Published online Aug 14, 2015. doi: 10.3748/wjg.v21.i30.9067
Peer-review started: December 26, 2014
First decision: April 23, 2015
Revised: May 14, 2015
Accepted: June 9, 2015
Article in press: June 10, 2015
Published online: August 14, 2015
Processing time: 234 Days and 17.1 Hours
AIM: To investigate the histological features of the liver in spontaneously diabetic Torii (SDT) fatty rats compared with age-matched Sprague-Dawley (SD) rats.
METHODS: Female SDT Leprfa (SDT fatty) rats and age-matched SD rats were fed ad libitum. Body weight and biochemical parameters, such as serum glucose, triglyceride (TG), total cholesterol (TC), alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels as well as fatty acid and TG accumulation in the liver were evaluated at 8 wk of age in the non-fasting state and at 8-wk intervals from 8 to 40 wk of age. Histopathological examinations of the liver were performed using hematoxylin and eosin and Sirius Red staining as well as double staining for ED-1 and toluidine blue. The expression of genes involved in TG synthesis, inflammation, and fibrosis was examined in the liver.
RESULTS: SDT fatty rats showed significantly increased body weight compared with SD rats. Serum glucose, TG, and TC levels were significantly higher in SDT fatty rats compared with SD rats. The serum AST and ALT levels in SDT fatty rats were significantly elevated at 8 wk of age compared with the levels in SD rats. Hepatic TG content was marked in SDT fatty rats from 8 to 32 wk of age. Histopathologically, severe hepatosteatosis accompanied by inflammation was observed at 8 wk of age, and fibrosis started to occur at 32 wk of age. Furthermore, Sirius Red and ED-1 staining were increased in the liver at 32 wk of age. Hepatic gene expression related to TG synthesis, inflammation and fibrosis tended to increase in SDT fatty rats compared with SD rats, and the gene expression related to TG secretion was decreased in SDT fatty rats compared with SD rats.
CONCLUSION: Female SDT fatty rats have the potential to become an important animal model of nonalcoholic steatohepatitis with type 2 diabetes and obesity.
Core tip: Nonalcoholic steatohepatitis (NASH) is recognized as a major risk for progression to cirrhosis and liver failure or to hepatocellular carcinoma. In this study, we investigated the histological features of the liver in female spontaneously diabetic Torii (SDT) fatty rats. The SDT fatty rats exhibited pathophysiological features of NASH, and fibrosis appeared in the liver without dietary manipulation. Female SDT fatty rats have the potential to become an important animal model of NASH with type 2 diabetes and obesity, a condition for which few models currently exist.
- Citation: Ishii Y, Motohashi Y, Muramatsu M, Katsuda Y, Miyajima K, Sasase T, Yamada T, Matsui T, Kume S, Ohta T. Female spontaneously diabetic Torii fatty rats develop nonalcoholic steatohepatitis-like hepatic lesions. World J Gastroenterol 2015; 21(30): 9067-9078
- URL: https://www.wjgnet.com/1007-9327/full/v21/i30/9067.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i30.9067
Nonalcoholic fatty liver disease (NAFLD) is presently well recognized as the most common chronic liver disease in the Western world[1]. NAFLD is strongly associated with central obesity, type 2 diabetes, dyslipidemia, hypertension, and insulin resistance[1]. NAFLD represents the pathology of fatty liver, including simple steatosis, nonalcoholic steatohepatitis (NASH), and cirrhosis[2]. In Western countries, 4%-22% of NAFLD cases lead to hepatocellular carcinoma[3-6]. The pathogenesis of NASH remains poorly defined, and effective pharmacological therapies for NASH have not been approved.
To understand the complicated features of NAFLD/NASH, animal models offer a source of important information. It has been reported that ob/ob mice, db/db mice, and KK-Ay mice, as NAFLD animal models, exhibit spontaneous hepatic steatosis based on insulin resistance and obesity, but these mice do not progress to liver fibrosis when fed a normal diet[7-9]. In contrast, methionine and choline-deficient (MCD) diet-induced NASH is accompanied by hepatic inflammation and fibrosis in ob/ob mice and db/db mice[10,11].
Spontaneously diabetic Torii Leprfa (SDT fatty) rats, which are generated by introducing the fa allele of the Zucker fatty rat into the SDT rat genome, represent a new model of obese type 2 diabetes. SDT fatty rats exhibit hyperphagia that leads to obesity associated with hyperglycemia, hyperinsulinemia, and hyperlipidemia at a young age compared with SDT rats[12,13]. Compared with SDT rats, this early onset of diabetes in SDT fatty rats is considered to be caused by obesity resulting from hyperphagia. Furthermore, female SDT rats also exhibit hyperglycemia at the same young age as male SDT fatty rats[14,15].
SDT fatty rats have been used to investigate the effects of a high-fat diet in a previous study. In this prior study, the rats fed a high-fat diet had improved hyperglycemia and increased serum glucagon-like peptide-1 (GLP-1) levels after refeeding[16]. Moreover, histopathological observations showed improvement in the fatty liver and pancreatic abnormalities. This previous report showed beneficial effects on diabetes in SDT fatty rats fed a high-fat diet.
With regard to the metabolic responses to a high-fat diet in Sprague-Dawley (SD) rats, steatohepatitis associated with hepatic fatty changes, inflammation, and mitochondrial lesions has been confirmed[17]. C57BL6 mice fed a high-fat diet also develop steatohepatitis[18]. However, the degree of hepatic fibrosis is less severe in animals fed a high-fat diet compared with choline-deficient diet models.
In the present study, we investigated the histological features of the liver in SDT fatty rats without manipulating the diet in comparison with age-matched SD rats. In addition to the pathophysiology, the biochemical parameters, hepatic lipid content, and expression of genes were examined.
This experiment was conducted in compliance with the Guidelines for Animal Experimentation of Japan Tobacco Biological/Pharmacological Research Laboratories. The animal protocol was designed to minimize pain or discomfort to the animals. Female SDT fatty rats from our colony were used in the study. At 8 wk of age, SDT fatty rats were divided into 5 groups for necropsy at 8, 16, 24, 32, and 40 wk of age. SD rats [Crj: CD (SD); Charles River Japan, Yokohama, Japan], as control animals, at 8 wk of age were also divided into the same 5 groups as the SDT fatty rats. In the experimental period, one SDT fatty rat in the 24-wk necropsy group died due to unidentified causes, leaving 4 animals in this group. The rats were housed individually in suspended bracket cages in a climate-controlled room with a temperature of 23 °C ± 3 °C, humidity 55% ± 15%, and a 12-h dark-light cycle, and the rats had free access to a commercial diet (CRF-1, Charles River Japan, Yokohama, Japan) and water. The rats were anesthetized with isoflurane inhalation before the procedures.
Body weight and biochemical parameters, such as serum glucose, triglyceride (TG), total cholesterol (TC), alanine aminotransferase (ALT), and aspartate aminotransferase (AST) levels, were measured at 8 wk of age and thereafter at 8-wk intervals until 40 wk of age. Blood samples were collected from the tail vein of the rats. The samples were stored in ice water until measurement. Serum glucose, TG, TC, ALT, and AST levels were measured as biochemical parameters using commercial kits (Roche Diagnostics, Basel, Switzerland) in an automatic analyzer (Hitachi 7170S; Hitachi, Tokyo, Japan).
Necropsy was performed at 8, 16, 24, 32, and 40 wk of age (n = 5 for each age, except 24 wk of age with n = 4). All animals were sacrificed by exsanguination under isoflurane anesthesia. The livers were sampled for measurement of gene expression, hepatic lipid content, and histopathology. Samples for determination of gene expression and hepatic lipid content were stored at -80 °C until analysis. For histopathology, the livers were immediately fixed in 10% neutral-buffered formalin. After resection, the tissue was paraffin-embedded by standard techniques and sectioned (3 to 5 μm). The sections were stained with hematoxylin and eosin (HE). The samples were all examined histopathologically in a blind manner, and the findings were graded from normal (-) to severe (3+).
The paraffin-embedded tissue sections were also stained with Sirius Red and toluidine blue (TB), and immunohistochemistry was performed for ED-1 (CD68: 1:100, BMA BIOMEDICALS, Switzerland) and alpha-smooth muscle actin (α-SMA; 1A4, 1:50, Dako, Denmark).
An approximate 100 mg portion of the liver, 0.5 mL of methanol, and zirconia beads were added to tubes. The liver portion was homogenized using a mixer mill (MM300 Retch) (25 Hz for 10 min). To the homogenized solution, 1 mL of chloroform was added and mixed thoroughly. The mixture was then centrifuged (10000 g for 5 min at 4 °C), and the resultant supernatant was collected. Solvents contained in 0.5 mL of the supernatant were dried under a stream of nitrogen gas. To the residue, 0.5 mL of 2-propanol was added, and the residue was then dissolved again. The TG concentration of the 2-propanol solution was determined using the biochemistry automatic analyzer (Hitachi 7170S; Hitachi, Tokyo, Japan). The free fatty acid (FFA) concentration was analyzed using a NEFA-C kit (Wako Pure Chemical Industries, Ltd., Osaka, Japan).
Total RNA was extracted from the liver with the GenElute Mammalian Total RNA Miniprep Kit (Sigma-Aldrich, St. Louis, MO, United States) at 8, 16, 24, 32, and 40 wk of age. RNA was transcribed into cDNA using M-MLV reverse transcriptase and random primers (Invitrogen, Carlsbad, CA, United States). The reaction mixture was incubated for 10 min at 25 °C, 1 h at 37 °C, and 5 min at 95 °C. Real-time PCR quantification was performed in a 50 μL reaction mixture with an automated sequence detector combined with ABI Prism 7700 Sequence Detection System software (Applied Biosystems, Foster City, CA, United States). The reaction mixture contained 50 ng of synthesized cDNA, 3.5 mmol/L MgCl2, 0.3 μmol/L primers, 0.1 μmol/L probes, and 1.25 units of Ampli Taq Gold®. The cycle parameters included 10 min at 95 °C followed by 40 cycles of 15 s at 95 °C and 60 s at 60 °C. The following primers and FAM-conjugated probes were designed using Primer Express software (Applied Biosystems): SREBP-1c (forward, CGACTACATCCGCTTCTTACAGC; reverse, TTTTGTGAGCACTTCGCAGG; probe, CAGCAACCAGAAACTCAAGCAGGAGAACC), DGAT2 (forward, GCTGATAGCTGCTCTCTACTTCACC; reverse, TGTGATCTCCTGCCACCTTTCT; probe, TGGCATTTGACTGGAACACGCCCA), FAS (forward, ACTGAACGGCATTACTCGGTCC; reverse, GTGTCCCATGTTGGATTTGGTG; probe, TTCCGCCAGAGCCCTTTGTTAATTGG), MTP (forward, GGACGTTGTGTTACTGTGGAGG; reverse, ACATTGACAGCCGTTATCGTGA; probe, GATCCCGACGGTGACGATGATCAACTG), and β-actin (purchased from Applied Biosystems). The expression of the following genes were confirmed using Taqman Gene Expression Assays on Demand and Universal Maser Mix: ACC (Rn00573474_m1), α-SMA (Rn01759928_g1), MCP-1 (Rn00580555_m1), TGF-β (Rn99999016_m1), TNF-α (Rn99999017_m1), and collagen type 1 (Rn01463848_m1).
The results of the biological parameters are expressed as the mean ± SD. Statistical analysis of differences between mean values was performed using an F-test followed by the Student t-test or the Aspin-Welch t-test. Differences were considered significant at P < 0.05. The statistical methods of this study were reviewed by Dr. Hisayo Morinaga from Biological/Pharmacological research laboratories, Central Pharmaceutical Research Institute, Japan Tobacco Inc.
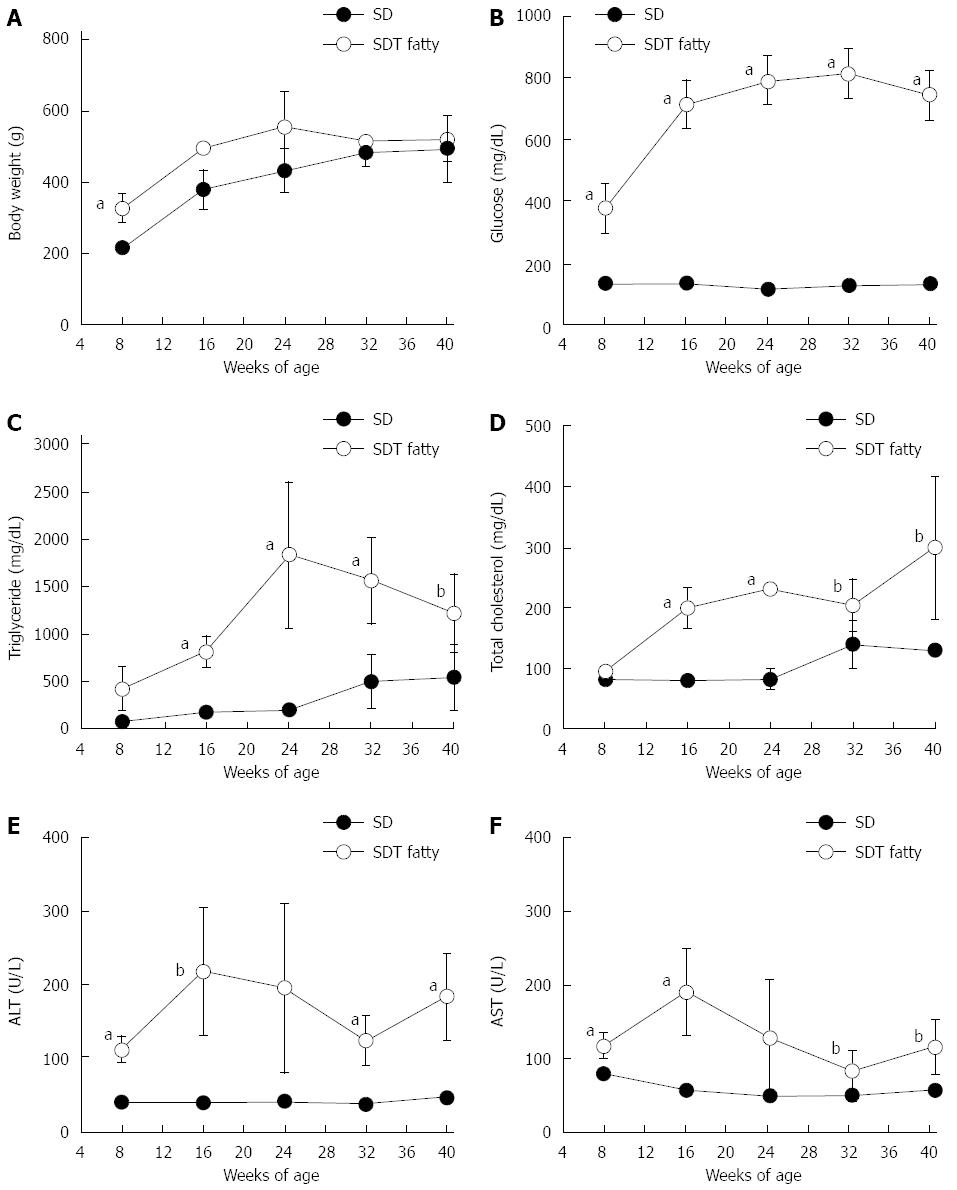
SDT fatty rats showed obesity at 8 wk of age. The mean bodyweight at 8 wk was significantly higher in SDT fatty rats compared with SD rats (328 ± 40 g vs 216 ± 8 g; P < 0.01). Obesity was sustained in SDT fatty rats until 24 wk of age (Figure 1A). The serum glucose levels in SDT fatty rats were higher than those in SD rats at 8 wk of age, and the increase was sustained until 40 wk of age (Figure 1B). The serum TG levels in SDT fatty rats increased until 24 wk of age, and they slightly decreased after 32 wk (Figure 1C). The serum TC levels in SDT fatty rats increased throughout the observation period (Figure 1D). The serum TG and TC levels in SDT fatty rats were higher than those in SD rats until 40 wk of age. Furthermore, the serum AST and ALT levels were significantly elevated at 8 wk, and these high levels were sustained until 40 wk of age (Figure 1E and F).
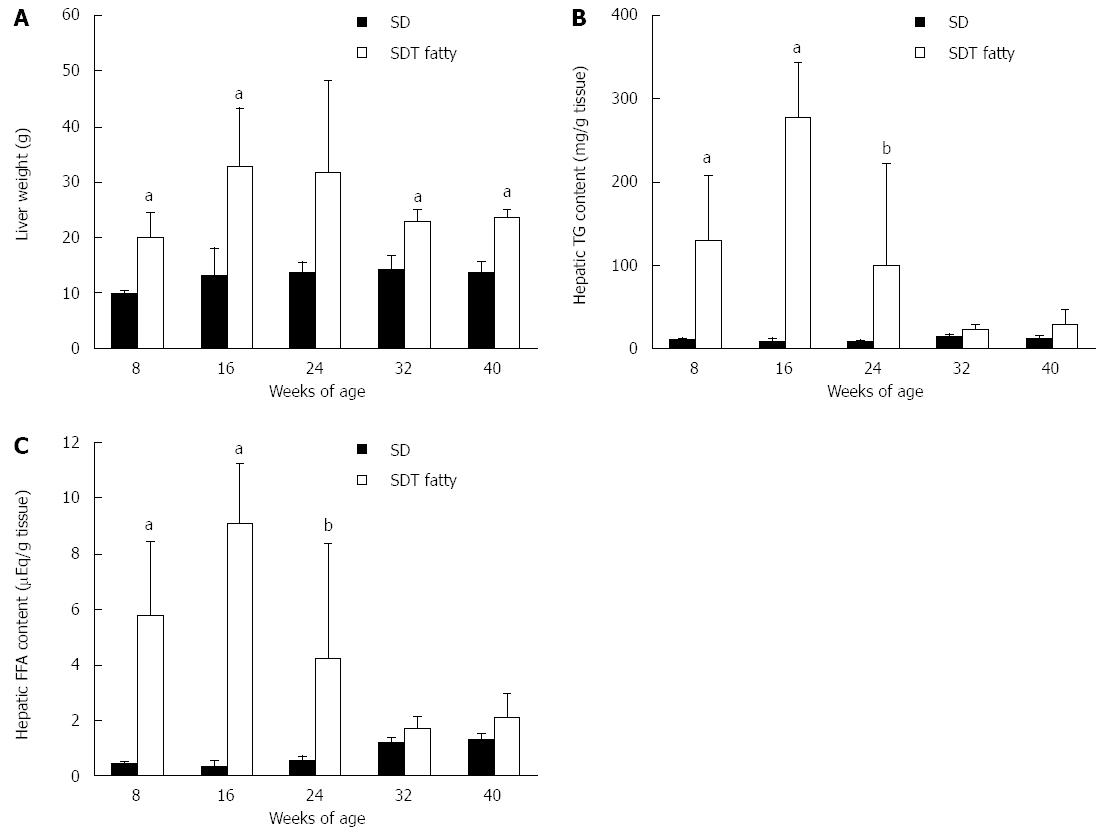
The liver weights were increased in SDT fatty rats until 24 wk of age and slightly decreased thereafter, but they were greater than those in SD rats throughout the experiment, except at 24 wk of age (Figure 2A). The hepatic TG content in SDT fatty rats was higher than that in SD rats at 8 wk of age (129 ± 77 mg/g tissue vs 10 ± 2 mg/g tissue; P < 0.01). The hepatic TG content was markedly elevated until 16 wk of age in SDT fatty rats, and gradually decreased to the level in SD rats (Figure 2B). The hepatic FFA content showed a similar change to hepatic TG content in SDT fatty rats because it increased until 16 wk and gradually decreased after 32 wk of age (Figure 2C).
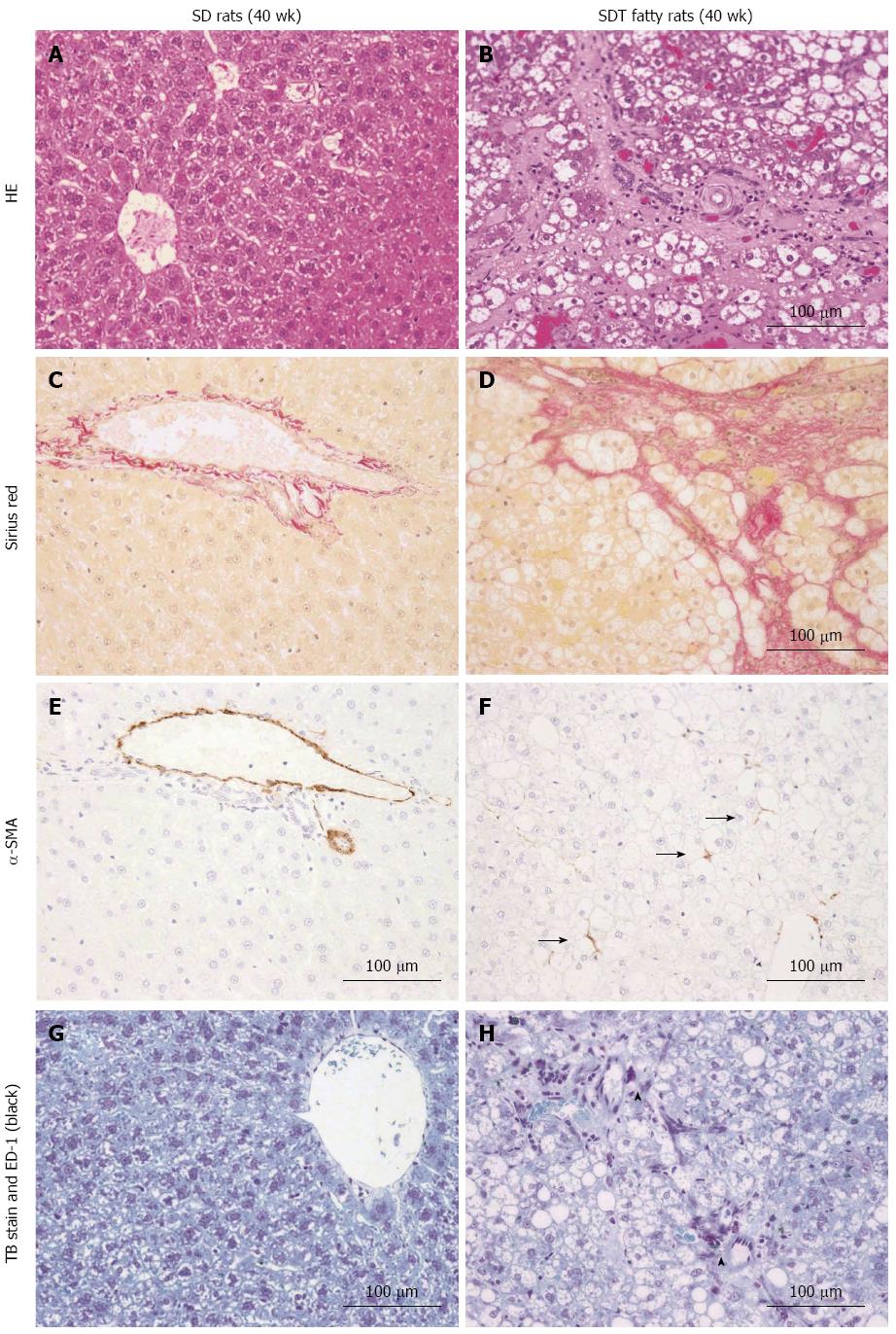
The liver histopathology was examined by HE staining. Histopathologically, severe changes (3+) of the liver, including fatty and vacuolar changes, were observed in SDT fatty rats at 8 wk of age. Moderate changes (1+-2+) of hypertrophy and very slight or slight changes in inflammation were also observed in SDT fatty rats at 8 wk of age (Table 1). Moreover, moderate changes (2+) indicating fibrosis were observed in SDT rats at 32 wk of age.
| 8 wk | 16 wk | 24 wk | 32 wk | 40 wk | ||||||
| SD | SDT fatty | SD | SDT fatty | SD | SDT fatty | SD | SDT fatty | SD | SDT fatty | |
| Hepatosteatosis (Vacuolar change/fatty change) | ||||||||||
| - | 5 | 0 | 5 | 0 | 5 | 0 | 5 | 0 | 5 | 0 |
| ± | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| + | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2+ | 0 | 4 | 0 | 2 | 0 | 2 | 0 | 3 | 0 | 3 |
| 3+ | 0 | 1 | 0 | 3 | 0 | 2 | 0 | 2 | 0 | 2 |
| Hypertrophy of hepatocytes (with vacuolation/fatty change) | ||||||||||
| - | 5 | 1 | 5 | 0 | 5 | 0 | 5 | 0 | 5 | 1 |
| ± | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| + | 0 | 2 | 0 | 2 | 0 | 2 | 0 | 1 | 0 | 2 |
| 2+ | 0 | 2 | 0 | 3 | 0 | 2 | 0 | 3 | 0 | 1 |
| 3+ | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 |
| Fibrosis, focal | ||||||||||
| - | 5 | 4 | 5 | 5 | 5 | 4 | 5 | 1 | 5 | 1 |
| ± | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| + | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 2 |
| 2+ | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 2 |
| 3+ | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Infiltration, inflammatory cell, periportal | ||||||||||
| - | 5 | 2 | 5 | 4 | 5 | 3 | 5 | 3 | 5 | 3 |
| ± | 0 | 3 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 |
| + | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 1 |
| 2+ | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 3+ | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Fibrosis was also confirmed by Sirius Red staining (Figure 3A-D). Consistent with the HE staining, similar findings of fibrosis were observed in SDT fatty rats. However, these changes were not observed in SD rats (Table 1). Activated hepatic stellate cells (HSCs) in the liver were investigated by α-SMA immunostaining. The SD rats showed few HSCs at 32 wk of age. Consistent with the presence of hepatic fibrosis, there were increased numbers of α-SMA-positive, activated HSCs in SDT fatty rats (Figure 3E and F). To examine the macrophages and mast cells in the liver, liver sections were immunostained with ED-1 and TB. Increased macrophage and mast cell counts, as indicated by ED-1- and TB-positive stained cells, respectively, were found in SDT fatty rats at 32 wk of age, and these cells were scarcely observed in SD rats (Figure 3G and H).
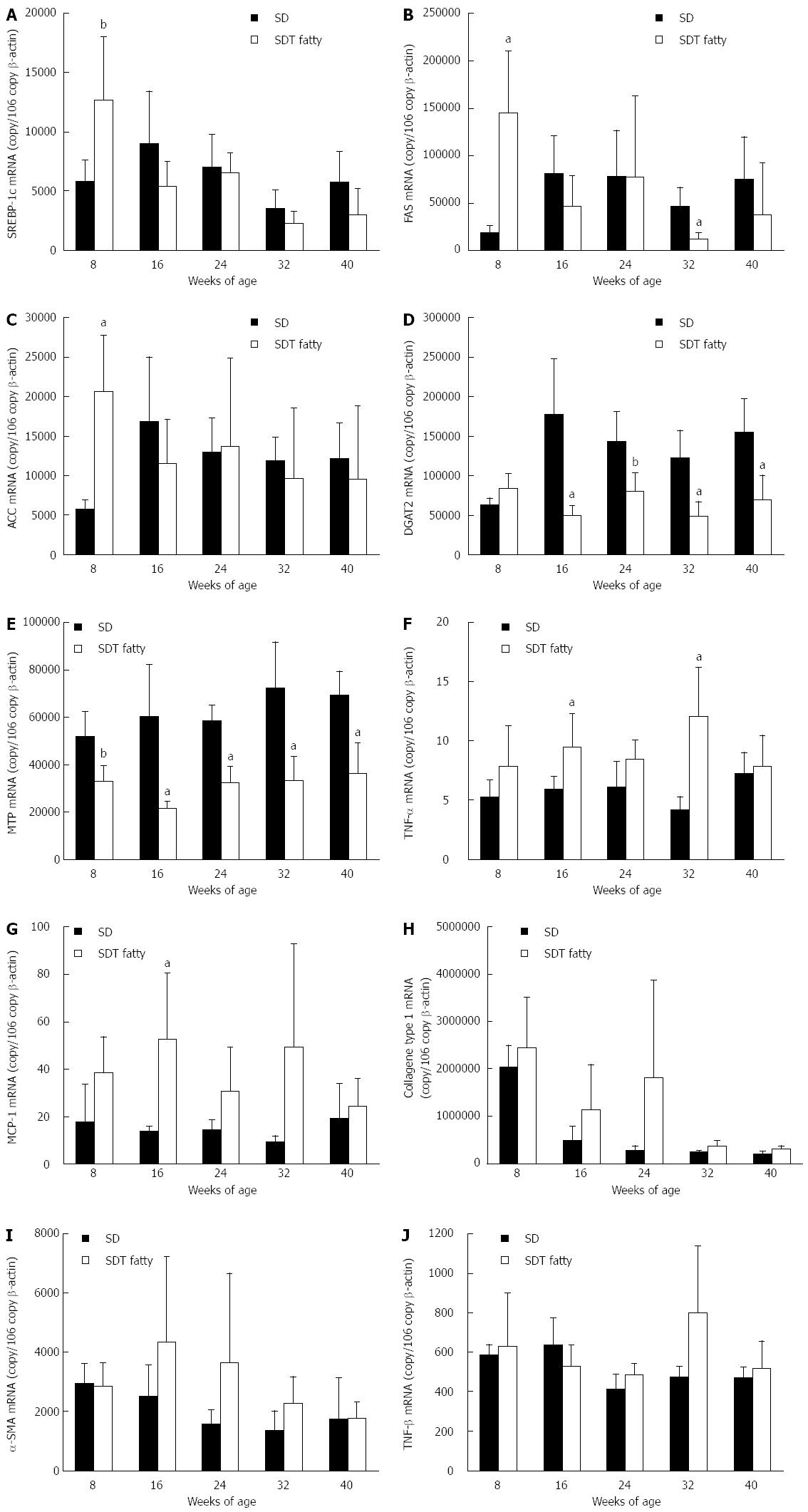
The mRNA levels of lipogenic genes (SREBP-1c, FAS, ACC, DGAT2, and MTP), inflammatory genes (MCP-1 and TNF-α), and fibrotic genes (collagen Type 1, TGF-β, and α-SMA) in the liver were measured by real-time PCR at 8-wk intervals. At 8 wk of age, the expression of lipogenic genes (such as SREBP-1c, FAS, and ACC) increased in SDT fatty rats compared with SD rats (Figure 4A-C). The DGAT2 mRNA level was not increased in SDT fatty rats (Figure 4D), and the MTP mRNA level, which is related to secretion of very low-density lipoprotein (VLDL), was decreased by 64% in SDT fatty rats compared with SD rats (Figure 4E). After 16 wk of age, SDT fatty rats showed decreased expression of lipogenic genes (such as SREBP-1c, FAS, and ACC). The expression of lipogenic genes was comparable between SDT fatty rats and SD rats, but the DGAT2 and MTP mRNA levels in SDT fatty rats were lower than those in SD rats.
The TNF-α and MCP-1 inflammatory genes were increased by 16.1% and 38.9%, respectively, in SDT fatty rats at 16 wk of age (Figure 4F and G). The mRNA levels of fibrotic genes, namely collagen type 1 and α-SMA, tended to increase in SDT fatty rats compared with SD rats at 16 and 24 wk of age. The TGF-β mRNA levels in SDT fatty rats also tended to increase at 32 wk of age.
In a previous study, we investigated diabetes mellitus in female SDT fatty rats by comparing these rats with age-matched female SDT rats. Female SDT fatty rats showed metabolic abnormalities such as hyperglycemia, hyperinsulinemia, and dyslipidemia at a young age, which were similar to the abnormalities observed in male SDT fatty rats[14]. Furthermore, diabetic complications such as renal lesion and cataracts were observed at 16 wk of age in female SDT fatty rats, and were comparable to the complications observed in male SDT fatty rats[15]. Sexual differences in SDT fatty rats did not exist in the progression of diabetes. Thus, female SDT fatty rats are an important animal model of metabolic syndrome with diabetes
In Zucker diabetic fatty rats, hepatic steatosis is observed without dietary manipulation, but hepatic fibrosis is not observed[19]. Although ob/ob and db/db mice develop hepatic steatosis associated with obesity and insulin resistance, these mice do not spontaneously progress to significant steatohepatitis[8,9]. Ob/ob and db/db mice show significant steatohepatitis development when fed a MCD diet, but only db/db mice fed a MCD diet have increased fibrosis in the liver[10,11]. Db/db mice require other stimulation in addition to the background condition, such as obesity, diabetes, and dyslipidemia, to trigger progression to fibrosis[10].
In the present study, we investigated the pathophysiological changes of the liver in female SDT fatty rats compared with SD rats. We used SD rats as the control, instead of SDT rats, because we wanted to focus on the investigation of pathophysiological changes of the liver in SDT fatty rats. In our previous study, we confirmed that female SDT rats only exhibit a fatty liver based on histopathology[16] but not fibrosis at 40 wk of age. Furthermore, male SDT rats, which are a severe type 2 diabetic model, do not exhibit a fatty liver and liver fibrosis at 24 wk of age[20].
SDT fatty rats showed hyperglycemia associated with increased body weight at 6 wk of age, and they exhibited severe hyperglycemia after 6 wk of age, which is considered to be due to a decrease in insulin levels. Indeed, our previous study has shown that insulin levels in SDT fatty rats increase until 6 wk of age and gradually decease thereafter, but the levels are significantly higher than those in SD rats until 20 wk of age (8.89 ± 7.07 ng/mL vs 1.42 ± 0.49 ng/mL; P < 0.05). From 20 to 40 wk of age, the insulin levels in SDT fatty rats are slightly greater than those in SD rats[14]. As expected, the hepatic TG content in SDT fatty rats was higher than those of SD rats, and it peaked at 16 wk of age. After 16 wk of age, it gradually decreased to the levels observed in the SD rats. The mRNA expression levels of MCP-1 and TNF-α at 16 wk of age were increased, suggesting that SDT fatty rats had accelerated liver injury and inflammation. The mRNA expression of collagen type 1 and α-SMA tended to increase in SDT fatty rats. With regard to the pathophysiology, hepatic fibrosis was observed in SDT fatty rats at 32 wk of age. These findings indicated that female SDT fatty rats spontaneously develop nonalcoholic steatohepatitis-like hepatic lesions without dietary manipulation.
The hepatic steatosis in SDT fatty rats observed at 8 wk of age resulted from increased hepatic TG synthesis and decreased VLDL secretion, and histopathological findings, such as fatty changes in the hepatocytes, showed hepatic steatosis. Indeed, the hepatic FFA content, which is a component of TG, was also increased. The expression of genes related to lipogenesis, FAS, and ACC1 was increased, and the expression of MTP, which is a gene related to VLDL secretion, was decreased in SDT fatty rats at 8 wk of age. Furthermore, the FFA content in the liver was attributed to the degradation of serum lipoproteins and uptake of serum FFA. The hyperlipidemia in SDT fatty rats is considered to supply fatty acids to the liver.
In terms of the hepatic steatosis, SDT fatty rats are comparable to other rat strains at 8 wk of age[19,21]. In contrast, the decrease in the hepatic TG content after 24 wk of age may be based on the decreased de novo lipogenesis. The mRNA expression of SREBP-1c, which regulates FAS and ACC1 mRNA expression, was decreased in SDT fatty rats after 16 wk of age. Some complex mechanisms involved in the decrease in SREBP-1c mRNA expression of liver should be considered. It is known that insulin activates the SREBP-1c promoter in liver, thereby increasing fatty acid and triglyceride synthesis[22]. A previous study has shown that the serum insulin levels are decreased in SDT fatty rats after 6 wk of age[14,16]. The reduction of SREBP-1c levels could contribute to the decrease in serum insulin levels. The reduction of hepatic steatosis has also been shown in Otsuka Long-Evans Tokushima fatty rats after 30 wk of age[21]. The change in hepatic steatosis is considered to be associated with a decrease in SREBP-1c expression[23]. The change in hepatic steatosis in SDT fatty rats may be partly due to decreased SREBP-1c expression based on the decreased serum insulin levels. Further study is required to confirm this mechanism.
The spontaneous development of steatohepatitis and hepatic fibrosis in SDT fatty rats could be explained by the increase in hepatic FFA. The excess FFA could serve as precursors for the production of lipid peroxidation[24], which can activate hepatic stellate cells and thereby result in the development of fibrosis[25,26]. Indeed, an increase in α-SMA level, which is an indicator of activated hepatic stellate cells, was observed. In addition to lipid peroxidation, CYP2E1 may be involved in hepatic fibrosis development in SDT fatty rats. It has been reported that fatty acids increase both CYP2E1 mRNA and protein levels in the liver of high-fat diet fed animals[27]. CYP2E1 produces high levels of reactive oxygen species[28,29], which play an important role in the development of NASH and fibrosis[30].
The difference in the pathophysiology of the liver between SDT fatty rats and other diabetic animal models remains unknown. However, the hepatic vulnerability in SDT fatty rats could be explained by the sensitivity to inflammation. SDT rats, which are the strain of origin of SDT fatty rats, show hyperglycemia caused by age-dependent degenerative changes in pancreatic islets[31,32]. Treatment with telmisartan ameliorates hyperglycemia and hypoinsulinemia due to the reduction in oxidative stress caused by renin angiotensin system activation in SDT rats[33], suggesting that a large amount of NO is produced by macrophages in the islets[34]. It has been suggested that SDT rats are under inflammatory conditions physiologically. In SDT fatty rats, ED-1 staining, which is a marker for activated macrophages, was increased in the liver at 40 wk of age. These results suggested that SDT fatty rats have increased inflammation as a steady condition in the liver. This state may enhance the stress sensitivity caused by lipid peroxidation, which induces hepatic fibrosis. Further study is needed to clarify the hepatic characteristics of SDT fatty rats. In addition, it is necessary to investigate whether SDT fatty rats develop hepatocarcinoma after 40 wk of age.
Compared with human NASH, SDT fatty rats have steatohepatitis accompanied by metabolic syndrome, including hyperglycemia, hyperinsulinemia, and obesity, which are similar features to those of human NASH. Human NASH is strongly associated with obesity, type 2 diabetes, and dyslipidemia[35,36]. In contrast, there is a difference between SDT fatty rats and human NASH. Pathophysiological features of NASH with fibrosis are observed in only female SDT fatty rats and not in male SDT fatty rats. Meanwhile, the prevalence of human NASH is common in men rather than women, which might be explained by sex hormones[37]. Further studies are needed to examine the molecular basis of this sex difference in SDT fatty rats.
In conclusion, female SDT fatty rats showed steatohepatitis (e.g., increased plasma ALT and hepatic steatosis) associated with hyperglycemia and dyslipidemia at 8 wk of age. Thereafter, hepatic fibrosis began to be observed at 32 wk of age. These results demonstrated that female SDT fatty rats exhibit pathophysiological features of NASH in the absence of dietary manipulation.
The precise mechanisms for developing NASH in humans remain unknown. However, female SDT fatty rats have the potential to become an important animal model of NASH with type 2 diabetes and obesity, a condition for which few models currently exist.
We wish to thank Mr. Nobuhiro Inaba and Mr. Naruhisa Ryumon (JT Creative Service Co., Ltd.) for long-term animal care.
Obesity and type 2 diabetes are well established risk factors for many chronic disorders, such as non-alcoholic steatohepatitis (NASH). Approximately 5%-10% of NASH patients are diagnosed with cirrhosis and/or hepatocellular carcinoma within 10 to 20 years. Currently, no medicines are approved for the treatment of NASH. To understand the complicated features of the disease, animal models of non-alcoholic fatty liver disease (NAFLD) offer important information for therapeutic intervention. Currently, there are no animal models of NASH accompanied by essential features of clinical conditions, such as obesity and insulin resistance, without dietary manipulation.
Spontaneously diabetic Torii Leprfa (SDT fatty) rats show several metabolic syndrome features, including obesity, hyperglycemia, hyperlipidemia, and insulin resistance. However, the features of liver lesions in SDT fatty rats have not been reported in detail. In the present study, the pathophysiological changes in the liver of SDT fatty rats were examined over time.
Many NASH studies have used animal models, such as diet-induced NASH models or mutant gene models, and these NASH animal models spontaneously progress to significant steatohepatitis accompanied by metabolic syndrome. This is the first study to show the time course of pathophysiological changes in the liver of SDT fatty rats. Furthermore, the in vivo studies suggest that female SDT fatty rats exhibit pathophysiological features of NASH in the absence of dietary manipulation.
Female SDT fatty rats may be useful to identify the complicated molecular mechanisms of the disease. In addition, this model may be a suitable preclinical model to evaluate candidate drugs for NASH.
NAFLD is presently well recognized as the most common chronic liver disease in the Western world. NAFLD is a broad-spectrum liver disease that ranges from simple steatosis to NASH and cirrhosis. Approximately 20%-30% of NALFD patients progress to NASH. Because NASH leads to serious diseases, such as cirrhosis of the liver and hepatocarcinoma, the development of therapeutic agents is desirable. The pathogenesis of NASH remains poorly defined, and effective pharmacological therapies have not been approved.
This is a good study in which the authors examined the pathophysiological changes in the liver of SDT fatty rats over time. The results are interesting and suggest that female SDT fatty rats spontaneously exhibit pathophysiological features of NASH. Female SDT fatty rats have the potential to become a suitable preclinical model of NASH.
P- Reviewer: Qiu LX, Sertoglu E, Shimizu Y S- Editor: Ma YJ L- Editor: Cant MR E- Editor: Ma S
| 1. | de Alwis NM, Day CP. Non-alcoholic fatty liver disease: the mist gradually clears. J Hepatol. 2008;48 Suppl 1:S104-S112. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 397] [Cited by in F6Publishing: 399] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 2. | Greenfield V, Cheung O, Sanyal AJ. Recent advances in nonalcholic fatty liver disease. Curr Opin Gastroenterol. 2008;24:320-327. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 80] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 3. | Malik SM, Gupte PA, de Vera ME, Ahmad J. Liver transplantation in patients with nonalcoholic steatohepatitis-related hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2009;7:800-806. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 4. | Ertle J, Dechêne A, Sowa JP, Penndorf V, Herzer K, Kaiser G, Schlaak JF, Gerken G, Syn WK, Canbay A. Non-alcoholic fatty liver disease progresses to hepatocellular carcinoma in the absence of apparent cirrhosis. Int J Cancer. 2011;128:2436-2443. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 335] [Cited by in F6Publishing: 361] [Article Influence: 27.8] [Reference Citation Analysis (1)] |
| 5. | Hucke F, Sieghart W, Schöniger-Hekele M, Peck-Radosavljevic M, Müller C. Clinical characteristics of patients with hepatocellular carcinoma in Austria - is there a need for a structured screening program? Wien Klin Wochenschr. 2011;123:542-551. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 6. | Yang JD, Kim B, Sanderson SO, St Sauver JL, Yawn BP, Pedersen RA, Larson JJ, Therneau TM, Roberts LR, Kim WR. Hepatocellular carcinoma in olmsted county, Minnesota, 1976-2008. Mayo Clin Proc. 2012;87:9-16. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 7. | Diehl AM. Lessons from animal models of NASH. Hepatol Res. 2005;33:138-144. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 109] [Cited by in F6Publishing: 120] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 8. | Ge F, Zhou S, Hu C, Lobdell H, Berk PD. Insulin- and leptin-regulated fatty acid uptake plays a key causal role in hepatic steatosis in mice with intact leptin signaling but not in ob/ob or db/db mice. Am J Physiol Gastrointest Liver Physiol. 2010;299:G855-G866. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 9. | Xu J, Kulkarni SR, Donepudi AC, More VR, Slitt AL. Enhanced Nrf2 activity worsens insulin resistance, impairs lipid accumulation in adipose tissue, and increases hepatic steatosis in leptin-deficient mice. Diabetes. 2012;61:3208-3218. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 130] [Cited by in F6Publishing: 128] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 10. | Sahai A, Malladi P, Pan X, Paul R, Melin-Aldana H, Green RM, Whitington PF. Obese and diabetic db/db mice develop marked liver fibrosis in a model of nonalcoholic steatohepatitis: role of short-form leptin receptors and osteopontin. Am J Physiol Gastrointest Liver Physiol. 2004;287:G1035-G1043. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 209] [Cited by in F6Publishing: 218] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 11. | Jha P, Knopf A, Koefeler H, Mueller M, Lackner C, Hoefler G, Claudel T, Trauner M. Role of adipose tissue in methionine-choline-deficient model of non-alcoholic steatohepatitis (NASH). Biochim Biophys Acta. 2014;1842:959-970. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 12. | Masuyama T, Katsuda Y, Shinohara M. A novel model of obesity-related diabetes: introgression of the Lepr(fa) allele of the Zucker fatty rat into nonobese Spontaneously Diabetic Torii (SDT) rats. Exp Anim. 2005;54:13-20. [PubMed] [Cited in This Article: ] |
| 13. | Katsuda Y, Ohta T, Miyajima K, Kemmochi Y, Sasase T, Tong B, Shinohara M, Yamada T. Diabetic complications in obese type 2 diabetic rat models. Exp Anim. 2014;63:121-132. [PubMed] [Cited in This Article: ] |
| 14. | Ishii Y, Ohta T, Sasase T, Morinaga H, Ueda N, Hata T, Kakutani M, Miyajima K, Katsuda Y, Masuyama T. Pathophysiological analysis of female Spontaneously Diabetic Torii fatty rats. Exp Anim. 2010;59:73-84. [PubMed] [Cited in This Article: ] |
| 15. | Ohta T, Katsuda Y, Miyajima K, Sasase T, Kimura S, Tong B, Yamada T. Gender differences in metabolic disorders and related diseases in Spontaneously Diabetic Torii-Lepr(fa) rats. J Diabetes Res. 2014;2014:841957. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 16. | Hata T, Ohta T, Ishii Y, Sasase T, Yamaguchi T, Mera Y, Miyajima K, Tanoue G, Sato E, Matsushita M. Elevated glucagon-like peptide-1 on a high-fat diet feeding prevents the incidence of diabetes mellitus in Spontaneously Diabetic Torii Leprfa rats. J Diabetes Mellit. 2012;2:170-178. [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 17. | Xu ZJ, Fan JG, Ding XD, Qiao L, Wang GL. Characterization of high-fat, diet-induced, non-alcoholic steatohepatitis with fibrosis in rats. Dig Dis Sci. 2010;55:931-940. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 119] [Cited by in F6Publishing: 124] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 18. | Ito M, Suzuki J, Tsujioka S, Sasaki M, Gomori A, Shirakura T, Hirose H, Ito M, Ishihara A, Iwaasa H. Longitudinal analysis of murine steatohepatitis model induced by chronic exposure to high-fat diet. Hepatol Res. 2007;37:50-57. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 151] [Cited by in F6Publishing: 163] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 19. | Forcheron F, Abdallah P, Basset A, del Carmine P, Haffar G, Beylot M. Nonalcoholic hepatic steatosis in Zucker diabetic rats: spontaneous evolution and effects of metformin and fenofibrate. Obesity (Silver Spring). 2009;17:1381-1389. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 20. | Ishii Y, Ohta T, Sasase T, Morinaga H, Hata T, Miyajima K, Katusda Y, Masuyama T, Shinohara M, Kakutani M. A high-fat diet inhibits the progression of diabetes mellitus in type 2 diabetic rats. Nutr Res. 2010;30:483-491. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Song YS, Fang CH, So BI, Park JY, Lee Y, Shin JH, Jun DW, Kim H, Kim KS. Time course of the development of nonalcoholic Fatty liver disease in the Otsuka long-evans Tokushima Fatty rat. Gastroenterol Res Pract. 2013;2013:342648. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 22. | Musso G, Gambino R, Cassader M. Recent insights into hepatic lipid metabolism in non-alcoholic fatty liver disease (NAFLD). Prog Lipid Res. 2009;48:1-26. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 461] [Cited by in F6Publishing: 506] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 23. | Matsuzaka T, Shimano H, Yahagi N, Kato T, Atsumi A, Yamamoto T, Inoue N, Ishikawa M, Okada S, Ishigaki N. Crucial role of a long-chain fatty acid elongase, Elovl6, in obesity-induced insulin resistance. Nat Med. 2007;13:1193-1202. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 386] [Cited by in F6Publishing: 405] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 24. | Mantena SK, Vaughn DP, Andringa KK, Eccleston HB, King AL, Abrams GA, Doeller JE, Kraus DW, Darley-Usmar VM, Bailey SM. High fat diet induces dysregulation of hepatic oxygen gradients and mitochondrial function in vivo. Biochem J. 2009;417:183-193. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 200] [Cited by in F6Publishing: 213] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 25. | Zern MA, Leo MA, Giambrone MA, Lieber CS. Increased type I procollagen mRNA levels and in vitro protein synthesis in the baboon model of chronic alcoholic liver disease. Gastroenterology. 1985;89:1123-1131. [PubMed] [Cited in This Article: ] |
| 26. | Li SY, Liu Y, Sigmon VK, McCort A, Ren J. High-fat diet enhances visceral advanced glycation end products, nuclear O-Glc-Nac modification, p38 mitogen-activated protein kinase activation and apoptosis. Diabetes Obes Metab. 2005;7:448-454. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 70] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 27. | Abdelmegeed MA, Banerjee A, Yoo SH, Jang S, Gonzalez FJ, Song BJ. Critical role of cytochrome P450 2E1 (CYP2E1) in the development of high fat-induced non-alcoholic steatohepatitis. J Hepatol. 2012;57:860-866. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 167] [Cited by in F6Publishing: 191] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 28. | Caro AA, Cederbaum AI. Oxidative stress, toxicology, and pharmacology of CYP2E1. Annu Rev Pharmacol Toxicol. 2004;44:27-42. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 379] [Cited by in F6Publishing: 378] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 29. | Aubert J, Begriche K, Knockaert L, Robin MA, Fromenty B. Increased expression of cytochrome P450 2E1 in nonalcoholic fatty liver disease: mechanisms and pathophysiological role. Clin Res Hepatol Gastroenterol. 2011;35:630-637. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 197] [Cited by in F6Publishing: 203] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 30. | Rolo AP, Teodoro JS, Palmeira CM. Role of oxidative stress in the pathogenesis of nonalcoholic steatohepatitis. Free Radic Biol Med. 2012;52:59-69. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 636] [Cited by in F6Publishing: 677] [Article Influence: 56.4] [Reference Citation Analysis (0)] |
| 31. | Shinohara M, Masuyama T, Shoda T, Takahashi T, Katsuda Y, Komeda K, Kuroki M, Kakehashi A, Kanazawa Y. A new spontaneously diabetic non-obese Torii rat strain with severe ocular complications. Int J Exp Diabetes Res. 2000;1:89-100. [PubMed] [Cited in This Article: ] |
| 32. | Masuyama T, Komeda K, Hara A, Noda M, Shinohara M, Oikawa T, Kanazawa Y, Taniguchi K. Chronological characterization of diabetes development in male Spontaneously Diabetic Torii rats. Biochem Biophys Res Commun. 2004;314:870-877. [PubMed] [Cited in This Article: ] |
| 33. | Hasegawa G, Fukui M, Hosoda H, Asano M, Harusato I, Tanaka M, Shiraishi E, Senmaru T, Sakabe K, Yamasaki M. Telmisartan, an angiotensin II type 1 receptor blocker, prevents the development of diabetes in male Spontaneously Diabetic Torii rats. Eur J Pharmacol. 2009;605:164-169. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 34. | Inokuchi C, Ueda H, Hamaguchi T, Miyagawa J, Shinohara M, Okamura H, Namba M. Role of macrophages in the development of pancreatic islet injury in spontaneously diabetic torii rats. Exp Anim. 2009;58:383-394. [PubMed] [Cited in This Article: ] |
| 35. | Kotronen A, Yki-Järvinen H. Fatty liver: a novel component of the metabolic syndrome. Arterioscler Thromb Vasc Biol. 2008;28:27-38. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 572] [Cited by in F6Publishing: 591] [Article Influence: 36.9] [Reference Citation Analysis (0)] |
| 36. | Cusi K. Nonalcoholic fatty liver disease in type 2 diabetes mellitus. Curr Opin Endocrinol Diabetes Obes. 2009;16:141-149. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 123] [Cited by in F6Publishing: 118] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 37. | Arun J, Clements RH, Lazenby AJ, Leeth RR, Abrams GA. The prevalence of nonalcoholic steatohepatitis is greater in morbidly obese men compared to women. Obes Surg. 2006;16:1351-1358. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 44] [Article Influence: 2.4] [Reference Citation Analysis (0)] |