Published online Jan 21, 2015. doi: 10.3748/wjg.v21.i3.919
Peer-review started: June 6, 2014
First decision: July 27, 2014
Revised: July 22, 2014
Accepted: September 18, 2014
Article in press: September 19, 2014
Published online: January 21, 2015
AIM: To identify the characteristics of gastric tube cancer (GTC) and the complications associated with endoscopic submucosal dissection (ESD) for GTC.
METHODS: Between 2007 and 2012, 11 individuals with early gastric cancer in the reconstructed gastric tube after esophagectomy who underwent ESD in this hospital were studied. The characteristics of GTC were identified, and the complications of ESD for GTC were analyzed at three phases: preoperative, intraoperative, and postoperative.
RESULTS: A total of 11 consecutive patients with 11 GTCs were selected for this study. All cases underwent en bloc resections by ESD. The median procedure time was 142 min. The average GTC diameter was 26.1 mm, and the average size of the resected lesions was 45.5 mm. The histopathological diagnosis in all cases was a differentiated adenocarcinoma. In the preoperative phase, anastomotic strictures (5/11, 45%) and food residues (4/11, 36.4%) in the gastric tube were the main complications. In the intraoperative phase, bleeding was observed in 5 cases (45%). The postoperative complications observed were delayed bleeding in 2 cases (18.2%) and stenosis in one case (9.1%). The case with stenosis was successfully treated using endoscopic balloon dilatation.
CONCLUSION: Minor complications were frequently observed. However, all GTCs underwent en bloc resection with ESD without any serious complications. ESD is considered a useful treatment for GTC.
Core tip: The pulled-up stomach conduit, when reconstructed and used as an esophageal substitute after esophagectomy, has the potential to develop gastric tube cancer (GTC). Although endoscopic submucosal dissection (ESD) for early gastric cancer is common, there are only a few reports on ESD for GTC. In this study, we identified the characteristics of GTC and the complications associated with ESD for GTC. Minor complications, such as anastomotic stricture, food residue, and intraoperative bleeding, were frequently observed. However, all GTCs were safely resected en bloc with ESD without serious complications. ESD can be considered a useful treatment modality for GTC.
- Citation: Mukasa M, Takedatsu H, Matsuo K, Sumie H, Yoshida H, Hinosaka A, Watanabe Y, Tsuruta O, Torimura T. Clinical characteristics and management of gastric tube cancer with endoscopic submucosal dissection. World J Gastroenterol 2015; 21(3): 919-925
- URL: https://www.wjgnet.com/1007-9327/full/v21/i3/919.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i3.919
Esophageal cancer is associated with metachronous malignancies in other organs, and its incidence rate ranges from 11.3% to 12.0%[1-4]. Gastric cancer and head and neck cancer are the most commonly identified malignancies in this regard[1,2]. The pulled-up stomach conduit, which can be used as an esophageal substitute after esophagectomy, has the potential to develop a second primary cancer known as gastric tube cancer (GTC). GTC has been increasingly observed in recent years as advancements in treatments for esophageal cancer have contributed to prolonged patient survival[5,6].
Surgical resection has been considered the standard treatment for GTC; however, surgery is not usually preferred because of its associated high morbidity and mortality[5,7]. As a result, it is necessary to find alternatives to the surgical treatment of GTC. At present, the use of endoscopic resection, including endoscopic submucosal dissection (ESD) for early gastric cancer (EGC), is accepted as a standard of care and is commonly performed[8-10]. ESD is a technique that was developed to enable the resection of large lesions that cannot be removed using conventional endoscopic mucosal resection (EMR). The other major advantage of ESD is its ability to achieve a higher rate of en bloc resection, which allows it to provide more accurate histological assessment than EMR. The indications for endoscopic resection and the histopathological criteria for the curative endoscopic resection of EGC were provided by the Japanese Gastric Cancer Association gastric cancer treatment guidelines in 2010[11].
Recently, the indications and criteria for ESD were expanded to include the treatment of GTC after esophagectomy[12-15]. ESD for GTC after esophagectomy is a technically difficult procedure because of the limited working space and unusual fluid-pooling area in the reconstructed gastric tube, in addition to the presence of severe gastric fibrosis with staples under the suture line. At present, there are very few data on the clinical significance of endoscopic treatment for GTC[5], and a therapeutic strategy for GTC has not yet been established.
During the period of this study, 11 cases of early GTC after esophagectomy were diagnosed at the hospital after detailed surveillance endoscopy. All GTCs were successfully resected en bloc with ESD. The aim of this retrospective study was to identify the clinical characteristics of GTC and the complications associated with ESD for GTC.
Between April 2007 and September 2012, all patients with EGC in the reconstructed gastric tube after esophagectomy who underwent ESD were retrospectively investigated at the Kurume University Hospital in Japan. The clinical characteristics of the patients with EGC are summarized in Table 1. The clinical indications of ESD for EGC were based on the Gastric Cancer Treatment Guidelines[11]. These indications were applied for GTC after esophagectomy with gastric tube reconstruction (GTR). The hospital ethics committee approved the study protocol, and all participating patients had provided prior written informed consent.
| Characteristics | |
| Patient | 11 |
| Gender (M/F) | 10/1 |
| Tumors | 11 |
| Mean age | 70.5 (65-78) |
| Median period from esophagectomy to initial ESD for GTC, in months (range) | 52.4 (4-144) |
| Gastric tube reconstruction | |
| Anterosternal subcutaneous route | 7 (63.6) |
| Posterior mediastinal route | 3 (27.3) |
| Substernal route | 1 (9.1) |
| Size (Major axis) | 26.1 mm (10-68 mm) |
| Macroscopic type | |
| 0-IIa | 4 (36.4) |
| 0-IIc | 5 (45.5) |
| 0-IIa + IIc | 1 (9.1) |
| Unclassified | 1 (9.1) |
| Histological diagnosis | |
| Differentiated | 11 (100) |
| Undifferentiated | 0 (0) |
| Depth | |
| Mucosal | 9 (81.8) |
| Submucosal | 2 (18.2) |
| Helicobacter pylori infection | |
| Positive | 9 (81.8) |
| Negative | 2 (18.2) |
All ESD procedures were performed by highly skilled endoscopists using video endoscopes and a standard video endoscope system (CV260SL/CLV260SL endoscopic system; Olympus Medical Systems, Tokyo, Japan). An insulation-tipped diathermic knife (IT knife; Olympus) was used for all ESD procedures[16]. A Dual knife (Olympus) or Flush knife (Fujifilm, Tokyo, Japan) was used to mark the dots for the initial incision, and the IT knife was used for the submucosal dissection. In addition, 0.4% sodium hyaluronate (MucoUp; Johnson & Johnson, Tokyo, Japan) diluted with normal saline solution containing epinephrine and a minute amount of indigo carmine dye (1:1) was used as the submucosal injection solution[17]. The resected GTC specimens were then extended on the boards with pins for fixation in 20% formalin. Each lesion, together with the surrounding mucosa, was cut into 2- to 5-mm-wide serial-step sections. The histopathological criteria for diagnosing GTC were based on the Japanese classifications of gastric carcinomas[11,18].
The parameters and outcome measures studied were patient characteristics, Helicobacter pylori infection, endoscopic findings, tumor size, histopathological results, operative time (from marking to complete lesion removal), and complications, such as stenosis, perforation, and bleeding during the three phases: preoperative; intraoperative; and postoperative. In this study, delayed bleeding was defined as cases with hematemesis or melena requiring endoscopic intervention in the postoperative phase.
Eleven consecutive patients with 11 lesions were included (10 men and 1 woman), having a mean age of 70.5 years. The characteristics of these patients are shown in Tables 1 and 2. All of the patients had earlier undergone esophagectomy with gastric tube reconstruction (GTR) for esophageal cancer of the squamous cell carcinoma type. Seven cases had GTR through the anterosternal subcutaneous route; 3 had GTR through the posterior mediastinal route; and 1 had a substernal GTR. GTC occurred an average length of 52.4 mo (4-144 mo) after esophagectomy. All cases underwent en bloc resections with ESD. The median procedure time was 142 min (range, 32-445 min). The average GTC diameter was 26.1 mm (10-68 mm), and the average size of the resected lesion was 45.5 mm in diameter (21-74 mm). During endoscopy, the cases were macroscopically classified as follows: 4 cases were 0-IIa type; 5 were 0-IIc type; 1 case was 0-IIa + IIc type; and 1 was unclassified. The histopathological diagnosis in all cases was differentiated adenocarcinoma. Based on the depth of invasion, 9 cases were observed to be limited to the mucosa (M), and in 2 cases, the tumor had penetrated into the submucosa (SM). HP infection was observed in most of the GTC patients (9/11, 81.9%).
| Case | Sex | Age | Gastric tube reconstruction | Wall | Location | Macroscopic finding | Period from esophagectomy (mo) | H. pylori infection | Tumor size (mm) | Resected size (mm) | Depth | Resection time (min) |
| 1 | M | 66 | Post | Left | Body | 0-IIa + IIc | 117 | Positive | 28 | 62 | SM | 184 |
| 2 | M | 77 | Ant | Left | Body | 0-IIa | 96 | Negative | 16 | 40 | M | 175 |
| 3 | M | 67 | Sub | Posterior | Body | 0-IIa | 50 | Positive | 10 | 21 | M | 180 |
| 4 | M | 62 | Ant | Right | Body | 0-IIa | 4 | Positive | 31 | 48 | M | 57 |
| 5 | M | 78 | Ant | Anterior | Antrum | 0-IIc | 38 | Positive | 15 | 28 | M | 65 |
| 6 | M | 66 | Post | Posterior | Antrum | unclassified | 144 | Negative | 68 | 74 | SM | 445 |
| 7 | M | 74 | Ant | Left | Body | 0-IIc | 12 | Positive | 32 | 45 | M | 208 |
| 8 | F | 78 | Ant | Anterior | Antrum | 0-IIa | 44 | Positive | 20 | 49 | M | 32 |
| 9 | M | 78 | Ant | Left | Body | 0-IIc | 6 | Positive | 12 | 31 | M | 32 |
| 10 | M | 65 | Ant | Posterior | Antrum | 0-IIc | 45 | Positive | 30 | 52 | M | 44 |
| 11 | M | 64 | Post | Left | Body | 0-IIc | 21 | Positive | 25 | 53 | M | 141 |
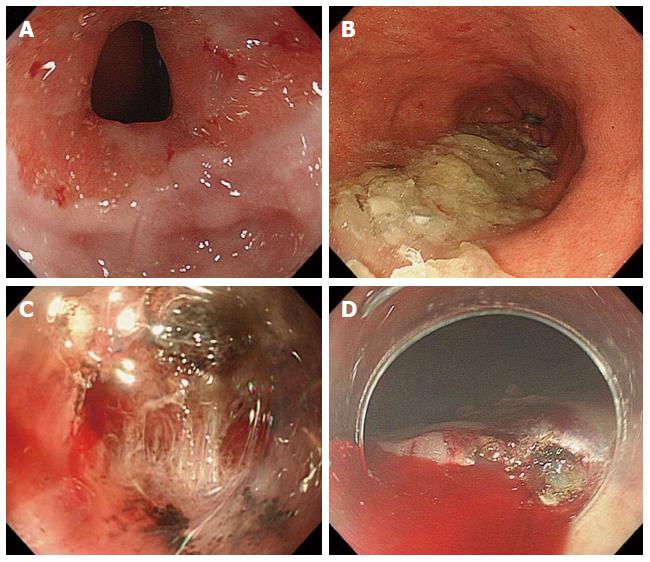
The complications of ESD for GTC were investigated at three phases: preoperative; intraoperative; and postoperative. In the preoperative phase, anastomotic strictures (Figure 1A) and food residue (Figure 1B) in the gastric tube were the main complications (Table 3). Anastomotic strictures were defined as an anastomotic narrowing that did not allow a standard endoscope with a diameter of 10 mm to pass without resistance. In this study, there were 5 cases (45%) of anastomotic stenosis in the preoperative phase, and balloon dilatation was required in all cases. Furthermore, overtube insertion was impossible in all 6 cases (including 5 preoperative cases and one postoperative) with anastomotic stenosis, although balloon dilatation for stenosis was performed. Migration of the overtube was frequently observed through the anterosternal subcutaneous route (4/7, 57.1%) compared with the other routes (2/4, 50.0%); however, this observation had no association with the insertion technique. The insertion of a scope-mounted hood was possible except in one case. In addition, food residue was observed in 4 cases (36.4%), although it is imperative to abstain from eating before treatment.
| Case | Anastomotic stenosis | Endoscopic dilation | Mounted attachment | Attaching overtube | Food residue |
| 1 | – | – | Possible | Possible | – |
| 2 | – | – | Possible | Possible | – |
| 3 | + | + | Possible | Impossible | + |
| 4 | + | + | Possible | Possible | – |
| 5 | + | + | Impossible | Impossible | – |
| 6 | – | – | Possible | Impossible | – |
| 7 | + | + | Possible | Impossible | – |
| 8 | – | – | Possible | Possible | + |
| 9 | – | – | Possible | Impossible | + |
| 10 | + | + | Possible | Impossible | + |
| 11 | – | – | Possible | Possible | – |
The intraoperative complications included were bleeding and perforation due to fibrosis of the submucosal layer (Figure 1C), an unusual fluid-pooling area, and limited working space (Table 4). Fibrosis of the submucosal layer at the site of the original stomach was frequently observed in the gastric tube and predominantly originated from the gastric body (5/7, 71.4%) more so than the antrum (1/4, 25%). As a result, intraoperative bleeding (Figure 1D) was more frequently observed in gastric tubes originating from the body of the stomach (body vs antrum, 5/7 vs 0/4). In addition, the resection time was longer in the gastric tube originating from the gastric body except for one case of the antrum, which developed SM infiltration (body vs antrum, 139.6 min vs 47 min). In this study, perforation was not observed as a complication (0%).
| Case | Submergence | Fibrosis of submucosa | Bleeding | Delayed bleeding | Stenosis |
| 1 | + | Moderate | Severe | + | – |
| 2 | + | Moderate | Severe | – | – |
| 3 | – | Moderate | Severe | + | – |
| 4 | – | Slight | None | – | – |
| 5 | – | Slight | None | – | – |
| 6 | – | Severe | None | – | + |
| 7 | + | Moderate | Severe | – | – |
| 8 | – | Slight | None | – | – |
| 9 | – | Slight | None | – | – |
| 10 | + | Slight | None | – | – |
| 11 | + | Severe | Severe | – | – |
The postoperative complications were postoperative bleeding and stenosis (Table 4). Emergency hemostasis was performed for postoperative bleeding in 2 cases (18.2%) of gastric tube cancers originating from the body of the stomach. Stenosis after ESD was observed in only one case of an extensive resection of a gastric tube cancer originating from the antrum (9.1%). This case was successfully treated with endoscopic balloon dilatation.
GTC is an important issue in the long-term follow-up of patients who undergo curative esophagectomy for esophageal cancer. Because recent advances in the diagnosis and treatment of esophageal cancer have improved patient survival after esophagectomy, the occurrence of GTC has been increasing[5,6]. The frequency of GTC in patients after esophagectomy is reported to be approximately 0.5%-6.3%[12,19,20]. Several reports have shown the efficacy and safety of EMR for GTC[3,21]. Compared with surgery, endoscopic treatment, including EMR, is considered a less invasive treatment for GTC. Several investigators have reported the application of ESD for GTC, although the indications of ESD for GTC have yet to be established[12,13]. ESD has advantages over EMR in terms of the size and depth of the resected specimens and has been successfully applied for the en bloc resection of various cancers[22,23]. Because the en bloc resection rate of ESD for GTC is reported to be 88%-95%, which is much higher than that of EMR (14.3%)[12,13,15], ESD provides a more accurate histological assessment than EMR. According to the treatment guidelines for gastric cancer in Japan[11], the indications for ESD in EGC tend to be mucosal cancer within 2 cm in size without ulcers. Furthermore, these guidelines have extended the indications for ESD in the following cases: (1) differentiated type, mucosal (M) cancer without ulcer, and larger than 2 cm; (2) differentiated type, M cancer with ulcer, and 3 cm or smaller; and (3) undifferentiated type, M cancer without ulcer, and 2 cm or smaller. The guidelines also state that an additional lymph node resection is not necessary when lymphovascular invasion is absent and when the tumor is not deeper than submucosal 1 (SM1; Approximately 500 μm). In the present study, we performed ESD for GTC following an extended indication for EGC, and the en bloc resection of all GTCs was successfully achieved using ESD.
Previous studies have shown a lower prevalence of HP infection in GTC patients[3,6]. Bile reflux was frequently observed in patients after esophagectomy and most likely contributed to intestinal metaplasia and the development of GTC[13,24]. On the other hand, Nonaka et al[20] observed that 98% of GTC patients had an HP infection, which is considered to be a major cause of gastric cancer. This result raises the possibility that HP infection is a major cause of GTC after esophagectomy, in addition to EGC occurring in the unresected stomach[25]. As for the results of this study, 81.8% of GTC patients were HP positive. Further studies, such as the prospective assessment of mucosal status and HP infection after surgery, will be required to clarify the activity of these carcinogenetic factors in the gastric tube.
Only a few reports have investigated the minor complications of ESD, such as anastomotic stricture, food residue, bleeding, and stenosis in the GTC. The precise incidence of complications has not been clearly elucidated in previous reports because these reports have involved a small number of patients or a mixed sample with surgical resections and ESD for EGC in the remnant stomach after gastrectomy[5,12,15]. Although anastomotic stenosis was observed at a high frequency (45.5%, 5/11) and the endoscopic extension was performed before the ESD, overtube insertion was impossible in most of those cases. Food residues were observed in 36.4% (4/11) of the cases and had to be fully removed before ESD. However, these complications were not associated with an extended resection time. Bleeding was observed in 45.5% of the patients during ESD. Intraoperative bleeding was more frequently observed in the gastric tube originating from the body of the stomach (71.4%, 5/7) than in those originating from the antrum (25%, 1/4). Emergency hemostasis was performed for postoperative bleeding in the gastric tube originating from the body in 2 cases (18.2%). Our results suggest that ESD for GTC originating from the body can be considered a high risk for intra- and postoperative bleeding. Stenosis after ESD was observed in only one case (9.1%) in the largest tumor size (68 mm) found in our study. Ono et al[26] have reported that resecting areas larger than three-quarters of the circumference of the esophageal lumen can be considered an important risk factor for postoperative stenosis in ESD of superficial esophageal cancer. The risk factors for postoperative stenosis may be associated with the tumor size of the GTC. A greater accumulation of cases will be required to assess the complications of ESD for GTC in detail.
Regarding major complications, Nonaka et al[20] showed that perforations and delayed bleeding occurred in 3.8% of patients, similar to the complication rates of ESD for EGC performed on unresected stomachs[16,27]. The influence of fibrosis and deformity due to the previous surgery would be one such explanation of the decrease in the en bloc resection rate. Moreover, the operative time was longer for ESD in the gastric tube than in the whole stomach. Anatomical deformities and a limited working space due to the anastomosis or stump line are possible reasons for the time-consuming nature of these procedures. Previous reports of perforation during ESD of a whole stomach specimen have found rates of 0% to 12%[28]. A high rate of perforation (18%) was reported during ESD in a gastric tube[15]. Once perforation has occurred in the gastric tube, pneumoperitoneum and pneumomediastinum are likely to occur, potentially leading to peritonitis or mediastinitis. In the current study, all patients who experienced perforations were successfully treated with immediate closure using endoclips and subsequent nasogastric suction.
In conclusion, minor complications, such as anastomotic stricture, food residue, and intraoperative bleeding, are frequently observed in ESD for GTC; however, all GTCs were safely resected en bloc using ESD without serious complications. ESD can be considered a useful treatment modality for GTC.
The pulled-up stomach conduit, which can be reconstructed and used as an esophageal substitute after esophagectomy, has the potential to develop gastric tube cancer (GTC). At present, the use of endoscopic resection including endoscopic submucosal dissection (ESD) for early gastric cancer (EGC) is accepted as a standard of care and is commonly performed. However, there are only a few reports of ESD for GTC. The aim of this study was to identify the characteristics of GTC and the complications associated with ESD for GTC.
Esophageal cancer is associated with metachronous malignancies in other organs, and the reported incidence rate ranges from 11.3% to 12.0%. The pulled-up stomach conduit that is used as an esophageal substitute after esophagectomy has the potential to develop a second primary cancer known as GTC. GTC has been increasingly observed in recent years as advancements in the treatment of esophageal cancer have contributed to prolonged patient survival. This research hotspot calls for the investigation of approaches for managing and treating GTC after esophagectomy.
Recently, the indications and criteria for ESD have been expanded to include the treatment of GTC after esophagectomy. ESD for GTC after esophagectomy is a technically difficult procedure because of the limited working space and unusual fluid-pooling area in the reconstructed gastric tube, as well as the presence of severe gastric fibrosis with staples under the suture line. At present, there are very few data on the clinical significance of endoscopic treatment for GTC, and no therapeutic strategy for GTC has yet been established. In the present study, the authors showed the usefulness of ESD for GTC following the extended indication of ESD for EGC.
GTCs underwent en bloc resection by ESD without any serious complications, although minor complications were frequently observed. The study’s results suggest that ESD can be considered a useful treatment for GTC.
GTC is a gastric cancer in the reconstructed gastric tube after esophagectomy. ESD is an advanced technique of therapeutic endoscopy for superficial gastrointestinal neoplasms. Three steps characterize this procedure, as follows: injecting fluid into the submucosa to elevate the lesion; cutting the surrounding mucosa of the lesion; and dissecting the submucosa beneath the lesion.
Mukasa et al describe the characteristics of GTC and the complications associated with ESD for GTC. Eleven consecutive patients with 11 GTC were selected for this study. All cases underwent en bloc resections with ESD. In the preoperative phase, anastomotic strictures and food residues in the gastric tube were the main complications. In the intraoperative phase, bleeding was the major complication, whereas in the postoperative phase, delayed bleeding and stenosis were observed. The authors concluded that ESD can be considered a useful treatment for GTC. Although surgical resection has been considered a standard treatment for GTC, more recently, the indications and criteria for ESD have been expanded to include the treatment of GTC after esophagectomy as well.
P- Reviewer: Herszenyi L S- Editor: Qi Y L- Editor: A E- Editor: Wang CH
| 1. | Kokawa A, Yamaguchi H, Tachimori Y, Kato H, Watanabe H, Nakanishi Y. Other primary cancers occurring after treatment of superficial oesophageal cancer. Br J Surg. 2001;88:439-443. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 2. | Nagasawa S, Onda M, Sasajima K, Takubo K, Miyashita M. Multiple primary malignant neoplasms in patients with esophageal cancer. Dis Esophagus. 2000;13:226-230. [PubMed] [Cited in This Article: ] |
| 3. | Okamoto N, Ozawa S, Kitagawa Y, Shimizu Y, Kitajima M. Metachronous gastric carcinoma from a gastric tube after radical surgery for esophageal carcinoma. Ann Thorac Surg. 2004;77:1189-1192. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 4. | Poon RT, Law SY, Chu KM, Branicki FJ, Wong J. Multiple primary cancers in esophageal squamous cell carcinoma: incidence and implications. Ann Thorac Surg. 1998;65:1529-1534. [PubMed] [Cited in This Article: ] |
| 5. | Sugiura T, Kato H, Tachimori Y, Igaki H, Yamaguchi H, Nakanishi Y. Second primary carcinoma in the gastric tube constructed as an esophageal substitute after esophagectomy. J Am Coll Surg. 2002;194:578-583. [PubMed] [Cited in This Article: ] |
| 6. | Kise Y, Kijima H, Shimada H, Tanaka H, Kenmochi T, Chino O, Tajima T, Makuuchi H. Gastric tube cancer after esophagectomy for esophageal squamous cell cancer and its relevance to Helicobacter pylori. Hepatogastroenterology. 2003;50:408-411. [PubMed] [Cited in This Article: ] |
| 7. | Ahn HS, Kim JW, Yoo MW, Park do J, Lee HJ, Lee KU, Yang HK. Clinicopathological features and surgical outcomes of patients with remnant gastric cancer after a distal gastrectomy. Ann Surg Oncol. 2008;15:1632-1639. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 74] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 8. | Gotoda T. Endoscopic resection of early gastric cancer. Gastric Cancer. 2007;10:1-11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 479] [Cited by in F6Publishing: 476] [Article Influence: 28.0] [Reference Citation Analysis (1)] |
| 9. | Soetikno R, Kaltenbach T, Yeh R, Gotoda T. Endoscopic mucosal resection for early cancers of the upper gastrointestinal tract. J Clin Oncol. 2005;23:4490-4498. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 408] [Cited by in F6Publishing: 443] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 10. | Rembacken BJ, Gotoda T, Fujii T, Axon AT. Endoscopic mucosal resection. Endoscopy. 2001;33:709-718. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 173] [Cited by in F6Publishing: 174] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 11. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer. 2011;14:113-123. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1723] [Cited by in F6Publishing: 1844] [Article Influence: 141.8] [Reference Citation Analysis (0)] |
| 12. | Osumi W, Fujita Y, Hiramatsu M, Kawai M, Sumiyoshi K, Umegaki E, Tokioka S, Yoda Y, Egashira Y, Abe S. Endoscopic submucosal dissection allows less-invasive curative resection for gastric tube cancer after esophagectomy - a case series. Endoscopy. 2009;41:777-780. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 13. | Bamba T, Kosugi S, Takeuchi M, Kobayashi M, Kanda T, Matsuki A, Hatakeyama K. Surveillance and treatment for second primary cancer in the gastric tube after radical esophagectomy. Surg Endosc. 2010;24:1310-1317. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 14. | Hoteya S, Yamashita S, Kikuchi D, Nakamura M, Fujimoto A, Matsui A, Nishida N, Mitani T, Kuroki Y, Iizuka T. Endoscopic submucosal dissection for submucosal invasive gastric cancer and curability criteria. Dig Endosc. 2011;23:30-36. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 15. | Nishide N, Ono H, Kakushima N, Takizawa K, Tanaka M, Matsubayashi H, Yamaguchi Y. Clinical outcomes of endoscopic submucosal dissection for early gastric cancer in remnant stomach or gastric tube. Endoscopy. 2012;44:577-583. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 16. | Ono H, Hasuike N, Inui T, Takizawa K, Ikehara H, Yamaguchi Y, Otake Y, Matsubayashi H. Usefulness of a novel electrosurgical knife, the insulation-tipped diathermic knife-2, for endoscopic submucosal dissection of early gastric cancer. Gastric Cancer. 2008;11:47-52. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 116] [Cited by in F6Publishing: 115] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 17. | Fujishiro M, Yahagi N, Kashimura K, Mizushima Y, Oka M, Matsuura T, Enomoto S, Kakushima N, Imagawa A, Kobayashi K. Different mixtures of sodium hyaluronate and their ability to create submucosal fluid cushions for endoscopic mucosal resection. Endoscopy. 2004;36:584-589. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 107] [Article Influence: 5.4] [Reference Citation Analysis (1)] |
| 18. | Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14:101-112. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2390] [Cited by in F6Publishing: 2656] [Article Influence: 204.3] [Reference Citation Analysis (0)] |
| 19. | Motoyama S, Saito R, Kitamura M, Suzuki H, Nakamura M, Okuyama M, Imano H, Inoue Y, Ogawa J. Prospective endoscopic follow-up results of reconstructed gastric tube. Hepatogastroenterology. 2003;50:666-669. [PubMed] [Cited in This Article: ] |
| 20. | Nonaka S, Oda I, Sato C, Abe S, Suzuki H, Yoshinaga S, Hokamura N, Igaki H, Tachimori Y, Taniguchi H. Endoscopic submucosal dissection for gastric tube cancer after esophagectomy. Gastrointest Endosc. 2014;79:260-270. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 21. | Suzuki H, Kitamura M, Saito R, Motoyama S, Ogawa J. Cancer of the gastric tube reconstructed through the posterior mediastinal route after radical surgery for esophageal cancer. Jpn J Thorac Cardiovasc Surg. 2001;49:466-469. [PubMed] [Cited in This Article: ] |
| 22. | Fujishiro M, Yahagi N, Nakamura M, Kakushima N, Kodashima S, Ono S, Kobayashi K, Hashimoto T, Yamamichi N, Tateishi A. Successful outcomes of a novel endoscopic treatment for GI tumors: endoscopic submucosal dissection with a mixture of high-molecular-weight hyaluronic acid, glycerin, and sugar. Gastrointest Endosc. 2006;63:243-249. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 184] [Cited by in F6Publishing: 204] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 23. | Onozato Y, Ishihara H, Iizuka H, Sohara N, Kakizaki S, Okamura S, Mori M. Endoscopic submucosal dissection for early gastric cancers and large flat adenomas. Endoscopy. 2006;38:980-986. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 117] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 24. | Dixon MF, Mapstone NP, Neville PM, Moayyedi P, Axon AT. Bile reflux gastritis and intestinal metaplasia at the cardia. Gut. 2002;51:351-355. [PubMed] [Cited in This Article: ] |
| 25. | Asaka M, Kimura T, Kato M, Kudo M, Miki K, Ogoshi K, Kato T, Tatsuta M, Graham DY. Possible role of Helicobacter pylori infection in early gastric cancer development. Cancer. 1994;73:2691-2694. [PubMed] [Cited in This Article: ] |
| 26. | Ono S, Fujishiro M, Niimi K, Goto O, Kodashima S, Yamamichi N, Omata M. Predictors of postoperative stricture after esophageal endoscopic submucosal dissection for superficial squamous cell neoplasms. Endoscopy. 2009;41:661-665. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 233] [Cited by in F6Publishing: 254] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 27. | Isomoto H, Shikuwa S, Yamaguchi N, Fukuda E, Ikeda K, Nishiyama H, Ohnita K, Mizuta Y, Shiozawa J, Kohno S. Endoscopic submucosal dissection for early gastric cancer: a large-scale feasibility study. Gut. 2009;58:331-336. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 470] [Cited by in F6Publishing: 496] [Article Influence: 33.1] [Reference Citation Analysis (1)] |
| 28. | Kakushima N, Fujishiro M. Endoscopic submucosal dissection for gastrointestinal neoplasms. World J Gastroenterol. 2008;14:2962-2967. [PubMed] [Cited in This Article: ] |