Published online Sep 28, 2015. doi: 10.3748/wjg.v21.i36.10418
Peer-review started: March 15, 2015
First decision: April 13, 2015
Revised: May 28, 2015
Accepted: June 15, 2015
Article in press: June 16, 2015
Published online: September 28, 2015
Processing time: 197 Days and 1.7 Hours
AIM: To investigate the relationship between contrast-enhanced ultrasound (CEUS), basic fibroblast growth factor (bFGF), endothelin-1 (ET-1), and hepatocellular carcinoma (HCC) recurrence after ablation.
METHODS: A total of 51 HCC patients (38 males and 13 females) who received radiofrequency ablation in our hospital from June 2012 to July 2014 were enrolled in this study. The patients were divided into two groups: recurrence group and non-recurrence group. Routine abdominal examination was first performed in the horizontal position. Then the patients underwent CEUS and immunohistochemical staining before receiving radiofrequency ablation. All patients were followed-up every three months for one year. The results of CEUS and serum tumor marker levels were evaluated and combined together to estimate HCC recurrence and metastasis. Patients were divided into two groups: recurrence group and non-recurrence group. Quantitative parameters of CEUS and tumor expression levels of bFGF and ET-1 were compared between the two groups, respectively. Binary logistic regression analysis was used to analyze the relationship between CEUS quantitative parameters, expression levels of ET-1 and bFGF, and HCC recurrence after ablation.
RESULTS: Based on the quantitative parameters of CEUS before patients received radiofrequency ablation, the levels of tumor rise time (tRT), tumor time to peak (tTTP), tumor peak intensity (tPI) and tumor-parenchymal peak intensity (t-pPI) in the recurrence group were significantly lower than those in the non-recurrence group (16.6 ± 6.1 vs 23.2 ± 7.0, P = 0.000; 41.2 ± 10.2 vs 59.6 ± 14.2, P = 0.000; 23.8 ± 6.7 vs 31.4 ± 6.4, P = 0.000; 7.1 ± 3.4 vs 14.6 ± 7.4, P = 0.000; respectively). The expression levels of bFGF in the recurrence group were significantly higher than those in the non-recurrence group (P < 0.05). Levels of tTTP showed a significant inverse correlation with the level of bFGF in tumors (r = -0.312, P = 0.037). The Binary logistic regression analysis results revealed that the levels of tRT, tTTP, tPI and the level of bFGF were associated with HCC recurrence after radiofrequency ablation (P < 0.05).
CONCLUSION: CEUS is a noninvasive and effective method for evaluating the angiogenesis of HCC, and predicting its recurrence and prognosis.
Core tip: This study quantitatively analyzed contrast-enhanced ultrasound (CEUS) images from patients with hepatocellular carcinoma (HCC). The results showed that HCC patients who have low levels of tumor time to peak (tTTP), tumor peak intensity and tumor-parenchymal peak intensity before ablation were more likely to relapse after ablation. Expression levels of basic fibroblast growth factor (bFGF) in the recurrence group were higher than those in the non-recurrence group, and tTTP levels were negatively correlated with bFGF expression levels. This was expected to be a predictable index for HCC recurrence after ablation. The results also revealed that CEUS is a non-invasive and effective method for evaluating HCC angiogenesis, its recurrence and metastasis.
- Citation: Gao Y, Zheng DY, Cui Z, Ma Y, Liu YZ, Zhang W. Predictive value of quantitative contrast-enhanced ultrasound in hepatocellular carcinoma recurrence after ablation. World J Gastroenterol 2015; 21(36): 10418-10426
- URL: https://www.wjgnet.com/1007-9327/full/v21/i36/10418.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i36.10418
There are currently various treatment options for hepatocellular carcinoma (HCC) such as surgery and radiofrequency ablation[1]. However, the high recurrence rate of HCC is a major problem. The prognosis related to HCC recurrence remains poor with a high mortality rate, resulting in a low long-term survival rate for HCC patients and an average five-year survival rate of approximately 60%[2]. Therefore, early prediction of HCC recurrence is of great importance.
The main method for predicting HCC recurrence is the detection of angiogenesis-related factors. Common angiogenesis-related factors include endothelin-1 (ET-1), basic fibroblast growth factor (bFGF) and vascular endothelial growth factor (VEGF)[3]. Starley[4] found that tumor angiogenesis is the most important mechanism in HCC recurrence. HCC invasion and metastasis depend on tumor angiogenesis. Kaseb et al[5] reported that the growth of tumor blood vessels, invasion and tumor cell metastasis may be regulated by ET-1. A study carried out by Feng et al[6] revealed that tumor cell migration, proliferation and angiogenesis were significantly inhibited by bFGF. However, the detection of angiogenesis-related factors involves invasive tests and the results cannot completely predict early HCC recurrence. Hence, a noninvasive, accurate and simple method for evaluating and predicting the recurrence and metastasis of HCC after surgery is necessary, but has been a difficult field of study in recent years.
Ultrasound is a noninvasive, convenient and inexpensive imaging technique and is the first choice in HCC diagnosis. The size, number, and boundary of tumor nodules can be identified by two-dimensional ultrasound. Vascular distribution and tumor vasculature flow can be observed using color Doppler ultrasound. However, these two ultrasonic methods cannot predict and diagnose early HCC recurrence[7-9]. In recent years, contrast-enhanced ultrasound (CEUS) has made a breakthrough in HCC blood flow imaging. Compared with other ultrasonic methods, CEUS can provide information on real-time dynamic tumor microcirculation. Its advanced time-intensity curve can quantitatively analyze tumor vasculature formation, as well as tumor invasion and metastasis tendencies. CEUS is a non-radioactive technique and has become an important method in evaluating the development and metastasis of HCC[10,11].
Therefore, this study aims to introduce CEUS to quantitatively analyze contrast images of HCC lesions. The predictive performance of CEUS for HCC recurrence and metastasis was explored by analyzing the correlation between CEUS and expression of angiogenesis-related factors. It is hoped that this study will be helpful in the early prediction of HCC recurrence and metastasis, and in selecting appropriate treatment programs. In addition, it is hoped that the results of this study will show that CEUS is a noninvasive, accurate, and convenient method for evaluating tumor angiogenesis and HCC prognosis.
HCC patients who received radiofrequency ablation in our hospital from June 2012 to July 2014 were enrolled in this study. The inclusion criteria were as follows: pathologically confirmed HCC, single lesions, a maximum tumor diameter < 50 mm; no extrahepatic metastasis; Child class A or B; no serious heart or lung disease. Finally, 51 cases (38 males and 13 females) were enrolled in this study. The average age of the patients was 54.2 ± 7.5 years.
