;226:13-26. [PubMed] |
| 3. | Reaven G. The metabolic syndrome or the insulin resistance syndrome? Different names, different concepts, and different goals. Endocrinol Metab Clin North Am. 2004;33:283-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 300] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 4. | Megyesi C, Samols E, Marks V. Glucose tolerance and diabetes in chronic liver disease. Lancet. 1967;2:1051-1056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 229] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 5. | Gragnoli G, Signorini AM, Tanganelli I. Plasma levels of insulin, C-peptide and glucagon in liver cirrhosis. J Endocrinol Invest. 1981;4:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 6. | Müller MJ, Willmann O, Rieger A, Fenk A, Selberg O, Lautz HU, Bürger M, Balks HJ, von zur Mühlen A, Schmidt FW. Mechanism of insulin resistance associated with liver cirrhosis. Gastroenterology. 1992;102:2033-2041. [PubMed] |
| 7. | Petrides AS, Stanley T, Matthews DE, Vogt C, Bush AJ, Lambeth H. Insulin resistance in cirrhosis: prolonged reduction of hyperinsulinemia normalizes insulin sensitivity. Hepatology. 1998;28:141-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 71] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 8. | Petrides AS, DeFronzo RA. Glucose and insulin metabolism in cirrhosis. J Hepatol. 1989;8:107-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 103] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 9. | Blei AT, Robbins DC, Drobny E, Baumann G, Rubenstein AH. Insulin resistance and insulin receptors in hepatic cirrhosis. Gastroenterology. 1982;83:1191-1199. [PubMed] |
| 10. | Gomis R, Fernández-Alvarez J, Pizcueta P, Fernández M, Casamitjana R, Bosch J, Rodés J. Impaired function of pancreatic islets from rats with portal hypertension resulting from cirrhosis and partial portal vein ligation. Hepatology. 1994;19:1257-1261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Kruszynska YT, Harry DS, Bergman RN, McIntyre N. Insulin sensitivity, insulin secretion and glucose effectiveness in diabetic and non-diabetic cirrhotic patients. Diabetologia. 1993;36:121-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 44] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | Nygren A, Adner N, Sundblad L, Wiechel KL. Insulin uptake by the human alcoholic cirrhotic liver. Metabolism. 1985;34:48-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237:E214-E223. [PubMed] |
| 14. | Araújo TG, Oliveira AG, Saad MJ. Insulin-resistance-associated compensatory mechanisms of pancreatic Beta cells: a current opinion. Front Endocrinol (Lausanne). 2013;4:146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Yi P, Park JS, Melton DA. Betatrophin: a hormone that controls pancreatic β cell proliferation. Cell. 2013;153:747-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 364] [Cited by in RCA: 358] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 16. | Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5490] [Cited by in RCA: 5728] [Article Influence: 110.2] [Reference Citation Analysis (2)] |
| 17. | Malinchoc M, Kamath PS, Gordon FD, Peine CJ, Rank J, ter Borg PC. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology. 2000;31:864-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1967] [Cited by in RCA: 2064] [Article Influence: 82.6] [Reference Citation Analysis (0)] |
| 18. | Ikeda Y, Suehiro T, Nakamura T, Kumon Y, Hashimoto K. Clinical significance of the insulin resistance index as assessed by homeostasis model assessment. Endocr J. 2001;48:81-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 104] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 19. | Francque S, Verrijken A, Mertens I, Hubens G, Van Marck E, Pelckmans P, Michielsen P, Van Gaal L. Visceral adiposity and insulin resistance are independent predictors of the presence of non-cirrhotic NAFLD-related portal hypertension. Int J Obes (Lond). 2011;35:270-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 20. | Vincent MA, Montagnani M, Quon MJ. Molecular and physiologic actions of insulin related to production of nitric oxide in vascular endothelium. Curr Diab Rep. 2003;3:279-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 167] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 21. | Iwakiri Y, Groszmann RJ. Vascular endothelial dysfunction in cirrhosis. J Hepatol. 2007;46:927-934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 207] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 22. | Svegliati-Baroni G, Ridolfi F, Di Sario A, Casini A, Marucci L, Gaggiotti G, Orlandoni P, Macarri G, Perego L, Benedetti A. Insulin and insulin-like growth factor-1 stimulate proliferation and type I collagen accumulation by human hepatic stellate cells: differential effects on signal transduction pathways. Hepatology. 1999;29:1743-1751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 248] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 23. | Paradis V, Perlemuter G, Bonvoust F, Dargere D, Parfait B, Vidaud M, Conti M, Huet S, Ba N, Buffet C. High glucose and hyperinsulinemia stimulate connective tissue growth factor expression: a potential mechanism involved in progression to fibrosis in nonalcoholic steatohepatitis. Hepatology. 