Published online Jun 21, 2015. doi: 10.3748/wjg.v21.i23.7242
Peer-review started: December 16, 2014
First decision: January 8, 2015
Revised: January 25, 2015
Accepted: February 13, 2015
Article in press: February 13, 2015
Published online: June 21, 2015
AIM: To investigate visceral fat accumulation in association with the risk of small bowel angioectasia.
METHODS: We retrospectively investigated 198 consecutive patients who underwent both capsule endoscopy and CT for investigation of obscure gastrointestinal bleeding (OGIB) from January 2009 to September 2013. The visceral fat area (VFA) and subcutaneous fat area were measured by CT, and information on comorbidities, body mass index, and medications was obtained from their medical records. Logistic regression analysis was used to evaluate associations.
RESULTS: Capsule endoscopy revealed small bowel angioectasia in 18/198 (9.1%) patients with OGIB. Compared to patients without small bowel angioectasia, those with small bowel angioectasia had a significantly higher VFA (96 ± 76.0 cm2vs 63.4 ± 51.5 cm2, P = 0.016) and a higher prevalence of liver cirrhosis (61% vs 22%, P < 0.001). The proportion of patients with chronic renal failure was higher in patients with small bowel angioectasia (22% vs 9%, P = 0.11). There were no significant differences in subcutaneous fat area or waist circumference. The prevalence of small bowel angioectasia progressively increased according to the VFA. Multivariate analysis showed that the VFA [odd ratio (OR) for each 10-cm2 increment = 1.1; [95% confidence interval (CI): 1.02-1.19; P = 0.021] and liver cirrhosis (OR = 6.1, 95%CI: 2.2-18.5; P < 0.001) were significant risk factors for small bowel angioectasia.
CONCLUSION: VFA is positively associated with the prevalence of small bowel angioectasia, for which VFA and liver cirrhosis are independent risk factors in patients with OGIB.
Core tip: Small bowel angioectasia is a major source of obscure gastrointestinal bleeding. The etiology and mechanism of the development of angioectasia are not fully understood. In this study, we elucidated the association of visceral fat accumulation with the risk of small bowel angioectasia. A positive association was observed between visceral fat accumulation and the prevalence of small bowel angioectasia. Visceral fat accumulation and liver cirrhosis are independent risk factors for small bowel angioectasia in patients with obscure gastrointestinal bleeding.
- Citation: Yamada A, Niikura R, Kobayashi Y, Suzuki H, Yoshida S, Watabe H, Yamaji Y, Hirata Y, Koike K. Risk factors for small bowel angioectasia: The impact of visceral fat accumulation. World J Gastroenterol 2015; 21(23): 7242-7247
- URL: https://www.wjgnet.com/1007-9327/full/v21/i23/7242.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i23.7242
Angioectasia is characterized by focal accumulation of dilated vessels in the mucosa and submucosa of the intestinal wall[1]. This condition can occur anywhere in the gastrointestinal (GI) tract, and most commonly occurs in the colon[2,3]; however, 15% of cases are thought to be located in the small bowel[4]. Small bowel angioectasia is a major source of obscure GI bleeding (OGIB)[5,6], which is detected by capsule endoscopy (CE).
The etiology and mechanism of the development of angioectasia are not fully understood. Pathogenic factors contributing to the formation of angioectasia are considered to be high intestinal wall tension causing chronic obstruction of submucosal veins with consecutive precapillary dilation[7], mucosal ischemia from chronic hypoxia or hypoperfusion[8,9], and vascular endothelial growth factor (VEGF)-related disorders of angiogenesis[10].
Visceral fat accumulation is thought to modulate a range of systemic and end-organ effects through the release of adipocytokines, growth factors, and inflammatory mediators[11]. VEGF is an important angiogenic factor. It is secreted by adipocytes and is implicated in both normal and pathologic vessel formation[12,13]. Thus, visceral fat accumulation may have a role in the pathogenesis of small bowel angioectasia through these secreted molecules. Visceral fat accumulation can be calculated using computer software based on CT images[14]. We conducted the present study to elucidate the association of visceral fat accumulation with the risk of small bowel angioectasia.
This study was a retrospective analysis conducted in accordance with the Declaration of Helsinki and with the approval of the ethics committee of the University of Tokyo.
Our study included consecutive patients who underwent CE at the University of Tokyo Hospital from January 2009 to September 2013. The inclusion criteria were: (1) an indication for investigation of OGIB; (2) performance of CT to investigate GI bleeding or for another purpose within 3 mo before or after the CE; and (3) informed consent provided. OGIB was defined as bleeding that persisted or recurred after a negative initial endoscopic result (obtained by colonoscopy and upper endoscopy)[15].
A Pillcam® SB or SB2 CE device (Given Imaging, Yoqneam, Israel) was used for small intestinal examination. Two experienced gastroenterologists, both of whom had the patients’ clinical background information, including GI endoscopy results, independently reviewed the CE images. After the independent review, the reviewers discussed all CE findings and reached a consensus. Small bowel angioectasia was defined as a circumscribed patchy, flat, sharply demarcated area of redness[16]. The small bowel visualized on CE during < 50% of the small bowel transit time was presumed to be jejunum, and > 50% of the small bowel transit time was designated as ileum.
The fat tissue area and waist circumference were measured in each patient by analyzing a CT image at the level of the umbilicus with the software Slimvision® (Cybernet Systems Co., Ltd., Tokyo, Japan)[17]. The subcutaneous fat area (SFA) was defined as the sum of the extraperitoneal fat area between the skin and muscle on a CT image that showed attenuation ranging from -150 to -50 Hounsfield units. Visceral fat area (VFA) was defined as the sum of the intraperitoneal fat area showing the same attenuation.
The medical records of the study patients were thoroughly reviewed, and data on comorbidities and ongoing medication were collected. Liver cirrhosis was diagnosed in each case based on the patient’s medical history, indicative physical findings (vascular spiders, palmar erythema, and leg edema), laboratory findings (impaired synthetic liver function and thrombocytopenia), radiographic features of the upper abdomen (nodular appearance, irregular liver contour, splenomegaly, and/or ascites), and/or liver biopsy findings. Chronic renal failure was defined as a successive estimated glomerular filtration rate of ≤ 60 mL/min per 1.73 m2. Ongoing medication indicated drug administration within 1 mo before CE examination.
All statistical analyses were performed with JMP software (version 10; SAS Institute, Cary, NC, United States). In all sample analyses, means were compared to an unpaired Student’s t-test and frequency distributions were compared with the Fisher’s exact probability test. Factors with a P < 0.2 in the univariate analysis were included in the multivariate analysis. The odds ratios (ORs) for small bowel angioectasia were calculated by logistic regression analysis. A P < 0.05 was considered statistically significant.
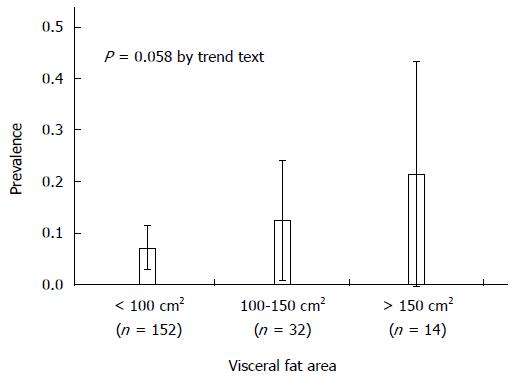
During the study period, 246 patients underwent CE after a diagnosis of OGIB. Of these 246 patients, 198 underwent both CE and CT examinations and satisfied the inclusion criteria, whereas the remaining 48 did not undergo CT. In total, 198 patients (117 male and 81 female) with the mean age 65.8 ± 12.8 years were analyzed. The mean VFA and SFA were 66.3 ± 54.8 cm2 and 98.3 ± 69.1 cm2, respectively. Small bowel angioectasia was found in 18/198 patients (9.1%). Small bowel angioectasia was detected more frequently in the jejunum than in the ileum (78% vs 33%, P = 0.018). The median number of small bowel angioectasia was 2 (interquartile range: 1-5). About half were cases of single angioectasia. Table 1 shows the comparison between patients with and without small bowel angioectasia. The mean VFA was significantly higher in patients with than without small bowel angioectasia (96.0 ± 76.0 cm2vs 63.4 ± 51.5 cm2, P = 0.016). The prevalence of small bowel angioectasia progressively increased according to the VFA: 7.2% in patients with a VFA of < 100 cm2, 12.5% in patients with a VFA of 100-150 cm2, and 21.4% in patients with a VFA of > 150 cm2 (P = 0.058 by trend test) (Figure 1). However, there were no significant differences in the SFA, waist circumference, or body mass index. The proportion of liver cirrhosis was higher in the group with small bowel angioectasia (61% vs 22%, P < 0.001). The proportion of patients with chronic renal failure was higher in those with than without small bowel angioectasia, but the difference was not statistically significant (22% vs 9%). Using three factors with a P < 0.2 in the univariate analysis, we performed multivariate unconditional logistic regression (Table 2). Multivariate analysis showed that the VFA (OR for each 10-cm2 increment = 1.1; 95%CI: 1.02-1.19; P = 0.021) and liver cirrhosis (OR = 6.1, 95%CI: 2.2-18.5; P > 0.001) were significant risk factors for small bowel angioectasia.
| Characteristics | With angioectasia | Without angioectasia | P value |
| (n = 18) | (n = 180) | ||
| Age (yr) | 64.7 ± 8.1 | 65.8 ± 13.2 | 0.701 |
| Sex (male/female) | 13/5 | 104/76 | 0.232 |
| Body mass index (kg/m2) | 22.6 ± 4.3 | 21.8 ± 3.9 | 0.391 |
| CT measurement | |||
| Waist circumference (cm) | 83.3 ± 11.6 | 80.0 ± 10.2 | 0.201 |
| VFA (cm2) | 96.0 ± 76.0 | 63.4 ± 51.5 | 0.021 |
| SFA (cm2) | 103.0 ± 68.6 | 97.8 ± 69.4 | 0.761 |
| Subtype of OGIB2 | |||
| Overt | 9 (50) | 114 (63) | 0.272 |
| Occult | 9 (50) | 66 (37) | |
| Comorbidities | |||
| Chronic renal failure | 4 (22) | 17 (9) | 0.112 |
| Diabetes mellitus | 5 (28) | 35 (19) | 0.402 |
| Hypertension | 7 (39) | 70 (39) | 1.002 |
| Hyperlipidemia | 3 (17) | 23 (13) | 0.712 |
| Liver cirrhosis | 11 (61) | 39 (22) | < 0.012 |
| Cause of hepatitis | |||
| HBV/HCV/non-B, non-C | 1/6/4 | 6/22/11 | |
| Aortic stenosis | 0 (0) | 10 (6) | 0.602 |
| Medication | |||
| NSAIDs | 1 (6) | 27 (15) | 0.482 |
| Antiplatelet drugs | 3 (5) | 15 (11) | 0.292 |
| Anticoagulant drugs | 3 (17) | 29 (16) | 1.002 |
Small bowel angioectasia was found in 9.1% of patients with OGIB. A positive association was observed between VFA and the prevalence of small bowel angioectasia. Multivariate analysis showed that VFA and liver cirrhosis were independent risk factors for small bowel angioectasia in patients with OGIB.
Small bowel angioectasia is a major source of OGIB[5,6]. Previous studies have reported that 30%-40% of patients with OGIB have small bowel angioectasia[5,15,18]. In fact, the prevalence of small bowel angioectasia was lower in the present study than in these previous reports. The reason for this discrepancy is the difference in the diagnostic criteria used for small bowel angioectasia. We regarded minimal red mucosa and red spots as insignificant findings because we cannot distinguish a CE picture of a nonspecific red spot from punctate angioectasia (Yano-Yamamoto classification type 1a)[19]. If we defined these changes as angioectasia, the prevalence of small bowel angioectasia would be 41%, which is concordant with other results[5,15,18].
The finding of liver cirrhosis is in accordance with data from previous reports. We previously reported that patients with liver cirrhosis and hepatocellular carcinoma have a high prevalence of small bowel angioectasia, and found a correlation between small bowel angioectasia and portal hypertension[20]. Patients with angioectasia had a larger spleen index and a more frequent history of band ligation or sclerotherapy for esophageal varices. De Palma et al[16] reported that 25/37 (67.5%) patients with liver cirrhosis with anemia and/or positive stool occult blood test results had vascular lesions, including angioectasia. Portal hypertension is a well-known major cause of vascular disease in patients with liver cirrhosis[21]. It is possible that small bowel mucosal changes caused by portal hypertension play an essential role in angioectasia formation.
The present study revealed a relationship between VFA and the risk of small bowel angioectasia. Visceral fat accumulation is a major determinant of insulin resistance, which causes obesity-related diseases, including diabetes mellitus, hypertension, and cardiovascular disease[22]. A previous study reported that patients with GI angioectasia are more likely to have underlying cardiovascular or pulmonary diseases, therefore suggesting that mucosal ischemia from chronic hypoxia or hypoperfusion may contribute to the development of angioectasia[8]. Moreover, adipose tissue expresses and releases various secretary molecules, such as leptin[23,24], tumor necrosis factor-α[25], plasminogen activator inhibitor-1[26], interleukin-6, and VEGF[12,13]. VEGF is an important angiogenic factor that reportedly induces migration and proliferation of endothelial cells, enhances vascular permeability, and modulates thrombogenicity[27]. VEGF is therefore implicated in vascular pathologies as well as normal blood vessel development. Although we had no data on cytokine levels, and although the relationship between VEGF and small bowel angioectasia was unclear, our results indicate that visceral fat accumulation may play a role in the pathogenesis of small bowel angioectasia.
Chronic renal failure[28], aortic stenosis[29], and von Willebrand disease[30] are associated with GI angioectasia. The present study indicated that VFA and liver cirrhosis are stronger risk factors for small bowel angioectasia than these known factors, although a causative relationship cannot be excluded.
There were several limitations to this study. First, it was a retrospective analysis, so selection bias for determining whether patients with OGIB should undergo CT may have been involved. Because the CE reviewers were aware of the patients’ clinical backgrounds, they might have been biased by their knowledge of the patients’ body weights or presence of cirrhosis. Second, we had no data on cytokine levels. Third, the number of study participants was small. Therefore, a larger prospective study is needed to confirm the contribution of these risk factors to small bowel angioectasia and to investigate the relationships between cytokine levels and small bowel angioectasia.
In conclusion, a positive association was observed between VFA and the prevalence of small bowel angioectasia. VFA and liver cirrhosis are independent risk factors for small bowel angioectasia in patients with OGIB.
Small bowel angioectasia is a major source of obscure gastrointestinal bleeding (OGIB), which is detected by capsule endoscopy. The etiology and mechanism of the development of angioectasia are not fully understood. Adipose tissue expresses and releases various secretary molecules. Vascular endothelial growth factor (VEGF) is an important angiogenic factor, which is secreted by adipocytes and is implicated in both normal and pathologic vessel formation. Visceral fat accumulation can be calculated using computer software based on computed tomography (CT) images.
Adipose tissue expresses and releases various secretary molecules, such as leptin, tumor necrosis factor-α, plasminogen activator inhibitor-1, interleukin-6, and VEGF. VEGF is an important angiogenic factor that reportedly induces migration and proliferation of endothelial cells, enhances vascular permeability, and modulates thrombogenicity.
This study indicated that a positive association is present between visceral fat accumulation and the prevalence of small bowel angioectasia. Visceral fat accumulation and liver cirrhosis are independent risk factors for small bowel angioectasia in patients with OGIB.
These study results suggest that visceral obesity and liver cirrhosis are useful for predicting small bowel angioectasia in patients with OGIB.
The subcutaneous fat area was defined as the sum of the extraperitoneal fat area between the skin and muscle at the level of the umbilicus on a CT image. The visceral fat area was defined as the sum of the intraperitoneal fat areas.
The authors investigated the association between visceral fat accumulation and risk for small bowel angioectasia, and report a positive association between them. Visceral fat accumulation and liver cirrhosis are independent risk factors for small bowel angioectasia in patients with OGIB.
P- Reviewer: Chen Z, Hassan M, Streba LAM S- Editor: Qi Y L- Editor: AmEditor E- Editor: Liu XM
| 1. | Boley SJ, DiBiase A, Brandt LJ, Sammartano RJ. Lower intestinal bleeding in the elderly. Am J Surg. 1979;137:57-64. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 160] [Cited by in F6Publishing: 151] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 2. | Ueno S, Nakase H, Kasahara K, Uza N, Kitamura H, Inoue S, Mikami S, Matsuura M, Chiba T. Clinical features of Japanese patients with colonic angiodysplasia. J Gastroenterol Hepatol. 2008;23:e363-e366. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 3. | Foutch PG, Rex DK, Lieberman DA. Prevalence and natural history of colonic angiodysplasia among healthy asymptomatic people. Am J Gastroenterol. 1995;90:564-567. [PubMed] [Cited in This Article: ] |
| 4. | Dray X, Camus M, Coelho J, Ozenne V, Pocard M, Marteau P. Treatment of gastrointestinal angiodysplasia and unmet needs. Dig Liver Dis. 2011;43:515-522. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 5. | Foutch PG. Angiodysplasia of the gastrointestinal tract. Am J Gastroenterol. 1993;88:807-818. [PubMed] [Cited in This Article: ] |
| 6. | Liao Z, Gao R, Xu C, Li ZS. Indications and detection, completion, and retention rates of small-bowel capsule endoscopy: a systematic review. Gastrointest Endosc. 2010;71:280-286. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 472] [Cited by in F6Publishing: 425] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 7. | Boley SJ, Sammartano R, Adams A, DiBiase A, Kleinhaus S, Sprayregen S. On the nature and etiology of vascular ectasias of the colon. Degenerative lesions of aging. Gastroenterology. 1977;72:650-660. [PubMed] [Cited in This Article: ] |
| 8. | Rogers BH. Endoscopic diagnosis and therapy of mucosal vascular abnormalities of the gastrointestinal tract occurring in elderly patients and associated with cardiac, vascular, and pulmonary disease. Gastrointest Endosc. 1980;26:134-138. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 94] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 9. | Heer M, Sulser H, Hany A. Angiodysplasia of the colon: an expression of occlusive vascular disease. Hepatogastroenterology. 1987;34:127-131. [PubMed] [Cited in This Article: ] |
| 10. | Junquera F, Saperas E, de Torres I, Vidal MT, Malagelada JR. Increased expression of angiogenic factors in human colonic angiodysplasia. Am J Gastroenterol. 1999;94:1070-1076. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 108] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 11. | Després JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444:881-887. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2823] [Cited by in F6Publishing: 2922] [Article Influence: 171.9] [Reference Citation Analysis (0)] |
| 12. | Miyazawa-Hoshimoto S, Takahashi K, Bujo H, Hashimoto N, Saito Y. Elevated serum vascular endothelial growth factor is associated with visceral fat accumulation in human obese subjects. Diabetologia. 2003;46:1483-1488. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 165] [Cited by in F6Publishing: 168] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 13. | Hausman GJ, Richardson RL. Adipose tissue angiogenesis. J Anim Sci. 2004;82:925-934. [PubMed] [Cited in This Article: ] |
| 14. | Kvist H, Chowdhury B, Grangård U, Tylén U, Sjöström L. Total and visceral adipose-tissue volumes derived from measurements with computed tomography in adult men and women: predictive equations. Am J Clin Nutr. 1988;48:1351-1361. [PubMed] [Cited in This Article: ] |
| 15. | Raju GS, Gerson L, Das A, Lewis B. American Gastroenterological Association (AGA) Institute medical position statement on obscure gastrointestinal bleeding. Gastroenterology. 2007;133:1694-1696. [PubMed] [Cited in This Article: ] |
| 16. | De Palma GD, Rega M, Masone S, Persico F, Siciliano S, Patrone F, Matantuono L, Persico G. Mucosal abnormalities of the small bowel in patients with cirrhosis and portal hypertension: a capsule endoscopy study. Gastrointest Endosc. 2005;62:529-534. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 126] [Cited by in F6Publishing: 123] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 17. | Zhao B, Colville J, Kalaigian J, Curran S, Jiang L, Kijewski P, Schwartz LH. Automated quantification of body fat distribution on volumetric computed tomography. J Comput Assist Tomogr. 2006;30:777-783. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 61] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 18. | Schmit A, Gay F, Adler M, Cremer M, Van Gossum A. Diagnostic efficacy of push-enteroscopy and long-term follow-up of patients with small bowel angiodysplasias. Dig Dis Sci. 1996;41:2348-2352. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 66] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 19. | Yano T, Yamamoto H, Sunada K, Miyata T, Iwamoto M, Hayashi Y, Arashiro M, Sugano K. Endoscopic classification of vascular lesions of the small intestine (with videos). Gastrointest Endosc. 2008;67:169-172. [PubMed] [Cited in This Article: ] |
| 20. | Yamada A, Watabe H, Obi S, Sugimoto T, Kondo S, Ohta M, Togo G, Ogura K, Yamaji Y, Okamoto M. Surveillance of small intestinal abnormalities in patients with hepatocellular carcinoma: a prospective capsule endoscopy study. Dig Endosc. 2011;23:124-129. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 21. | Aoyama T, Oka S, Aikata H, Nakano M, Watari I, Naeshiro N, Yoshida S, Tanaka S, Chayama K. Small bowel abnormalities in patients with compensated liver cirrhosis. Dig Dis Sci. 2013;58:1390-1396. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 22. | Kissebah AH, Krakower GR. Regional adiposity and morbidity. Physiol Rev. 1994;74:761-811. [PubMed] [Cited in This Article: ] |
| 23. | MacDougald OA, Hwang CS, Fan H, Lane MD. Regulated expression of the obese gene product (leptin) in white adipose tissue and 3T3-L1 adipocytes. Proc Natl Acad Sci USA. 1995;92:9034-9037. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 342] [Cited by in F6Publishing: 345] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 24. | Caro JF, Sinha MK, Kolaczynski JW, Zhang PL, Considine RV. Leptin: the tale of an obesity gene. Diabetes. 1996;45:1455-1462. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 633] [Cited by in F6Publishing: 647] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 25. | Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87-91. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5334] [Cited by in F6Publishing: 5219] [Article Influence: 168.4] [Reference Citation Analysis (0)] |
| 26. | Shimomura I, Funahashi T, Takahashi M, Maeda K, Kotani K, Nakamura T, Yamashita S, Miura M, Fukuda Y, Takemura K. Enhanced expression of PAI-1 in visceral fat: possible contributor to vascular disease in obesity. Nat Med. 1996;2:800-803. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 652] [Cited by in F6Publishing: 627] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 27. | Neufeld G, Cohen T, Gengrinovitch S, Poltorak Z. Vascular endothelial growth factor (VEGF) and its receptors. FASEB J. 1999;13:9-22. [PubMed] [Cited in This Article: ] |
| 28. | Karagiannis S, Goulas S, Kosmadakis G, Galanis P, Arvanitis D, Boletis J, Georgiou E, Mavrogiannis C. Wireless capsule endoscopy in the investigation of patients with chronic renal failure and obscure gastrointestinal bleeding (preliminary data). World J Gastroenterol. 2006;12:5182-5185. [PubMed] [Cited in This Article: ] |
| 29. | Bhutani MS, Gupta SC, Markert RJ, Barde CJ, Donese R, Gopalswamy N. A prospective controlled evaluation of endoscopic detection of angiodysplasia and its association with aortic valve disease. Gastrointest Endosc. 1995;42:398-402. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 59] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 30. | Veyradier A, Balian A, Wolf M, Giraud V, Montembault S, Obert B, Dagher I, Chaput JC, Meyer D, Naveau S. Abnormal von Willebrand factor in bleeding angiodysplasias of the digestive tract. Gastroenterology. 2001;120:346-353. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 121] [Article Influence: 5.3] [Reference Citation Analysis (0)] |