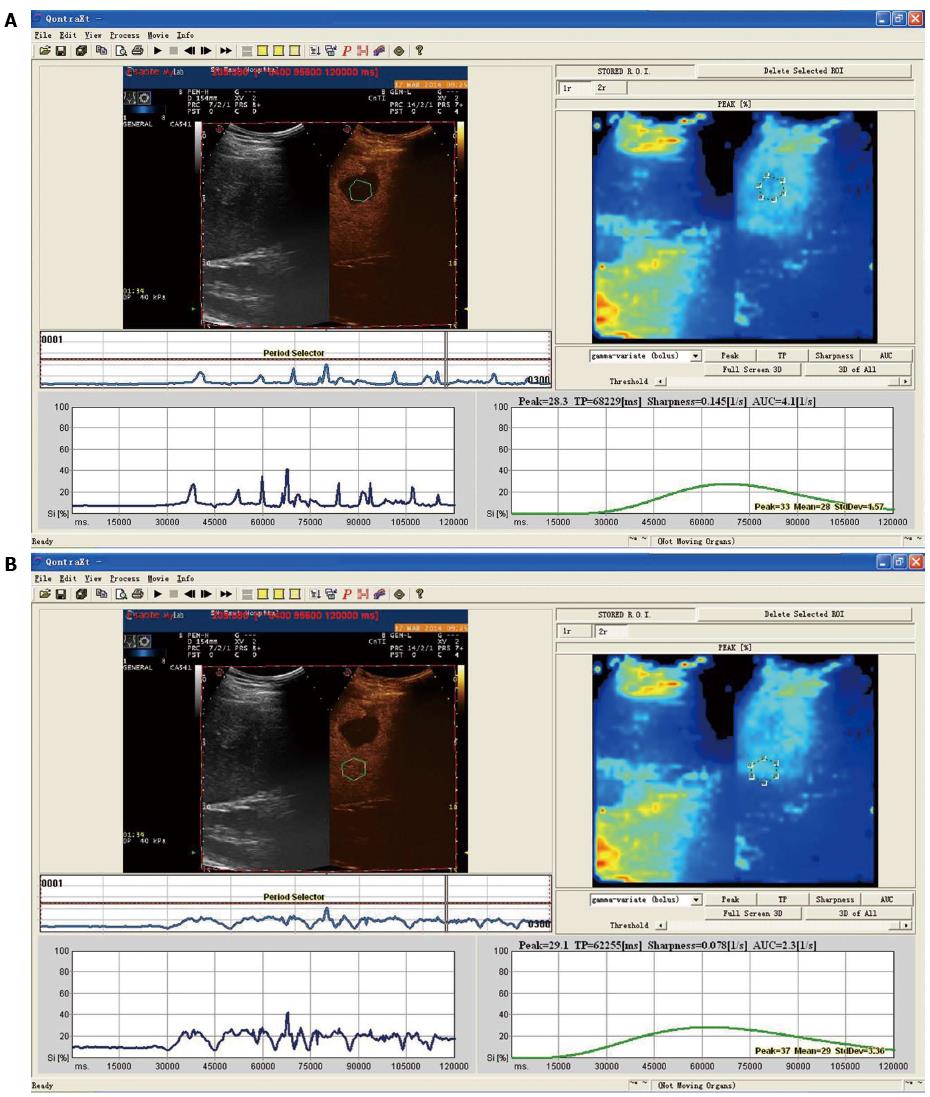
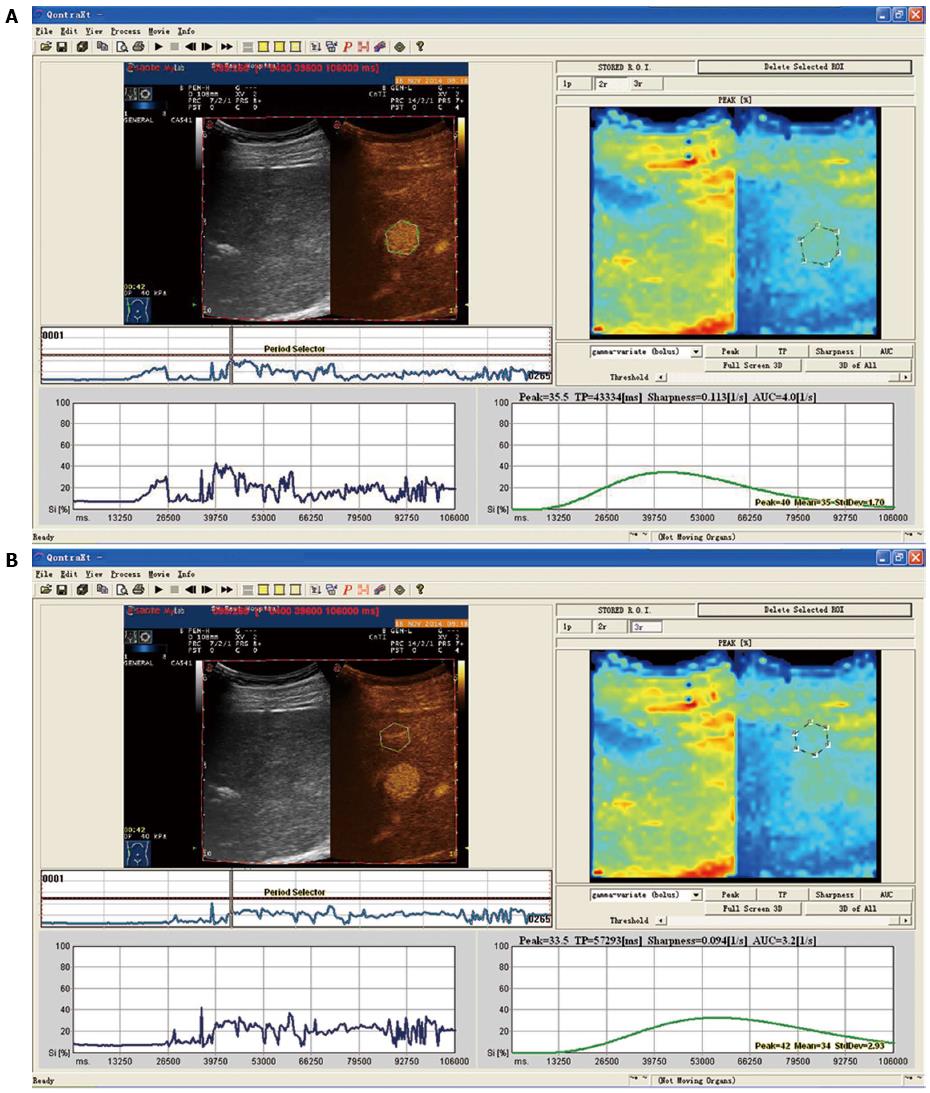
CEUS before ablation: The CEUS (MyLab Twice system, Esaote, Genoa, Italy) procedure was carried out as follows: Routine abdominal examination was first performed in the horizontal position. Each section of the liver tumor was observed; and tumor size, shape, boundary echo and blood flow signals were recorded. The acoustic contrast agent, SonoVue, was then injected into the median cubital vein. Subsequently, a five-minute continuous contrast process was observed, and radiographic contrast images were sent to the software. In the focal area of the tumor and surrounding areas of normal liver parenchyma, a uniform and obvious part of the tumor was chosen as the region of interest (ROI); and the liver parenchyma 2 cm beyond the tumor was set as the control ROI. The time-intensity curve was obtained from the time-intensity curve analysis after motion compensation. Tumor peak intensity (tPI), tumor rise time (tRT), parenchymal rise time (pRT), tumor time to peak (tTTP), parenchymal time to peak (pTTP), parenchymal-tumor time to peak (p-tTTP), and tumor-parenchymal peak intensity (t-pPI) levels of the time-intensity curves were calculated and recorded in Microsoft Excel (Microsoft, Redmond, WA, United States).
Biopsy procedure: Tumor tissue samples were obtained by liver puncture under ultrasound guidance. The puncture target was determined, and the entry point and route were selected. Local anesthesia with lidocaine was administered. Specimens were obtained when the needle punctured and reached the leading edge of the tumor. Samples were sent to pathology for examination.
Radiofrequency ablation: The patients were positioned in the supine or left-lateral position, and puncture positioning was determined under ultrasound guidance. First, the needle was sterilized and draped. Then, local anesthesia with 1% lidocaine hydrochloride was administered. Patients were asked to hold their breath before implanting the bipolar radiofrequency needle to the pre-set position of the tumor lesions. Ablation therapy began under intravenous anesthesia with propofol. At the end of treatment, the needle tract was solidified during needle withdrawal to avoid bleeding.
Follow-up after ablation: All patients were followed-up every three months for one year. The results of CEUS, enhanced CT/MRI, and serum tumor marker levels were combined to evaluate HCC recurrence and metastasis. For CEUS, no recurrence was defined as a “black hole” on the screen for three periods after ablation. High arterial phase enhancement and low delayed phase enhancement suggested tumor recurrence.
Immunohistochemistry: (1) paraffin sections were cut, deparaffinized and rehydrated, then washed for five minutes with running water and five minutes with distilled water; (2) sections underwent antigen repair; (3) sections were dripped with 30% H2O2 at room temperature to deactivate endogenous enzymes, and washed three times with distilled water; (4) sections underwent primary antibody incubation; (5) sections underwent secondary antibody incubation; (6) sections were stained with DAB; and (7) sections were lightly re-stained by hematoxylin, dehydrated and vitrified, then the slices were sealed and examined with a microscope.
SPSS 18.0 statistical software was used to measure numerical variable data (mean ± SD) and categorical variable data (n). All data were compared using t-test or χ2 test. Spearman correlation analysis was used to analyze CEUS quantitative parameters and expression levels of ET-1 and bFGF. Binary logistic regression analysis was used to analyze the relationship between CEUS quantitative parameters, expression levels of ET-1 and bFGF, and HCC recurrence after ablation. P < 0.05 indicates that the differences were statistically significant.
All 51 patients were followed-up for one year; wherein, 37 patients had no recurrence and 14 patients were found to have recurrence. Gender, age, tumor size and location, Child grade, and AFP level differences, as well as the degree of tumor differentiation, between the recurrence and non-recurrence groups were not statistically significant (P > 0.05) (Table 1).
| Non-recurrence group (n = 37) | Recurrence group (n = 14) | χ2/t | P-value | |
| Gender | ||||
| Male | 28 | 10 | 0.002 | 0.756 |
| Female | 9 | 4 | ||
| Age (yr) | 54.1 ± 7.7 | 54.3 ± 8.2 | 0.453 | 0.653 |
| Tumor location | ||||
| Left lobe of liver | 6 | 3 | 0.001 | 0.663 |
| Right lobe of liver | 31 | 11 | ||
| Maximum diameter of tumor (cm) | ||||
| ≤3 | 28 | 11 | 0.023 | 0.828 |
| 3-5 | 9 | 3 | ||
| Child grade | ||||
| A | 34 | 12 | 0.018 | 0.508 |
| B | 3 | 2 | ||
| AFP (ug/L) | ||||
| > 200 | 6 | 4 | 0.356 | 0.321 |
| ≤200 | 31 | 10 | ||
| Degree of tumor differentiation | ||||
| High | 12 | 4 | 0.672 | 0.437 |
| Medium | 23 | 9 | ||
| Low | 2 | 1 | ||
Differences between time-intensity curves of patients in the recurrence and non-recurrence groups are shown in Figures 1 and 2. The curve was steeper, tRT and tTTP were shorter, and tPI was higher for patients in the recurrence group, compared with patients in the non-recurrence group. Differences between time-intensity curves for patients in the recurrence and non-recurrence group before ablation are shown in Table 2. Levels of tRT, tTTP, tPI and t-pPI for patients in the recurrence group were significantly lower than those in the non-recurrence group (P < 0.05); but pRT, pTTP and p-tTTP level differences were not statistically significant between the two groups (P > 0.05).
| Parameters | Non-recurrence group (37) | Recurrence group (14) | t | P-value |
| tRT (s) | 23.2 ± 7.0 | 13.6 ± 6.1 | 4.517 | 0.000 |
| pRT (s) | 20.7 ± 10.2 | 18.5 ± 9.9 | 0.693 | 0.492 |
| tTTP (s) | 59.6 ± 14.2 | 41.2 ± 10.2 | 4.423 | 0.000 |
| pTTP (s) | 66.4 ± 17.3 | 56.8 ± 13.2 | 1.876 | 0.067 |
| tPI (%) | 31.4 ± 6.4 | 23.8 ± 6.7 | 3.737 | 0.000 |
| p-tTTP (s) | -6.3 ± 10.7 | -12.3 ± 10.2 | 1.809 | 0.077 |
| t-pPI (%) | 14.6 ± 7.4 | 7.1 ± 3.4 | 3.632 | 0.000 |
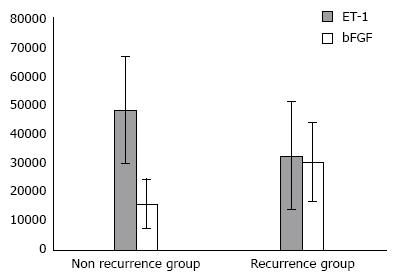
Expression levels of bFGF in patients in the recurrence group were significantly higher than those in the non-recurrence group, and the difference was statistically significant (t = 4.323, P = 0.000). However, ET-1 expression levels in these two groups were not significantly different (t = 0.781, P = 0.175), as shown in Figure 3.
The level of tTTP was negatively correlated with the expression level of bFGF, and the difference was statistically significant (P < 0.05). No correlations between tRT, pTTP, tPI and t-pTTP levels, and between bFGF and ET-1 expression levels (P > 0.05) were observed, as shown in Table 3.
| Parameters | ET-1 | bFGF | ||
| r | P-value | r | P-value | |
| tRT | 0.211 | 0.302 | -0.317 | 0.143 |
| tTTP | 0.133 | 0.570 | -0.312 | 0.037 |
| pTTP | 0.132 | 0.671 | -0.344 | 0.214 |
| tPI | -0.412 | 0.293 | -0.063 | 0.715 |
| p-tTTP | -0.005 | 0.913 | -0.075 | 0.702 |
Binary logistic regression analysis was carried out, recurrence was taken as the dependent variable, and both the CEUS quantitative parameters (tRT, pRT, tTTP, pTTP, tPI, t-pTTP and t-pPI) and expression levels of ET-1 and bFGF were taken as independent variables. The results showed that low levels of tRT, tPI and tTTP, as well as high levels of bFGF, were risk factors for HCC recurrence after ablation, as shown in Table 4.
| Parameters | β | SE | Wald | P-value | OR | 95%CI | |
| Lower limit | Upper limit | ||||||
| Low level of tRT | -0.207 | 0.062 | 5.326 | 0.037 | 0.813 | 0.720 | 0.918 |
| Low level of tPI | -0.076 | 0.007 | 7.392 | 0.006 | 0.927 | 0.914 | 0.940 |
| Low level of tTTP | -0.312 | 0.008 | 5.892 | 0.019 | 0.732 | 0.721 | 0.744 |
| High level of bFGF | 0.071 | 0.029 | 4.872 | 0.011 | 1.074 | 1.015 | 1.137 |
| Low level of pRT | -0.091 | 0.091 | 5.322 | 0.137 | 0.913 | 0.764 | 1.091 |
| Low level of pTTP | -0.057 | 0.032 | 4.352 | 0.086 | 0.945 | 0.888 | 1.006 |
| Low level of t-pTTP | -0.066 | 0.042 | 4.892 | 0.136 | 0.936 | 0.862 | 1.016 |
| Low level of t-pPI | -0.013 | 0.032 | 3.842 | 0.187 | 0.987 | 0.927 | 1.051 |
| High level of ET-1 | 0.031 | 0.032 | 5.762 | 0.879 | 1.032 | 0.969 | 1.099 |
This study introduced the use of CEUS, and quantitatively analyzed ultrasonic images of HCC patients using its software. The results showed that HCC patients with low levels of tRT, tTTP, tPI and t-pPI before ablation had a high probability of relapse after ablation. Expression levels of bFGF in patients in the recurrence group were significantly higher than those in the non-recurrence group. Levels of tTTP were negatively correlated with expression levels of bFGF, suggesting that low levels of tTTP can easily cause HCC patients to relapse after ablation. This was expected to be the predictable index for HCC recurrence after ablation. It was also revealed that CEUS is a noninvasive and effective method for evaluating HCC angiogenesis, and its recurrence and metastasis.
This study revealed that bFGF expression levels in patients in the recurrence group were significantly higher than those in the non-recurrence group. A high bFGF expression level is a risk factor for HCC recurrence after ablation; demonstrating that bFGF expression levels are correlated with the prognosis of HCC patients, and that this could predict the recurrence of HCC after ablation. A possible reason for this may be due to the widespread distribution of bFGF in the body, as its expression level increases with ischemia and hypoxia. Previous studies have shown that increasing bFGF expression levels in the liver are closely related to HCC invasion and metastasis[12,13]. Basic research has indicated that bFGF can activate the Ras-Raf-MARK system in angiogenesis[14,15]. The cooperation of bFGF with VEGF promotes vascular endothelial cell generation, and provides nutrition for the invasion and metastasis of tumor cells. Therefore, bFGF could be used to predict HCC metastasis and recurrence. However, this study found that ET-1 expression levels in the recurrence and non-recurrence groups were not significantly different, which did not conform to previous studies[16]. Vasoactive substances such as ET-1 have an important regulatory role in the angiogenesis, proliferation, invasion and metastasis of malignant tumors[17-20]. It was found that ET-l did not only reflect tumor angiogenesis, but also determined tumor proliferation and metastasis[11,21]. In this study, no differences in ET-1 expression levels were found, which was probably because ET-1 expression levels are related to the degree of malignancy. ET-1 expression levels were low in tumors that had high differentiation and low malignancy, while expression levels were only high in tumors that had low differentiation and high malignancy[22-24]. In this study, only three patients showed low differentiation, causing the overall ET-l expression level to be low. Hence, the difference between the recurrence and non-recurrence groups was not statistically significant.
This study revealed that tTTP levels were negatively correlated with bFGF expression levels, suggesting that tTTP may predict HCC recurrence. In principle, tTTP is mainly determined through hepatic artery and portal vein blood flow[25]. The more blood is supplied to the hepatic artery, the earlier the tTTP level is reached. Conversely, the more blood is supplied to the portal vein, the tTTP level is delayed. These results indicate that tTTP may reflect the tumor blood supply component. Angiogenesis was prevalent in tumors with a rich arterial blood supply, causing HCC invasion and metastasis. Tumor angiogenesis was closely related to bFGF expression levels[26]. Therefore, we believe that as bFGF expression levels increase in HCC patients, tumor angiogenesis becomes more prevalent and arterial blood supply increases; which is followed by a decrease in tTTP levels. On the other hand, when bFGF expression levels were low in tumors, angiogenesis and arterial blood supply were slow, and tTTP levels increased.
The results showed that tRT, tTTP, tPI and t-pPI levels in the recurrence group were significantly lower than those in the non-recurrence group. Low levels of tRT, tPI and tTTP were risk factors of HCC recurrence after ablation, suggesting that tRT, tPI and tTTP levels before ablation could be used to predict HCC recurrence after treatment. Levels of tPI decreased in patients in the recurrence group due to liver parenchyma changes close to the tumor tissue. Levels of tRT and tTTP were low, because tRT and tTTP reflected the tumor’s blood supply component. These were determined by the hepatic artery blood supply and tumor portal vein component ratio[27]. The larger the proportion of the hepatic artery blood supply, the faster the tumor’s rise time, and the earlier the time to peak is reached. Conversely, the larger the proportion of the portal vein blood supply, the slower the tumor rise time, and the time to peak is delayed. In CEUS, HCC was closely related to tumor hemodynamic characteristics[28]. There was more blood supply for HCC than in benign lesions. Filling time was shorter, and the rate of increase of peak intensity per unit-time was higher. HCC lesions had a rich blood supply and the hepatic artery was the main blood supply. The contrast agent completely filled the early and middle artery phase. Peak time was short, showing a “fast-forward” movement[29]. The lower the levels of tRT, tPI and tTTP, the larger the proportion of the hepatic artery, and the more dangerous the recurrence would be after ablation.
There are some limitations in this research. First, the sample size was small, and a larger sample size is needed to validate our conclusions. Secondly, the relationship between the CEUS index and molecular biological index needs to be explored further. Other or more detailed biological indices are needed, combined with clinical practice.
In conclusion, CEUS is a simple and noninvasive method for tumor angiogenesis assessment. Quantitative parameters analyzed by CEUS software were more objective; where tTTP was found to be a special predictor of HCC recurrence and is worthy of further study. CEUS is important for determining tumor tissue hemodynamics in detail, and can be used to evaluate tumor angiogenesis and predict tumor recurrence.
There are currently various treatment options for hepatocellular carcinoma (HCC) such as surgery and radiofrequency ablation. However, the high recurrence rate of HCC is a major problem. The prognosis of HCC recurrence remains poor with a high mortality rate; resulting in a low long-term survival rate for HCC patients, and an average five-year survival rate of approximately 60%. Therefore, early prediction of HCC recurrence is of great importance. The main method for predicting HCC recurrence is the detection of angiogenesis-related factors. Common angiogenesis-related factors include endothelin-1 (ET-1), basic fibroblast growth factor (bFGF) and vascular endothelial growth factor.
Starley found that tumor angiogenesis is the most important mechanism in HCC recurrence. HCC invasion and metastasis depend on tumor angiogenesis. Kaseb et al reported that the growth of tumor blood vessels, invasion and tumor cell metastasis may be regulated by ET-1. The study by Feng et al revealed that tumor cell migration, proliferation and angiogenesis were significantly inhibited by bFGF. However, the detection of angiogenesis-related factors involves invasive tests and the results cannot completely predict early HCC recurrence. Hence, a noninvasive, accurate and simple method for evaluating and predicting the recurrence and metastasis of HCC after surgery is necessary, but has been a difficult field of study in recent years.
In recent years, contrast-enhanced ultrasound (CEUS) has made a breakthrough in HCC blood flow imaging. CEUS provides information on real-time dynamic tumor microcirculation. Its advanced time-intensity curve can quantitatively analyze tumor vasculature formation, as well as tumor invasion and metastasis tendencies. CEUS is a non-radioactive technique and has become an important method in evaluating the development and metastasis of HCC. In this study, quantitative parameters were more objective; CEUS is important for determining tumor tissue hemodynamics in detail, and can be used to evaluate tumor angiogenesis and predict tumor recurrence.
CEUS will be helpful in the early prediction of HCC recurrence and metastasis, and in selecting appropriate treatment programs. It is hoped that this noninvasive, accurate, and convenient method will be used to evaluate tumor angiogenesis and HCC prognosis.