2001;34:738-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 389] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 24. | Rockey DC. Hepatic fibrosis, stellate cells, and portal hypertension. Clin Liver Dis. 2006;10:459-479, vii-viii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 82] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 25. | Cammà C, Petta S, Di Marco V, Bronte F, Ciminnisi S, Licata G, Peralta S, Simone F, Marchesini G, Craxì A. Insulin resistance is a risk factor for esophageal varices in hepatitis C virus cirrhosis. Hepatology. 2009;49:195-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 59] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 26. | Cavallo-Perin P, Cassader M, Bozzo C, Bruno A, Nuccio P, Dall’Omo AM, Marucci M, Pagano G. Mechanism of insulin resistance in human liver cirrhosis. Evidence of a combined receptor and postreceptor defect. J Clin Invest. 1985;75:1659-1665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 105] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 27. | Aytug S, Reich D, Sapiro LE, Bernstein D, Begum N. Impaired IRS-1/PI3-kinase signaling in patients with HCV: a mechanism for increased prevalence of type 2 diabetes. Hepatology. 2003;38:1384-1392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 187] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 28. | Bosch J, Gomis R, Kravetz D, Casamitjana R, Terés J, Rivera F, Rodés J. Role of spontaneous portal-systemic shunting in hyperinsulinism of cirrhosis. Am J Physiol. 1984;247:G206-G212. [PubMed] |
| 29. | Merli M, Leonetti F, Riggio O, Valeriano V, Ribaudo MC, Strati F, Tisone G, Casciani CU, Capocaccia L. Glucose intolerance and insulin resistance in cirrhosis are normalized after liver transplantation. Hepatology. 1999;30:649-654. [PubMed] |
| 30. | Ishikawa T, Shiratsuki S, Matsuda T, Iwamoto T, Takami T, Uchida K, Terai S, Yamasaki T, Sakaida I. Occlusion of portosystemic shunts improves hyperinsulinemia due to insulin resistance in cirrhotic patients with portal hypertension. J Gastroenterol. 2014;49:1333-1341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (1)] |
| 31. | Erice E, Llop E, Berzigotti A, Abraldes JG, Conget I, Seijo S, Reverter E, Albillos A, Bosch J, García-Pagán JC. Insulin resistance in patients with cirrhosis and portal hypertension. Am J Physiol Gastrointest Liver Physiol. 2012;302:G1458-G1465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 32. | Petrides AS, Groop LC, Riely CA, DeFronzo RA. Effect of physiologic hyperinsulinemia on glucose and lipid metabolism in cirrhosis. J Clin Invest. 1991;88:561-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 113] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 33. | Greco AV, Mingrone G, Mari A, Capristo E, Manco M, Gasbarrini G. Mechanisms of hyperinsulinaemia in Child’s disease grade B liver cirrhosis investigated in free living conditions. Gut. 2002;51:870-875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 38] [Article Influence: 1.7] [Reference Citation Analysis (1)] |
| 34. | Goswami A, Bhargava N, Dadhich S, Kulamarva G. Insulin resistance in euglycemic cirrhosis. Ann Gastroenterol. 2014;27:237-243. [PubMed] |
| 35. | Flier SN, Kulkarni RN, Kahn CR. Evidence for a circulating islet cell growth factor in insulin-resistant states. Proc Natl Acad Sci USA. 2001;98:7475-7480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 113] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 36. | Bonner-Weir S. Perspective: Postnatal pancreatic beta cell growth. Endocrinology. 2000;141:1926-1929. [PubMed] |
| 37. | Fu Z, Berhane F, Fite A, Seyoum B, Abou-Samra AB, Zhang R. Elevated circulating lipasin/betatrophin in human type 2 diabetes and obesity. Sci Rep. 2014;4:5013. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 143] [Cited by in RCA: 177] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 38. | Espes D, Martinell M, Carlsson PO. Increased circulating betatrophin concentrations in patients with type 2 diabetes. Int J Endocrinol. 2014;2014:323407. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 90] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 39. | Hu H, Sun W, Yu S, Hong X, Qian W, Tang B, Wang D, Yang L, Wang J, Mao C. Increased circulating levels of betatrophin in newly diagnosed type 2 diabetic patients. Diabetes Care. 2014;37:2718-2722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 137] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 40. | Espes D, Lau J, Carlsson PO. Increased circulating levels of betatrophin in individuals with long-standing type 1 diabetes. Diabetologia. 2014;57:50-53. [PubMed] |
| 41. | Gómez-Ambrosi J, Pascual E, Catalán V, Rodríguez A, Ramírez B, Silva C, Gil MJ, Salvador J, Frühbeck G. Circulating betatrophin concentrations are decreased in human obesity and type 2 diabetes. J Clin Endocrinol Metab. 2014;99:E2004-E2009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 142] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 42. | Chen X, Lu P, He W, Zhang J, Liu L, Yang Y, Liu Z, Xie J, Shao S, Du T. Circulating betatrophin levels are increased in patients with type 2 diabetes and associated with insulin resistance. J Clin Endocrinol Metab. 2015;100:E96-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 99] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 43. | Fu Z, Abou-Samra AB, Zhang R. An explanation for recent discrepancies in levels of human circulating betatrophin. Diabetologia. 2014;57:2232-2234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 74] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 44. | Raghow R. Betatrophin: A liver-derived hormone for the pancreatic β-cell proliferation. World J Diabetes. 2013;4:234-237. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |