Published online Sep 28, 2015. doi: 10.3748/wjg.v21.i36.10385
Peer-review started: April 24, 2015
First decision: June 2, 2015
Revised: June 17, 2015
Accepted: July 18, 2015
Article in press: July 18, 2015
Published online: September 28, 2015
AIM: To investigate the anti-tumor effects of equol in gastric cancer cells and the underlying molecular mechanisms.
METHODS: MGC-803 cells were employed for in vitro experiments in this study. Cells were treated with control (vehicle, 0.1% DMSO) or equol under specified dose titration or time courses. Cell viability was examined by MTS assay, and the levels of Ki67 were determined by qPCR and immunofluorescent assay. Changes in cell cycle distribution and apoptosis rate were detected by flow cytometry. The mRNA expression of cyclin E1 and P21WAF1 was determined by qPCR. The protein levels of cell cycle regulators, PARP and Caspase-3 cleavage, and the phosphorylation of Akt were examined by Western blot. In addition, to characterize the role of elevated Akt activation in the anti-tumor effect exerted by equol, Ly294002, a PI3K/AKT pathway inhibitor, was used to pretreat MGC-803 cells.
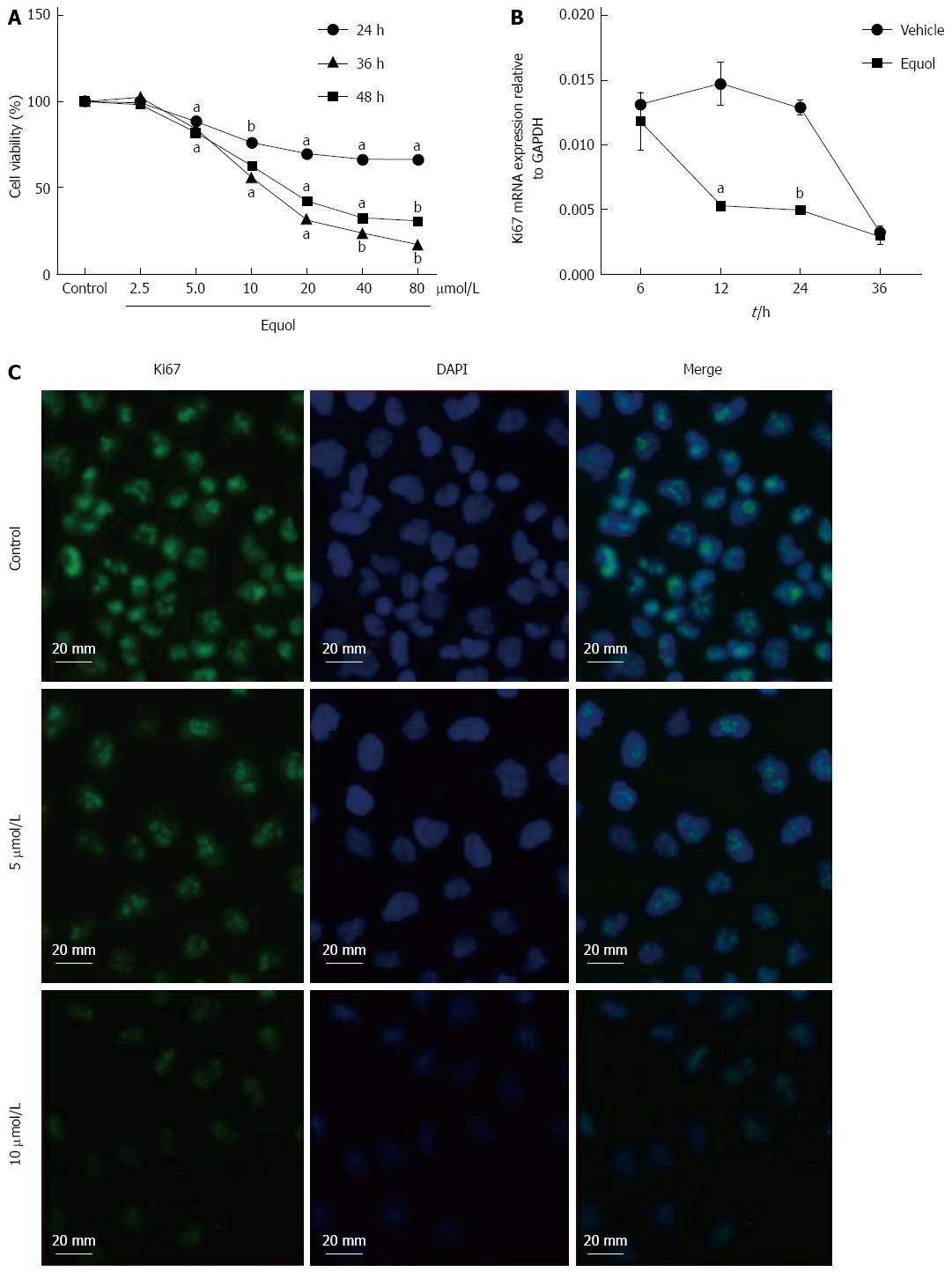
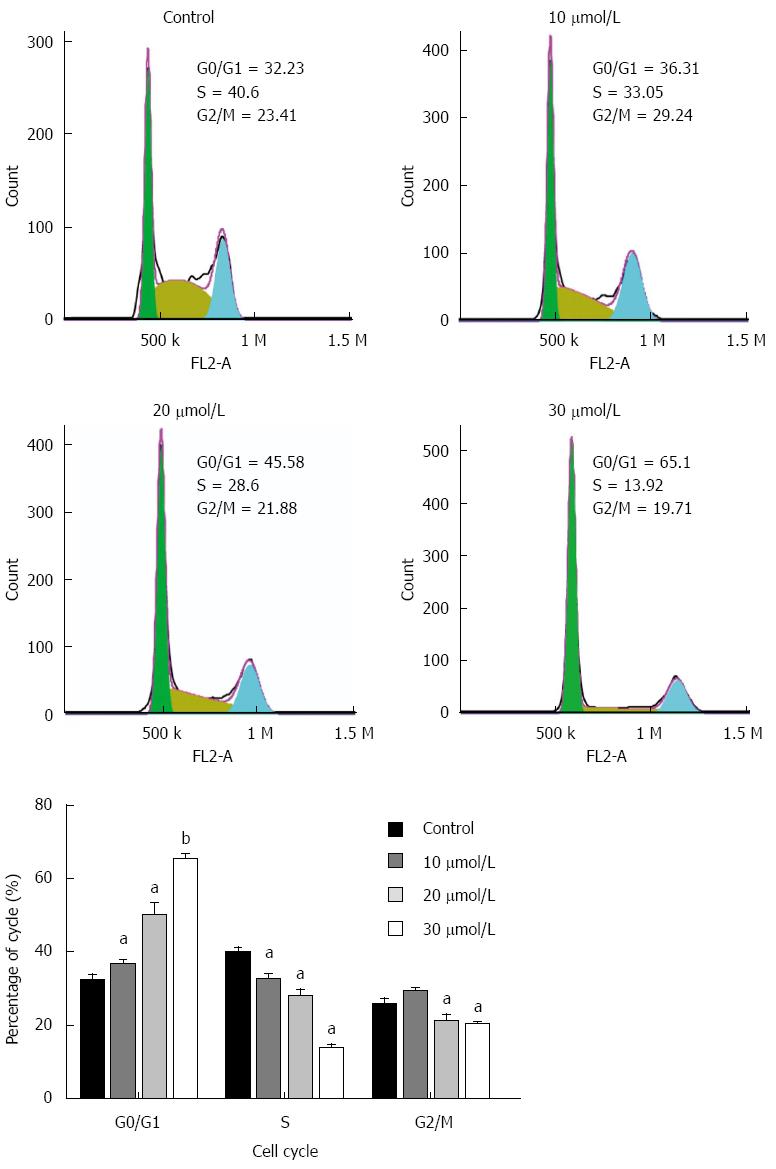
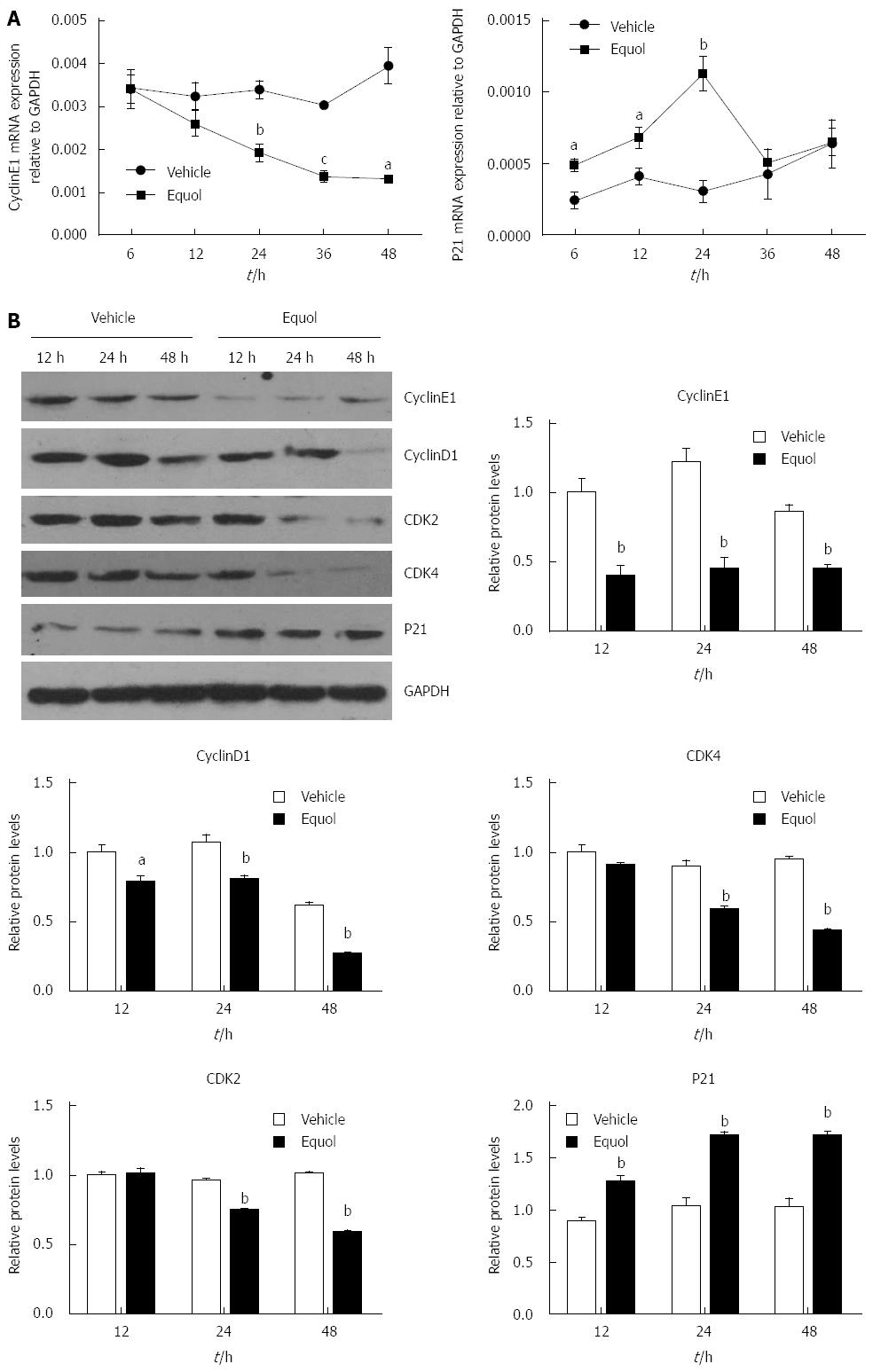
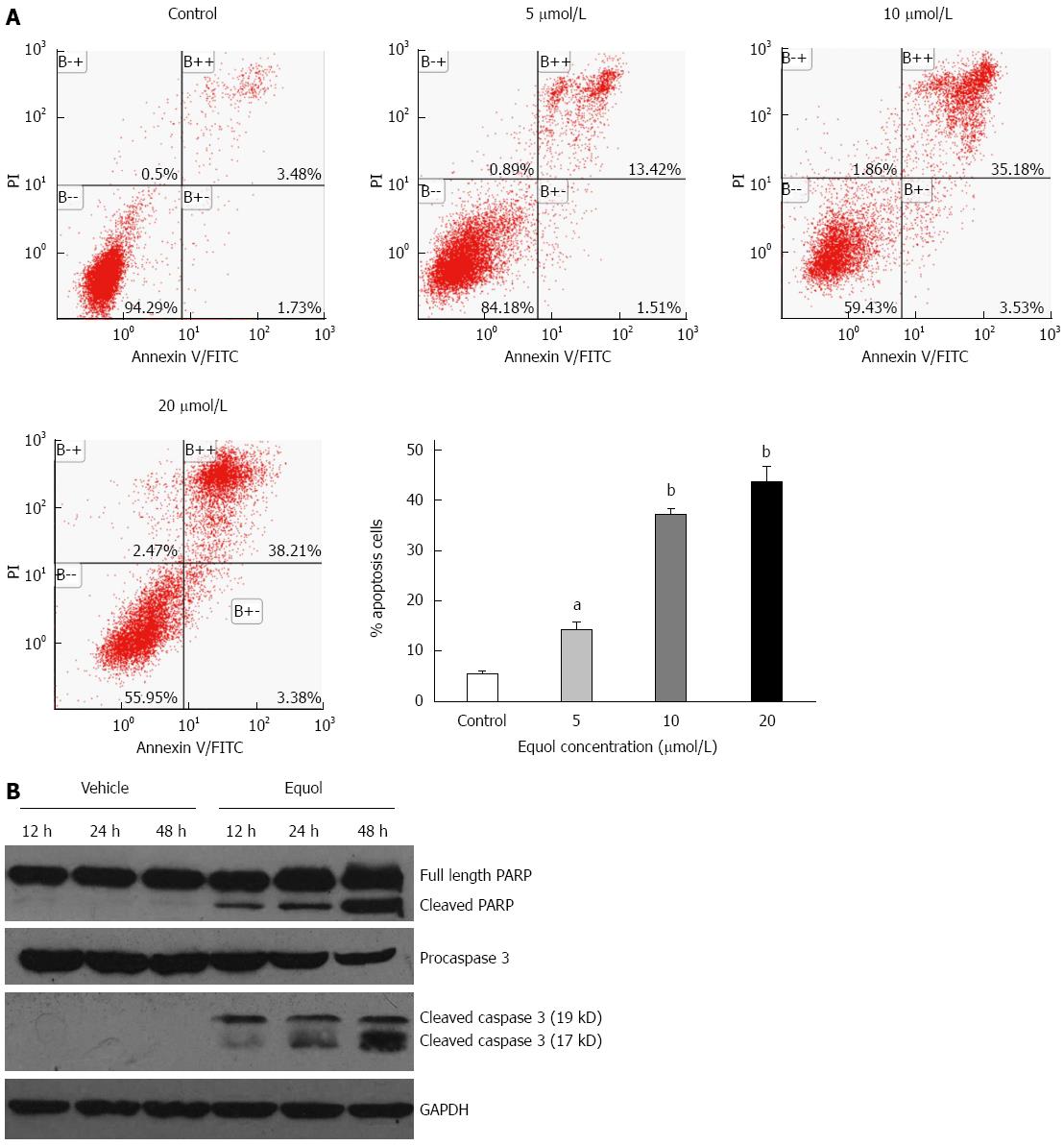
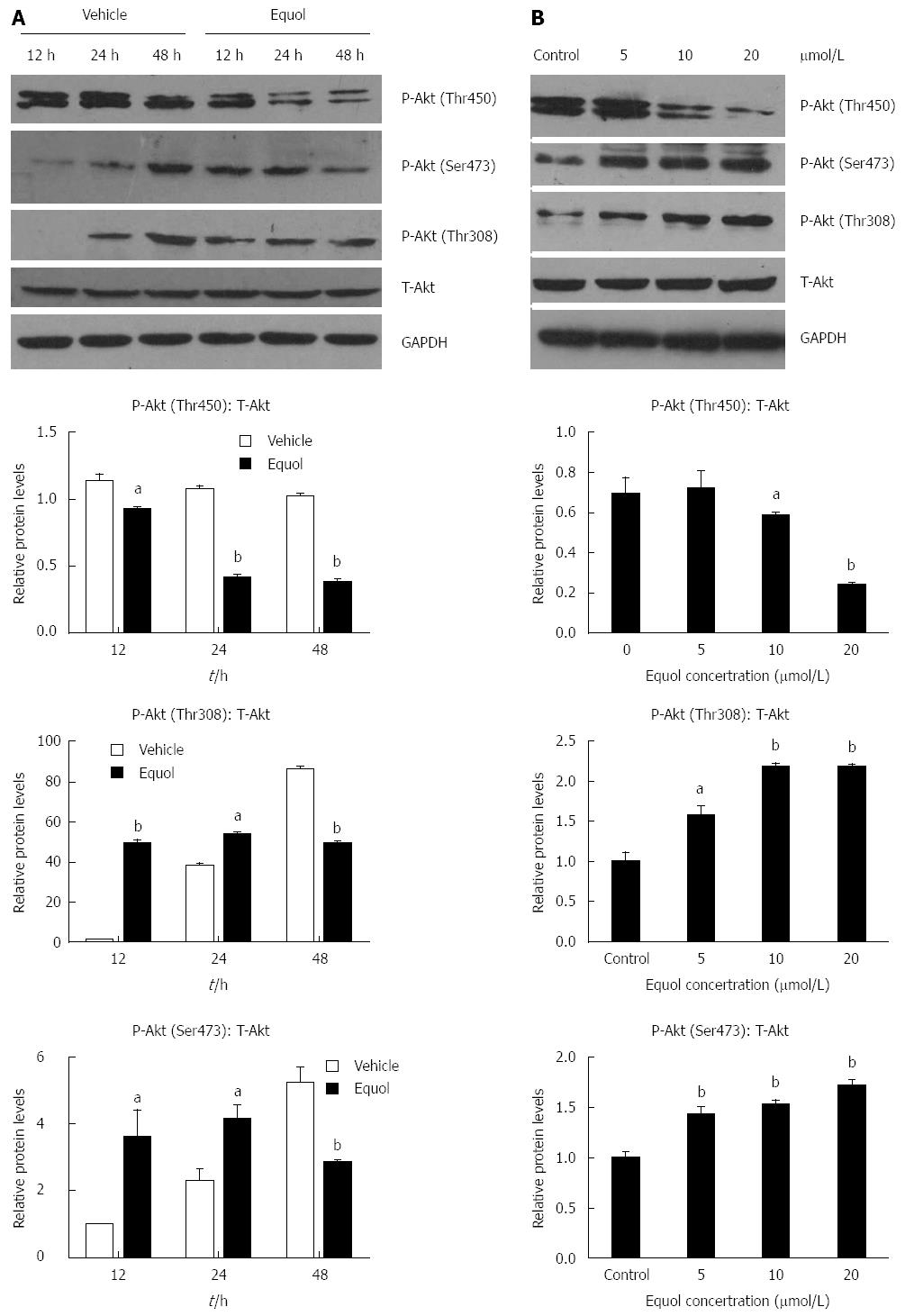
RESULTS: Equol (5, 10, 20, 40, or 80 μmol/L) inhibited viability of MGC-803 cells in a dose- and time-dependent manner after treatment for 24, 36, or 48 h (P < 0.05 for all). Equol also decreased the mRNA (P < 0.05 for 12 and 24 h treatment) and protein levels of Ki67. Equol treatment significantly induced G0/G1 cell cycle arrest (P < 0.05), with the percentages of G0/G1 cells of 32.23% ± 3.62%, 36.31% ± 0.24%, 45.58% ± 2.29%, and 65.10% ± 2.04% for equol (0, 10, 20, or 30 μmol/L) treatment, respectively, accompanied by a significant decrease of CDK2/4 (P < 0.05 for 24 and 48 h treatment) and Cyclin D1/Cyclin E1 (P < 0.05), and an increased level of P21WAF1 (P < 0.05). A marked increase of apoptosis was observed, with the percentages of apoptotic cells of 5.01% ± 0.91%, 14.57% ± 0.99%, 37.40% ± 0.58%, and 38.46% ± 2.01% for equol (0, 5, 10, or 20 μmol/L) treatment, respectively, accompanied by increased levels of cleaved PARP and caspase-3. In addition, we found that equol treatment increased P-Akt (Ser473 and Thr308) at 12 and 24 h compared to vehicle-treated control; longer treatment for 48 h decreased P-Akt (Ser473 and Thr308). P-Akt at Thr450, however, was decreased by equol treatment at all time points examined (P < 0.05 for all). Moreover, Akt inhibition by Ly294002 could not prevent but led to enhanced G0/G1 arrest and apoptosis.
CONCLUSION: Equol inhibits MGC-803 cells proliferation by induction of G0/G1 arrest and apoptosis. Its anti-cancer effects are likely mediated by dephosphorylation of Akt at Thr450.
Core tip: The results of our study demonstrated that equol could effectively inhibit the proliferation of human gastric cancer MGC-803 cells, which was associated with cell cycle arrest and apoptosis. Our data also suggested that the Akt signaling pathway may play a role in equol-mediated cell cycle arrest. To our knowledge, this is the first study demonstrating that equol exerts anticancer effect in human gastric cancer cells and that blockade of the Akt signaling pathway may be an antitumor mechanism of equol.
-
Citation: Yang ZP, Zhao Y, Huang F, Chen J, Yao YH, Li J, Wu XN. Equol inhibits proliferation of human gastric carcinoma cells
via modulating Akt pathway. World J Gastroenterol 2015; 21(36): 10385-10399 - URL: https://www.wjgnet.com/1007-9327/full/v21/i36/10385.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i36.10385
Gastric cancer, a leading cause of cancer death worldwide, constitutes a serious threat to human health[1]. Although many therapies, including radiotherapy, chemotherapy, and biotherapy, have been developed for gastric cancer treatment, the recurrence and subsequent resistance to chemoradiation therapy are unavoidable. Dietary modification is a helpful strategy for reducing cancer risk. It has been suggested that high consumption of fresh vegetables, fruits, and soy products may be associated with a decreased risk for gastric cancer[2-4].
Many epidemiologic studies have reported the health benefits of soy products for gastric cancer[3-8]. The majority of these studies demonstrate a decreased tendency for gastric cancer risk, although the results seem to be inconsistent. The isoflavones in soy possess many biological activities, such as estrogenic and anti-estrogenic[9], antioxidative[10], and anti-proliferative[11] effects. Genistein and daidzein are two major isoflavones in soy, and equol is a metabolite of daidzein produced by gut bacteria. Equol is different from its parent compounds in biologic activity. In general, equol has a longer half-life in the body than daidzein[12]; moreover, equol is more potent than daidzein in terms of its estrogenicity[12], antioxidative effects[10], and anti-proliferative abilities[13,14]. Therefore, equol seems to be biologically more active than its precursor, daidzein. As equol has estrogenic and antiestrogenic activities, its anti-carcinogenic property on hormone-dependent carcinomas including breast and prostate cancers has been widely discussed[15-21]. Additionally, increasing evidence suggested that equol has a protective effect on hormone-independent cancers, such as colorectal cancer[22], hepatocellular carcinoma[23], skin cancer[24], and pancreatic cancer[25]. As to the effect of equol on gastric cancer, a previous study demonstrated that overall plasma concentrations of equol were lower in cancer patients than in healthy controls; however, no statistical significance was found[26]. Another study[27] reported that a higher equol concentration was associated with a decreased risk for gastric cancer. However, little information, if any, is available in terms of the antitumor effect of equol on human gastric cancer cells and the molecular mechanisms for its antitumor activities remain poorly understood.
In the present study, we aimed to elucidate the chemopreventive effects of equol and the underlying mechanisms in gastric cancer cells.
Equol (racemic mixture) was purchased from LC Laboratories (Woburn, MA, United States). Reagents also included the CellTiter 96 Aqueous One Solution Cell Proliferation Assay (MTS) reagent (Promega, Madison, WI), and DAPI (Sigma, United States). Rabbit polyclonal antibodies specific to P-Akt (Thr450), P-Akt (Thr308), P-Akt (Ser473), Akt, PARP, CDK4, CDK2 and mouse monoclonal antibodies specific to P21WAF1 were obtained from Cell Signaling Technology (Danvers, MA, United States). Rabbit monoclonal antibodies specific to Cyclin D1, Cyclin E1 and Ki67 were obtained from Abcam (Cambridge, MA, United States). Rabbit polyclonal antibodies specific to GAPDH and Caspase-3 and all secondary antibodies were from Santa Cruz Biotechnology (CA, United States). Annexin V/PI apoptosis kit was obtained from BD Biosciences (San Jose, CA) and Cell Cycle Staining Kit was purchased from MultiScience Biotech (Hangzhou, China).
The human gastric cancer cell line MGC-803 was obtained from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). Cells were cultured at 37 °C in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS), 1% penicillin/streptomycin in a fully humidified atmosphere of 95% O2 and 5% CO2.
Cells were treated with control (vehicle, 0.1% DMSO) or equol in RPMI-1640 medium containing 2% FBS under specified dose titration or time courses.
MGC-803 cell viability was examined by MTS assay, which is based on the conversion of a tetrazolium salt into a coloured, aqueous soluble formazan product by mitochondrial enzyme activity of viable cells at 37 °C. The amount of formazan produced by dehydrogenase enzymes, which are present in living cells, is directly proportional to the number of living cells in culture and can be measured at 490 nm by a microplate reader. The reagent utilized for this study was CellTite 96 Aqueous One Solution. The detection was carried out according to the manufacturer’s instructions. Briefly, MGC-803 cells were seeded into 96-well plates (4 × 103 cells/well) and treated with various concentrations of equol (0-80 μmol/L) in RPMI-1640 medium with 2% FBS for 24, 36 and 48 h. Subsequently, the MTS reagent was added (1:5 dilution) to each well and incubated in darkness for 2 h at 37 °C. Finally, the absorbance at 490 nm was recorded by a microplate reader (Bio-Rad, United States). Cell viability was shown as the percentages of viable cells with vehicle-treated cells arbitrarily set as 100% viability.
MGC-803 cells were treated with vehicle or various concentrations of equol (10-30 μmol/L) for 24 h. At the end of incubation, cells (1 × 106) were harvested and fixed with 70% ethanol at -20 °C and stained with propidium iodide utilizing a Cell Cycle Staining Kit according to the manufacturer’s protocol. A total number of 10000 cells were collected and subjected to flow cytometry using a FACScan flow cytometer equipped with the Cellquest acquisition and analysis program (BD FACS Calibur, San Jose, CA).
Cells were seeded into 60-mm plates and treated with vehicle or desired concentrations of equol (5-20 μmol/L). Then, cells were harvested 48 h post-treatment and stained with annexin-V and PI, as described by the manual for the Annexin V/PI apoptosis kit. The samples were analyzed by flow cytometry utilizing a FACScan flow cytometer (BD FACS Calibur), and 10000 events were collected for each sample. For analysis, the annexin-V+/PI- cells were identified as early apoptotic cells, the annexin-V+/PI+ cells as late apoptotic ones, and the annexin-V-/PI- cells as viable.
MGC-803 cells were seeded onto glass coverslips placed in 24-well plates and treated with various concentrations of equol (0-10 μmol/L) for 24 h. The slips were rinsed with PBS and fixed in 4% paraformaldehyde for 15 min. After two rinses in PBS, the coverslips were permeabilized with 0.5% Triton X-100, blocked in 5% BSA and incubated overnight at 4 °C with anti-Ki67 antibody. After three rinses in PBS, coverslips were further incubated with FITC conjugated secondary antibody for 1 h at room temperature in dark and stained with DAPI solution. For negative controls, a set of coverslips was performed under similar conditions but only incubated without the primary antibodies. Finally, all the samples were mounted with Fluorescent Mounting Medium and observed under a fluorescent microscope (Olympus, Japan).
Total RNA was extracted from cells treated with vehicle or equol for desired time (6-48 h) utilizing an RNAsimple Total Kit, according to the manufacturer’s instructions. The RNA concentration and purity were detected utilizing a NanoDrop (ThermoScientific, Wilmington, DE). For single-stranded cDNA synthesis, 1 μg of total RNA was used utilizing a FastQuant RT Kit. The qRT-PCR assay was performed utilizing SuperReal PreMix Plus (SYBR Green), and the fluorescence signal was detected with LightCycler480-II (Roche Applied Science, Mannheim, Germany). PCR reactions were conducted with an initial PCR activation at 95 °C for 15 min, followed by 45 cycles of 95 °C for 2 s, 58 °C for 5 s, and 72 °C for 10 s. The results were normalized with the detected value for GAPDH. Each measurement was repeated three times. Real-time PCR primers were as follows: Ki67, forward (5′-GTGGTAAGCACCAGAGACCC-3′) and reverse (5′-GGAGCAACCCTCTGCTTCTT-3′); Cyclin E1 forward (5′-ATACTTGCTGCTTCGGCCTT-3′) and reverse (5′-TCAGTTTTGAGCTCCCCGTC-3′); GAPDH forward (5′-TTGCCATCAATGACCCCTTCA-3′) and reverse (5′-CGCCCCACTTGATTTTGGA-3′); P21 forward (5′-GCGACTGTGATGCGCTAATG-3′) and reverse (5′-GAAGGTAGAGCTTGGGCAGG-3′).
Cells were washed with ice-cold PBS and lysed with RIPA Lysing Buffer. The total protein concentration in the extracts was measured utilizing a BCA protein assay kit. Equal amounts of proteins (30-60 μg/lane) were resolved on 10% or 12% SDS-PAGE and electrotransferred to PVDF membranes. Then, the membranes were blocked with 5% BSA or non-fat dry milk in TBST for 1 h and probed with appropriate primary antibodies at 4 °C with gentle shaking overnight, followed by incubation with HRP conjugated anti-rabbit or anti-mouse secondary antibodies for 1 h at room temperature. The signal was detected by chemiluminescence utilizing the ECL reagent and recorded on an X-ray film.
Cells were treated with 20 μmol/L of equol for 12, 24 and 48 h or various concentrations of equol for 24 h. The protein expression and phosphorylation levels of Akt at Thr450, Ser473, and Thr 308 sites were detected by Western blot analysis. The detailed steps were conducted as we described above.
Statistical analyses were conducted utilizing GraphPad Prism software. All of the values are expressed as the mean ± SE. Significant differences between groups were determined via one-way analysis of variance (ANOVA) followed by Tukey’s post hoc test with a 0.05 significance level. Each experiment was repeated at least three times.
The effect of equol on the viability of MGC-803 cells was determined by MTS assay. As shown in Figure 1A, equol treatment caused a dose- and time-dependent decrease in cell viability. Equol significantly inhibited cell growth at concentrations higher than 2.5 μmol/L. The 50% inhibitory concentrations (IC50) of equol in MGC-803 cells were 180.10, 19.98 and 13.93 μmol/L, respectively, for 24, 36 and 48 h incubation.
To further demonstrate the anti-proliferative effect of equol on MGC-803 cells, the mRNA and protein expression of Ki67 was examined. As shown in Figure 1B, the mRNA expression of Ki67, a general marker of cellular proliferation in tumor[28], was down-regulated after treatment with 20 μmol/L of equol for 12 and 24 h. In addition, immunofluorescence assay showed that equol obviously decreased the protein expression of Ki67 after 24 h treatment (Figure 1C).
We further tested whether the anti-proliferative effect of equol on MGC-803 cells was due to cell cycle arrest. As shown in Figure 2, equol treatment resulted in a significantly increased proportion of cells in the G0/G1 phase compared to the vehicle-treated controls (P < 0.05). The S values were obviously decreased from 40.6% in the control group to 13.92% in 30 μmol/L of equol treated one, and the G2/M phase was only minimally changed.
To explore the molecular mechanisms underlying equol-induced cell cycle arrest, the expression of cell cycle regulators involved in the G0/G1 progression of the cell cycle was examined. The results of qPCR (Figure 3A) demonstrated that equol (20 μmol/L) treatment resulted in a time-dependent decrease in the Cyclin E1 gene expression, whereas an increase in the levels of P21WAF1. Moreover, Western blot analysis revealed that equol obviously down-regulated the protein expression of Cyclin E1, Cyclin D1, CDK2, and CDK4, and up-regulated the levels of P21WAF1 (Figure 3B). These molecular changes were consistent with the promoting effect of equol on G0/G1 cell cycle arrest.
After 48 h incubation, equol induced apoptosis of MGC-803 cells in a dose-dependent manner, as determined by Annexin V/PI apoptosis assay (Figure 4A). The percentages of apoptotic cells, including the early (Annexin V+/PI-) and late (Annexin V+/PI+) apoptotic cells, were 5.22%, 14.93%, 38.61%, and 41.59% for the vehicle control, 5, 10, and 20 μmol/L of equol treatment, respectively.
To further confirm the apoptotic effect of equol, Western blot analysis was performed to analyze the expression of cleaved PARP and caspase-3. As shown in Figure 4B, equol time-dependently increased the levels of cleaved PARP and caspase-3. These findings certify the induction of apoptosis in equol-treated cells.
To gain further insights into the molecular basis for the capacity of equol to induce cell cycle arrest and apoptosis, we examined its effect on the activities of Akt. As shown in Figure 5A and B, equol treatment significantly induced dephosphorylation of Akt at Thr450 in a dose and time-dependent manner, without affecting the expression of total Akt. However, equol modulated the phosphorylation of Akt at Ser473 and Thr308 in a different way. As shown in Figure 5A, a dramatic dephosphorylation of Akt at these two sites was observed after 48 h incubation, whereas equol led to elevated activation of Akt at Ser473 and Thr308 after 12 and 24 h treatment in comparison with the vehicle-treated control. Meanwhile, we found that equol induced hypophosphorylation of Akt at Ser473 and Thr308 in a dose-dependent manner after 24 h treatment (Figure 5B). These results provided evidence that equol modulated Akt signaling in MGC-803 cells.
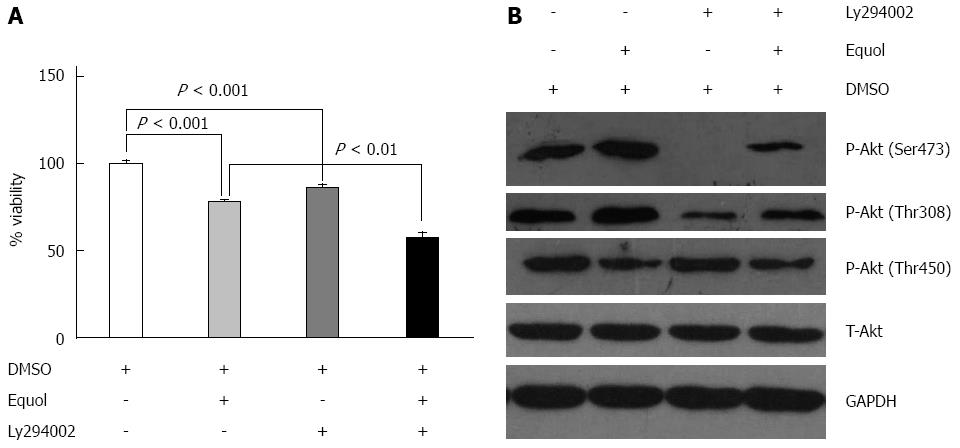
In general, Akt is the downstream target of PI3K and PI3K inhibition could play a role in suppressing phosphorylation of Akt. In our study, to characterize the role of elevated Akt activation in the anti-proliferative effect exerted by equol, MGC-803 cells were incubated with 20 μmol/L equol in the presence or absence of Ly294002 (a specific PI3K inhibitor) for 24 h and cell viability was determined by MTS assay. As shown in Figure 6A, at a concentration of 20 μmol/L, equol inhibited cell growth, with cell viability of 78% compared with the vehicle-treated control. Ly294002 alone also inhibited MGC-803 cell growth (with cell viability of 86%), and the combination treatment of Ly294002 and equol had a stronger inhibitory effect, with cell viability of 57%.
Next, the effect of Ly294002 on the levels of phosphorylated Akt was examined. As shown in Figure 6B, Ly294002 was effective in inhibiting Akt phosphorylation at Ser473 and Thr308, as expected. However, treatment with Ly294002 alone or in combination with equol did not affect the phosphorylation of Akt at Thr450. These results demonstrated that the phosphorylation of Akt at Thr308 and Ser473 was likely dependent on PI3K activity, whereas phosphorylation at Thr450 was independent of PI3K.
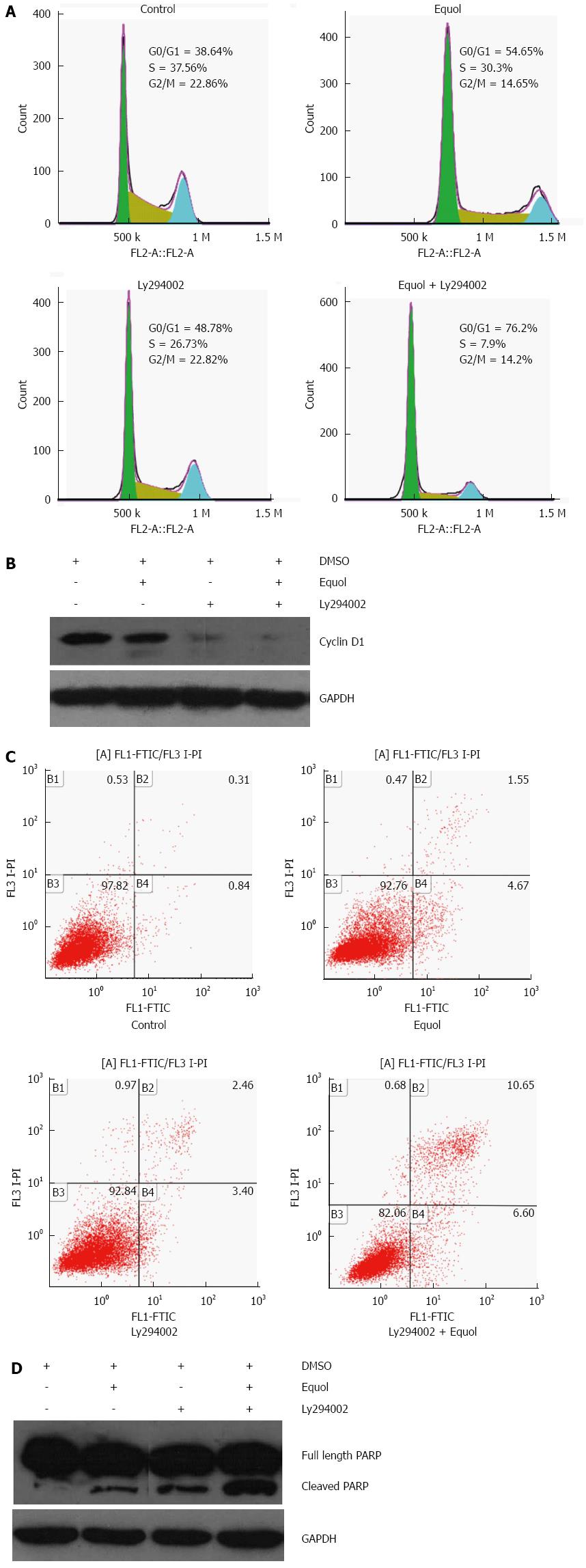
Akt activation has been found to facilitate the G1/S progression of the cell cycle[29], but there are also studies reporting its cell cycle arrest inducing effect in some cellular systems[30,31]. To clarify the role of activated Akt in equol-induced G0/G1 phase arrest, MGC-803 cells were incubated with 20 μmol/L equol in the presence or absence of Ly294002 for 24 h, and cell cycle assay was determined. As shown in Figure 7A, equol effectively triggered cell cycle arrest at G0/G1 phase, and Ly294002 did not prevent but led to enhanced G0/G1 phase cell cycle arrest induced by equol.
Our study demonstrated that equol induced G0/G1 phase cell cycle arrest accompanied by inhibition of Cyclin D1. Next, the effect of Ly294002 on equol- mediated Cyclin D1 inhibition was investigated. As shown in Figure 7B, Ly294002 alone decreased the levels of Cyclin D1 in comparison with untreated controls. Moreover, Ly294002 enhanced equol-mediated Cyclin D1 inhibition. These results indicated that hypophosphorylation of Akt at Ser473 and Thr308 did not contribute to equol-mediated G0/G1 arrest but facilitated the G1/S progression of the cell cycle.
Akt activation has been shown to exhibit both anti-apoptotic and pro-apoptotic effects in a variety of cellular systems[32-35]. To determine the role of elevated Akt phosphorylation in equol-induced apoptosis, MGC-803 cells were treated for 24 h with equol alone or combined with Ly294002 to inhibit equol-induced signaling through Akt. The results are illustrated in Figure 7C and 7D; Ly294002 effectively enhanced equol-induced apoptotic death and PARP cleavage. These results indicated that the increase in Akt activation was not responsible for cell apoptosis signaling in equol-treated MGC-803 cells.
The results of our study demonstrated that equol could effectively inhibit the proliferation of human gastric cancer MGC-803 cells, which was associated with cell cycle arrest. Equol triggered cell cycle arrest at G0/G1 phase by regulating cell cycle regulators, such as CDK2/4, Cyclin D1/E1, and P21WAF1. Furthermore, equol induced apoptotic cell death that was evidenced by the cleavage of PARP and Caspase-3. Our data also suggested that the Akt signaling pathway may play a role in equol-mediated cell cycle arrest and apoptosis. To our knowledge, this is the first study demonstrating that equol exerts anticancer effect in human gastric cancer cells and that blockade of the Akt signaling pathway may be an antitumor mechanism of equol.
Chemopreventive effects of equol have been widely discussed among a variety of human tumors[15,17,19,23,24,36], including breast and prostate cancers, hepatocellular carcinoma, skin cancer, cervical cancer, and so on. Several studies[36-38] reported that equol could inhibit proliferation of human breast cancer cells (MDA-MB-453 and MCF-7), human cervical cancer cells (Hela) and human prostate tumor cells (LNCaP) at varying concentrations (<10 μmol/L to 100 μmol/L). Whether equol is effective in inhibition of human gastric carcinoma cells, however, remains unclear. In our study, we found that equol (>2.5 μmol/L) effectively inhibited the proliferation of MGC-803 cells, with the IC50 of 13.93 μmol/L after 48 h of incubation, which are lower than the values reported in LNCaP and LAPC-4 cells[39], with IC50 values of 53.8 and 35.1 μmol/L, respectively, even after 96 h of incubation. Our in vitro data suggest that equol may be an effective chemopreventive agent for human gastric carcinoma.
The results in the present study demonstrated that equol obviously triggered G0/G1 phase cell cycle arrest in MGC-803 cells. The G0/G1 phase is the first phase of the cell cycle that takes place in eukaryotic cell division and also a crucial checkpoint of cell cycle. Progression through G0/G1 phase requires both Cyclin D-dependent CDK4/CDK6 and CDK2/Cyclin E holoenzymes[40]. The results of this study demonstrated that equol treatment down-regulated the expression of Cyclin D1, CDK4, Cyclin E1, and CDK2, a finding that confirmed a possible G0/G1 phase arrest in the treated MGC-803 cells. Cell cycle progression is also coordinated by the relative balance between the cellular concentrations of Cyclin-CDK complexes and CDK inhibitors. It has become apparent that P21WAF1 is a universal inhibitor of Cyclin/CDK catalytic activity[41]. Our data clearly demonstrated that equol treatment induced the up-regulation of P21WAF1 expression, thus accounting for its effect on reduction in CDK activity and the subsequent induction of cell cycle arrest. Taken together, the data in our study suggest that the inhibitory effect of equol on the proliferation of MGC-803 cells might result from cell cycle arrest during the G0/G1 phase.
It is well established that apoptosis and the genes that control it have a profound effect on the malignant phenotype and can be targeted for the therapy of various malignancies[42]. Accordingly, our results demonstrating induction of apoptotic death by equol could also be the mechanism that accounts for the anti-proliferating effect of equol in MGC-803 cells. The increase in the levels of Caspase-3 and PARP cleavage suggested that equol-induced apoptosis induction might be mediated by Caspases activation.
Although many studies have indicated the antiproliferative activity of equol in terms of its capacity to induce cell cycle arrest and cell apoptosis[21,38,43], the underlying molecular mechanism is still unclear. The Akt signaling pathway plays a critical role in controlling cell survival and apoptosis. In recent years, increasing data have proved that Akt signaling is frequently activated in gastrointestinal tumors[44]. Therefore, in this study, we explored the effect of equol on the Akt pathway. Usually, there are three major sites (Thr308, Thr450, and Ser473) on Akt that are phosphorylated in vivo. Akt appears to be basally phosphorylated at Thr450, which is independent of PI3K, whereas Thr308 and Ser473 are inducibly phosphorylated after treatment of cells with extracellular stimuli and dependent on PI3K activity[45]. Our study demonstrated that equol treatment increased P-Akt (Ser473 and Thr308) at 12 and 24 h compared to vehicle-treated control. Increased Akt activation was shown to be associated with cell proliferation and survival[46,47]. In certain circumstances, however, up-regulation of Akt signaling could also play a role in cell cycle arrest and apoptotic death[31,34], and the exact effect of which may be dependent on the cell type and the stress condition[48]. Our study demonstrated that Ly294002, a specific PI3K inhibitor, effectively inhibited Akt phosphorylation at Ser473 and Thr308, as expected. In addition, Akt inhibition by Ly294002 could not prevent but led to enhanced G0/G1 arrest and apoptosis. These results suggested that increased Akt phosphorylation at Ser473 and Thr308 did not contribute to equol-exerted antitumor activity. Although equol could induce dephosphorylation of Akt at Ser473 and Thr308 after 48 h treatment, since MGC-803 cells underwent cell cycle arrest and apoptotic death early from 24 h, there was a time lag of 24 h. Therefore, we speculated that equol exerted antitumor activity in MGC-803 cells possibly not by dephosphorylation of Akt at Thr308 and Ser473. On the contrary, equol induced dephosphorylation of Akt at Thr450 as early as 12 h, and the cells underwent a parallel cell cycle arrest and apoptosis after 24 h. Therefore, it is possible that equol induced cell cycle arrest and apoptosis by dephosphorylation of Akt at Thr450. Our study also demonstrated that equol triggered degradation of Cyclin D1 and up-regulated the levels of P21WAF1. Such a finding is consistent with previous studies demonstrating that the activation of Akt increased the expression of Cyclin D1, facilitating the G1/S progression of cell cycle[49], and that the inhibition of Akt pathway led to transcriptional induction of P21WAF1[50]. Therefore, we speculated that Akt-CyclinD1 and Akt-P21WAF1 signaling axes might at least partly account for the antiproliferative effect of equol on MGC-803 cells. The detailed mechanisms of how equol leads to dephosphorylation of Akt at Thr450 remains to be investigated.
Taken together, these findings indicate that equol exhibits anti-tumor effects on human gastric carcinoma cells by leading to G0/G1 cell cycle arrest and apoptotic death, which might be mediated by dephosphorylation of Akt at Thr450. The present study also demonstrated the involvement of cell cycle regulators and end point markers of apoptosis, including CDK2/4, Cyclin D1/E1, P21WAF1, Caspase-3 and PARP cleavage. These findings suggested that equol may be a novel candidate for gastric cancer chemoprevention and therapy.
Gastric cancer, a leading cause of cancer death worldwide, constitutes a serious threat to human health. Although many therapies, including radiotherapy, chemotherapy, and biotherapy, have been developed for gastric cancer treatment, the recurrence and subsequent resistance to chemoradiation therapy are unavoidable.
Equol is a metabolite of daidzein, which is one of the major isoflavones in soy. Chemopreventive effects of equol have been demonstrated in a wide variety of human tumors; however, few studies have been conducted in gastric cancer.
The results of this study demonstrated that equol could effectively inhibit the proliferation of human gastric cancer MGC-803 cells, which was associated with cell cycle arrest and apoptosis. The data also suggested that the Akt signaling pathway may play a role in equol-mediated cell cycle arrest. Until now, this is the first study demonstrating that equol exerts anticancer effect in human gastric cancer cells and that blockade of the Akt signaling pathway may be an antitumor mechanism of equol.
Equol may be a novel candidate for gastric cancer chemoprevention and therapy.
The Akt signaling pathway plays a critical role in controlling cell survival and apoptosis. Usually, there are three major sites (Thr308, Thr450, and Ser473) on Akt that are phosphorylated in vivo. Akt appears to be basally phosphorylated at Thr450, which is independent of PI3K, whereas Thr308 and Ser473 are inducibly phosphorylated after treatment of cells with extracellular stimuli and dependent on PI3K activity.
This is a well written and designed study. The authors give a sufficient overview about the study background and raised clearly the hypothesis of the study. The aim of the study is fulfilled. This study makes a contribution to better understanding the molecular and intracellular mechanisms of carcinogenesis, and the authors suggest equol as a novel candidate for gastric cancer chemoprevention and therapy.
P- Reviewer: Vorobjova T S- Editor: Ma YJ L- Editor: Wang TQ E- Editor: Ma S
| 1. | Hartgrink HH, Jansen EP, van Grieken NC, van de Velde CJ. Gastric cancer. Lancet. 2009;374:477-490. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 692] [Cited by in F6Publishing: 758] [Article Influence: 50.5] [Reference Citation Analysis (0)] |
| 2. | Hoshiyama Y, Sasaba T. A case-control study of stomach cancer and its relation to diet, cigarettes, and alcohol consumption in Saitama Prefecture, Japan. Cancer Causes Control. 1992;3:441-448. [PubMed] [Cited in This Article: ] |
| 3. | Kim HJ, Chang WK, Kim MK, Lee SS, Choi BY. Dietary factors and gastric cancer in Korea: a case-control study. Int J Cancer. 2002;97:531-535. [PubMed] [Cited in This Article: ] |
| 4. | Wu AH, Yang D, Pike MC. A meta-analysis of soyfoods and risk of stomach cancer: the problem of potential confounders. Cancer Epidemiol Biomarkers Prev. 2000;9:1051-1058. [PubMed] [Cited in This Article: ] |
| 5. | Ito LS, Inoue M, Tajima K, Yamamura Y, Kodera Y, Hirose K, Takezaki T, Hamajima N, Kuroishi T, Tominaga S. Dietary factors and the risk of gastric cancer among Japanese women: a comparison between the differentiated and non-differentiated subtypes. Ann Epidemiol. 2003;13:24-31. [PubMed] [Cited in This Article: ] |
| 6. | Nagata C, Takatsuka N, Kawakami N, Shimizu H. A prospective cohort study of soy product intake and stomach cancer death. Br J Cancer. 2002;87:31-36. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 62] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 7. | Sauvaget C, Lagarde F, Nagano J, Soda M, Koyama K, Kodama K. Lifestyle factors, radiation and gastric cancer in atomic-bomb survivors (Japan). Cancer Causes Control. 2005;16:773-780. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 52] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 8. | Takezaki T, Gao CM, Wu JZ, Li ZY, Wang JD, Ding JH, Liu YT, Hu X, Xu TL, Tajima K. hOGG1 Ser(326)Cys polymorphism and modification by environmental factors of stomach cancer risk in Chinese. Int J Cancer. 2002;99:624-627. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 83] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 9. | Adlercreutz CH, Goldin BR, Gorbach SL, Höckerstedt KA, Watanabe S, Hämäläinen EK, Markkanen MH, Mäkelä TH, Wähälä KT, Adlercreutz T. Soybean phytoestrogen intake and cancer risk. J Nutr. 1995;125:757S-770S. [PubMed] [Cited in This Article: ] |
| 10. | Arora A, Nair MG, Strasburg GM. Antioxidant activities of isoflavones and their biological metabolites in a liposomal system. Arch Biochem Biophys. 1998;356:133-141. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 299] [Cited by in F6Publishing: 272] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 11. | Choi EJ, Kim GH. Antiproliferative activity of daidzein and genistein may be related to ERα/c-erbB-2 expression in human breast cancer cells. Mol Med Rep. 2013;7:781-784. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 12. | Kelly GE, Joannou GE, Reeder AY, Nelson C, Waring MA. The variable metabolic response to dietary isoflavones in humans. Proc Soc Exp Biol Med. 1995;208:40-43. [PubMed] [Cited in This Article: ] |
| 13. | Dubey RK, Gillespie DG, Imthurn B, Rosselli M, Jackson EK, Keller PJ. Phytoestrogens inhibit growth and MAP kinase activity in human aortic smooth muscle cells. Hypertension. 1999;33:177-182. [PubMed] [Cited in This Article: ] |
| 14. | Verma SP, Goldin BR. Effect of soy-derived isoflavonoids on the induced growth of MCF-7 cells by estrogenic environmental chemicals. Nutr Cancer. 1998;30:232-239. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 31] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 15. | Zheng W, Zhang Y, Ma D, Shi Y, Liu C, Wang P. (±)Equol inhibits invasion in prostate cancer DU145 cells possibly via down-regulation of matrix metalloproteinase-9, matrix metalloproteinase-2 and urokinase-type plasminogen activator by antioxidant activity. J Clin Biochem Nutr. 2012;51:61-67. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 16. | Ward HA, Kuhnle GG, Mulligan AA, Lentjes MA, Luben RN, Khaw KT. Breast, colorectal, and prostate cancer risk in the European Prospective Investigation into Cancer and Nutrition-Norfolk in relation to phytoestrogen intake derived from an improved database. Am J Clin Nutr. 2010;91:440-448. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 94] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 17. | Onoda A, Ueno T, Uchiyama S, Hayashi S, Kato K, Wake N. Effects of S-equol and natural S-equol supplement (SE5-OH) on the growth of MCF-7 in vitro and as tumors implanted into ovariectomized athymic mice. Food Chem Toxicol. 2011;49:2279-2284. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 18. | Lund TD, Blake C, Bu L, Hamaker AN, Lephart ED. Equol an isoflavonoid: potential for improved prostate health, in vitro and in vivo evidence. Reprod Biol Endocrinol. 2011;9:4. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 19. | Ju YH, Fultz J, Allred KF, Doerge DR, Helferich WG. Effects of dietary daidzein and its metabolite, equol, at physiological concentrations on the growth of estrogen-dependent human breast cancer (MCF-7) tumors implanted in ovariectomized athymic mice. Carcinogenesis. 2006;27:856-863. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 109] [Cited by in F6Publishing: 111] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 20. | Goodman MT, Shvetsov YB, Wilkens LR, Franke AA, Le Marchand L, Kakazu KK, Nomura AM, Henderson BE, Kolonel LN. Urinary phytoestrogen excretion and postmenopausal breast cancer risk: the multiethnic cohort study. Cancer Prev Res (Phila). 2009;2:887-894. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 59] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 21. | Choi EJ, Kim GH. Anticancer mechanism of equol in 7,12-dimethylbenz(a)anthracene-treated animals. Int J Oncol. 2011;39:747-754. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 22. | Vrieling A, Rookus MA, Kampman E, Bonfrer JM, Korse CM, van Doorn J, Lampe JW, Cats A, Witteman BJ, van Leeuwen FE. Isolated isoflavones do not affect the circulating insulin-like growth factor system in men at increased colorectal cancer risk. J Nutr. 2007;137:379-383. [PubMed] [Cited in This Article: ] |
| 23. | Liang XL, Li M, Li J, Wang XL. Equol induces apoptosis in human hepatocellular carcinoma SMMC-7721 cells through the intrinsic pathway and the endoplasmic reticulum stress pathway. Anticancer Drugs. 2014;25:633-640. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 24. | Widyarini S, Husband AJ, Reeve VE. Protective effect of the isoflavonoid equol against hairless mouse skin carcinogenesis induced by UV radiation alone or with a chemical cocarcinogen. Photochem Photobiol. 2005;81:32-37. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 12] [Reference Citation Analysis (0)] |
| 25. | Lyn-Cook BD, Stottman HL, Yan Y, Blann E, Kadlubar FF, Hammons GJ. The effects of phytoestrogens on human pancreatic tumor cells in vitro. Cancer Lett. 1999;142:111-119. [PubMed] [Cited in This Article: ] |
| 26. | Yang JJ, Cho LY, Ko KP, Shin A, Ma SH, Choi BY, Han DS, Song KS, Kim YS, Lee JY. Genetic susceptibility on CagA-interacting molecules and gene-environment interaction with phytoestrogens: a putative risk factor for gastric cancer. PLoS One. 2012;7:e31020. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 27. | Ko KP, Park SK, Park B, Yang JJ, Cho LY, Kang C, Kim CS, Gwack J, Shin A, Kim Y. Isoflavones from phytoestrogens and gastric cancer risk: a nested case-control study within the Korean Multicenter Cancer Cohort. Cancer Epidemiol Biomarkers Prev. 2010;19:1292-1300. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 69] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 28. | de Azambuja E, Cardoso F, de Castro G, Colozza M, Mano MS, Durbecq V, Sotiriou C, Larsimont D, Piccart-Gebhart MJ, Paesmans M. Ki-67 as prognostic marker in early breast cancer: a meta-analysis of published studies involving 12,155 patients. Br J Cancer. 2007;96:1504-1513. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 587] [Cited by in F6Publishing: 651] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 29. | Chen XL, Ren KH, He HW, Shao RG. Involvement of PI3K/AKT/GSK3beta pathway in tetrandrine-induced G1 arrest and apoptosis. Cancer Biol Ther. 2008;7:1073-1078. [PubMed] [Cited in This Article: ] |
| 30. | Alvarez B, Martínez-A C, Burgering BM, Carrera AC. Forkhead transcription factors contribute to execution of the mitotic programme in mammals. Nature. 2001;413:744-747. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 214] [Cited by in F6Publishing: 216] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 31. | Chen KC, Yang TY, Wu CC, Cheng CC, Hsu SL, Hung HW, Chen JW, Chang GC. Pemetrexed induces S-phase arrest and apoptosis via a deregulated activation of Akt signaling pathway. PLoS One. 2014;9:e97888. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 32. | Cardone MH, Roy N, Stennicke HR, Salvesen GS, Franke TF, Stanbridge E, Frisch S, Reed JC. Regulation of cell death protease caspase-9 by phosphorylation. Science. 1998;282:1318-1321. [PubMed] [Cited in This Article: ] |
| 33. | del Peso L, González-García M, Page C, Herrera R, Nuñez G. Interleukin-3-induced phosphorylation of BAD through the protein kinase Akt. Science. 1997;278:687-689. [PubMed] [Cited in This Article: ] |
| 34. | Lu B, Wang L, Stehlik C, Medan D, Huang C, Hu S, Chen F, Shi X, Rojanasakul Y. Phosphatidylinositol 3-kinase/Akt positively regulates Fas (CD95)-mediated apoptosis in epidermal Cl41 cells. J Immunol. 2006;176:6785-6793. [PubMed] [Cited in This Article: ] |
| 35. | van Gorp AG, Pomeranz KM, Birkenkamp KU, Hui RC, Lam EW, Coffer PJ. Chronic protein kinase B (PKB/c-akt) activation leads to apoptosis induced by oxidative stress-mediated Foxo3a transcriptional up-regulation. Cancer Res. 2006;66:10760-10769. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 52] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 36. | Kim EY, Shin JY, Park YJ, Kim AK. Equol induces mitochondria-mediated apoptosis of human cervical cancer cells. Anticancer Res. 2014;34:4985-4992. [PubMed] [Cited in This Article: ] |
| 37. | Mitchell JH, Duthie SJ, Collins AR. Effects of phytoestrogens on growth and DNA integrity in human prostate tumor cell lines: PC-3 and LNCaP. Nutr Cancer. 2000;38:223-228. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 56] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 38. | Choi EJ, Kim T. Equol induced apoptosis via cell cycle arrest in human breast cancer MDA-MB-453 but not MCF-7 cells. Mol Med Rep. 2008;1:239-244. [PubMed] [Cited in This Article: ] |
| 39. | Raschke M, Wähälä K, Pool-Zobel BL. Reduced isoflavone metabolites formed by the human gut microflora suppress growth but do not affect DNA integrity of human prostate cancer cells. Br J Nutr. 2006;96:426-434. [PubMed] [Cited in This Article: ] |
| 40. | Sherr CJ. G1 phase progression: cycling on cue. Cell. 1994;79:551-555. [PubMed] [Cited in This Article: ] |
| 41. | Harper JW, Adami GR, Wei N, Keyomarsi K, Elledge SJ. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell. 1993;75:805-816. [PubMed] [Cited in This Article: ] |
| 42. | Lowe SW, Lin AW. Apoptosis in cancer. Carcinogenesis. 2000;21:485-495. [PubMed] [Cited in This Article: ] |
| 43. | Charalambous C, Pitta CA, Constantinou AI. Equol enhances tamoxifen’s anti-tumor activity by induction of caspase-mediated apoptosis in MCF-7 breast cancer cells. BMC Cancer. 2013;13:238. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 44. | Michl P, Downward J. Mechanisms of disease: PI3K/AKT signaling in gastrointestinal cancers. Z Gastroenterol. 2005;43:1133-1139. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 78] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 45. | Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes Dev. 1999;13:2905-2927. [PubMed] [Cited in This Article: ] |
| 46. | Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655-1657. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4101] [Cited by in F6Publishing: 4147] [Article Influence: 188.5] [Reference Citation Analysis (0)] |
| 47. | Liang J, Slingerland JM. Multiple roles of the PI3K/PKB (Akt) pathway in cell cycle progression. Cell Cycle. 2003;2:339-345. [PubMed] [Cited in This Article: ] |
| 48. | Shack S, Wang XT, Kokkonen GC, Gorospe M, Longo DL, Holbrook NJ. Caveolin-induced activation of the phosphatidylinositol 3-kinase/Akt pathway increases arsenite cytotoxicity. Mol Cell Biol. 2003;23:2407-2414. [PubMed] [Cited in This Article: ] |
| 49. | Ma X, Hu Y. Targeting PI3K/Akt/mTOR cascade: the medicinal potential, updated research highlights and challenges ahead. Curr Med Chem. 2013;20:2991-3010. [PubMed] [Cited in This Article: ] |
| 50. | Choi HJ, Chung TW, Kang SK, Lee YC, Ko JH, Kim JG, Kim CH. Ganglioside GM3 modulates tumor suppressor PTEN-mediated cell cycle progression--transcriptional induction of p21(WAF1) and p27(kip1) by inhibition of PI-3K/AKT pathway. Glycobiology. 2006;16:573-583. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 46] [Article Influence: 2.6] [Reference Citation Analysis (0)] |