This study introduced the use of CEUS and quantitatively analyzed ultrasonic images of HCC patients by using its software. Results have shown that HCC patients with low levels of tRT, tTTP, tPI and t-pPI before ablation have a high probability of relapse after ablation. Expression levels of bFGF in patients in the recurrence group were significantly higher than in the non-recurrence group. Levels of tTTP were negatively correlated with expression levels of bFGF, suggesting that low tTTP levels can easily cause HCC patients to relapse after ablation. This was expected to be the predictable index for HCC recurrence after ablation. It also reveals that CEUS is expected to be a noninvasive and effective method for evaluating the angiogenesis of HCC, and the prognosis of its recurrence and metastasis.
P- Reviewer: Faloppi L, Sharma M S- Editor: Ma YJ L- Editor: Webster JR E- Editor: Zhang DN
| 1. | Zocco MA, Garcovich M, Lupascu A, Di Stasio E, Roccarina D, Annicchiarico BE, Riccardi L, Ainora ME, Ponziani F, Caracciolo G. Early prediction of response to sorafenib in patients with advanced hepatocellular carcinoma: the role of dynamic contrast enhanced ultrasound. J Hepatol. 2013;59:1014-1021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 69] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 2. | Forner A, Llovet JM, Bruix J. Chemoembolization for intermediate HCC: is there proof of survival benefit? J Hepatol. 2012;56:984-986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 93] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 3. | Tsuchiya K, Asahina Y, Matsuda S, Muraoka M, Nakata T, Suzuki Y, Tamaki N, Yasui Y, Suzuki S, Hosokawa T. Changes in plasma vascular endothelial growth factor at 8 weeks after sorafenib administration as predictors of survival for advanced hepatocellular carcinoma. Cancer. 2014;120:229-237. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 4. | Starley BQ, Calcagno CJ, Harrison SA. Nonalcoholic fatty liver disease and hepatocellular carcinoma: a weighty connection. Hepatology. 2010;51:1820-1832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 968] [Cited by in RCA: 1013] [Article Influence: 67.5] [Reference Citation Analysis (0)] |
| 5. | Kaseb AO, Garrett-Mayer E, Morris JS, Xiao L, Lin E, Onicescu G, Hassan MM, Hassabo HM, Iwasaki M, Deaton FL. Efficacy of bevacizumab plus erlotinib for advanced hepatocellular carcinoma and predictors of outcome: final results of a phase II trial. Oncology. 2012;82:67-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 70] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 6. | Feng YX, Wang T, Deng YZ, Yang P, Li JJ, Guan DX, Yao F, Zhu YQ, Qin Y, Wang H. Sorafenib suppresses postsurgical recurrence and metastasis of hepatocellular carcinoma in an orthotopic mouse model. Hepatology. 2011;53:483-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 89] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 7. | Liu Y, Chen Z, Liu C, Yu D, Lu Z, Zhang N. Gadolinium-loaded polymeric nanoparticles modified with Anti-VEGF as multifunctional MRI contrast agents for the diagnosis of liver cancer. Biomaterials. 2011;32:5167-5176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 99] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 8. | Floriani I, Torri V, Rulli E, Garavaglia D, Compagnoni A, Salvolini L, Giovagnoni A. Performance of imaging modalities in diagnosis of liver metastases from colorectal cancer: a systematic review and meta-analysis. J Magn Reson Imaging. 2010;31:19-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 184] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 9. | Welzel TM, Graubard BI, Zeuzem S, El-Serag HB, Davila JA, McGlynn KA. Metabolic syndrome increases the risk of primary liver cancer in the United States: a study in the SEER-Medicare database. Hepatology. 2011;54:463-471. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 426] [Cited by in RCA: 423] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 10. | Strobel D, Bernatik T, Blank W, Schuler A, Greis C, Dietrich CF, Seitz K. Diagnostic accuracy of CEUS in the differential diagnosis of small (≤ 20 mm) and subcentimetric (≤ 10 mm) focal liver lesions in comparison with histology. Results of the DEGUM multicenter trial. Ultraschall Med. 2011;32:593-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 76] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 11. | Rennert J, Georgieva M, Schreyer AG, Jung W, Ross C, Stroszczynski C, Jung EM. Image fusion of contrast enhanced ultrasound (CEUS) with computed tomography (CT) or magnetic resonance imaging (MRI) using volume navigation for detection, characterization and planning of therapeutic interventions of liver tumors. Clin Hemorheol Microcirc. 2011;49:67-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 12. | Sakuma K, Aoki M, Kannagi R. Transcription factors c-Myc and CDX2 mediate E-selectin ligand expression in colon cancer cells undergoing EGF/bFGF-induced epithelial-mesenchymal transition. Proc Natl Acad Sci USA. 2012;109:7776-7781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 127] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 13. | Lin CC, Huang CY, Mong MC, Chan CY, Yin MC. Antiangiogenic potential of three triterpenic acids in human liver cancer cells. J Agric Food Chem. 2011;59:755-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 76] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 14. | Liu L, Yang Z, Xu Y, Li J, Xu D, Zhang L, Sun J, Xia S, Zou F, Liu Y. Inhibition of oxidative stress-elicited AKT activation facilitates PPARγ agonist-mediated inhibition of stem cell character and tumor growth of liver cancer cells. PLoS One. 2013;8:e73038. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 15. | Xi J, Wang Y, Zhang P, He L, Nan X, Yue W, Pei X. Human fetal liver stromal cells that overexpress bFGF support growth and maintenance of human embryonic stem cells. PLoS One. 2010;5:e14457. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 16. | Zhou JH, Cao LH, Liu JB, Zheng W, Liu M, Luo RZ, Han F, Li AH. Quantitative assessment of tumor blood flow in mice after treatment with different doses of an antiangiogenic agent with contrast-enhanced destruction-replenishment US. Radiology. 2011;259:406-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 17. | Williams R, Hudson JM, Lloyd BA, Sureshkumar AR, Lueck G, Milot L, Atri M, Bjarnason GA, Burns PN. Dynamic microbubble contrast-enhanced US to measure tumor response to targeted therapy: a proposed clinical protocol with results from renal cell carcinoma patients receiving antiangiogenic therapy. Radiology. 2011;260:581-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 100] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 18. | Lassau N, Chami L, Koscielny S, Chebil M, Massard C, Benatsou B, Bidault S, Cioffi A, Blay JY, Le Cesne A. Quantitative functional imaging by dynamic contrast enhanced ultrasonography (DCE-US) in GIST patients treated with masatinib. Invest New Drugs. 2012;30:765-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 19. | Chen LD, Xu HX, Xie XY, Xie XH, Xu ZF, Liu GJ, Wang Z, Lin MX, Lu MD. Intrahepatic cholangiocarcinoma and hepatocellular carcinoma: differential diagnosis with contrast-enhanced ultrasound. Eur Radiol. 2010;20:743-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 121] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 20. | Arii S, Tanaka S, Mitsunori Y, Nakamura N, Kudo A, Noguchi N, Irie T. Surgical strategies for hepatocellular carcinoma with special reference to anatomical hepatic resection and intraoperative contrast-enhanced ultrasonography. Oncology. 2010;78 Suppl 1:125-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 21. | Cao XL, Bao W, Zhu SG, Wang LH, Sun MH, Wang L, Men YM, Xue J. Contrast-enhanced ultrasound characteristics of breast cancer: correlation with prognostic factors. Ultrasound Med Biol. 2014;40:11-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 22. | Luo J, Guo RP, Lai EC, Zhang YJ, Lau WY, Chen MS, Shi M. Transarterial chemoembolization for unresectable hepatocellular carcinoma with portal vein tumor thrombosis: a prospective comparative study. Ann Surg Oncol. 2011;18:413-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 273] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 23. | Loss M, Schneider J, Uller W, Wiggermann P, Scherer MN, Jung W, Schlitt HJ, Stroszczynski C, Jung EM. Intraoperative high resolution linear contrast enhanced ultrasound (IOUS) for detection of microvascularization of malignant liver lesions before surgery or radiofrequeny ablation. Clin Hemorheol Microcirc. 2012;50:65-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 24. | Kudo M, Hatanaka K, Maekawa K. Newly developed novel ultrasound technique, defect reperfusion ultrasound imaging, using sonazoid in the management of hepatocellular carcinoma. Oncology. 2010;78 Suppl 1:40-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 74] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 25. | Omata M, Lesmana LA, Tateishi R, Chen PJ, Lin SM, Yoshida H, Kudo M, Lee JM, Choi BI, Poon RT. Asian Pacific Association for the Study of the Liver consensus recommendations on hepatocellular carcinoma. Hepatol Int. 2010;4:439-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 797] [Cited by in RCA: 841] [Article Influence: 56.1] [Reference Citation Analysis (0)] |
| 26. | Kawada N, Ohkawa K, Tanaka S, Matsunaga T, Uehara H, Ioka T, Takano Y, Takakura R, Imanaka K, Tamai C. Improved diagnosis of well-differentiated hepatocellular carcinoma with gadolinium ethoxybenzyl diethylene triamine pentaacetic acid-enhanced magnetic resonance imaging and Sonazoid contrast-enhanced ultrasonography. Hepatol Res. 2010;40:930-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 27. | Han ML, Chen CC, Kuo SH, Hsu WF, Liou JM, Wu MS, Wang HP. Predictors of in-hospital mortality after acute variceal bleeding in patients with hepatocellular carcinoma and concurrent main portal vein thrombosis. J Gastroenterol Hepatol. 2014;29:344-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 28. | Liu LN, Xu HX, Zhang YF, Xu JM. Hepatocellular carcinoma after ablation: the imaging follow-up scheme. World J Gastroenterol. 2013;19:797-801. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 29. | Liu F, Yu X, Liang P, Cheng Z, Han Z, Dong B. Contrast-enhanced ultrasound-guided microwave ablation for hepatocellular carcinoma inconspicuous on conventional ultrasound. Int J Hyperthermia. 2011;27:555-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |