Published online Jun 21, 2015. doi: 10.3748/wjg.v21.i23.7065
Peer-review started: January 28, 2015
First decision: March 10, 2015
Revised: March 23, 2015
Accepted: April 28, 2015
Article in press: April 28, 2015
Published online: June 21, 2015
Neuropilins (NRPs) are highly conserved transmembrane glycoproteins that possess pleiotropic functions. Neuropilin-1 (NRP1) and its homologue neuropilin-2 interact as coreceptors with both class 3 semaphorins and vascular endothelial growth factor and are involved in neuronal guidance and angiogenesis, respectively. The contribution of NRPs to tumor angiogenesis has been highlighted in previous studies, leading to the development of NRP antagonists as novel anti-angiogenesis therapies. However, more recent studies have demonstrated that NRPs have a much broader spectrum of activity in the integration of different pathways in physiological and pathological conditions. A few studies investigated the role of NRPs in both malignant and non-neoplastic liver diseases. In normal liver, NRP1 is expressed in hepatic stellate cells and liver sinusoidal endothelial cells. NRP1 expression in hepatocytes has been associated with malignant transformation and may play an important role in tumor behavior. A contribution of NRPs in sinusoidal remodeling during liver regeneration has been also noted. Studies in chronic liver diseases have indicated that, besides its influence on angiogenesis, NRP1 might contribute to the progression of liver fibrosis owing to its effects on other growth factors, including transforming growth factor β1. As a result, NRP1 has been identified as a promising therapeutic target for future antifibrotic therapies based on the simultaneous blockade of multiple growth factor signaling pathways. In this review, the structure of NRPs and their interactions with various ligands and associated cell surface receptors are described briefly. The current understanding of the roles of the NRPs in liver diseases including tumors, regeneration and fibrogenesis, are also summarized.
Core tip: The contribution of neuropilins (NRPs) to tumor angiogenesis has been identified in previous research, which has led to the development of NRP-targeted therapies. Although the number of relevant studies is too small to ascertain the precise role of NRPs in liver, the evidence implies an association with tumor behavior, liver regeneration and the progression of fibrosis. The interplay between NRPs and vascular endothelial growth factor, platelet-derived growth factor and transforming growth factor-β support NRPs as potential targets in the prevention of fibrogenesis in chronic liver diseases. However, further studies are needed to clarify whether NRPs may fill the large gaps in our understanding and ability to treat liver diseases.
- Citation: Elpek G&. Neuropilins and liver. World J Gastroenterol 2015; 21(23): 7065-7073
- URL: https://www.wjgnet.com/1007-9327/full/v21/i23/7065.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i23.7065
Neuropilins (NRPs) are highly conserved single-spanning transmembrane glycoproteins that are involved in a wide range of physiological and pathological processes[1]. To date, two NRP homologues have been identified in vertebrates: NRP1 and NRP2[1,2]. NRP1 was discovered by Takagi et al[2] in 1987 as an antigen to a monoclonal antibody (A5) that binds neuronal cell-surface proteins in the Xenopus nervous system. A decade later, NRP2 was identified as an alternative neuronal receptor[3,4]. NRPs were initially characterized as regulators of nervous system development because of their ability to act as coreceptors with plexins for specific secreted members of the semaphorin family (SEMAs), which possess neuronal guidance functions[4-9]. The role of NRPs in the development of the nervous system during embryogenesis is noteworthy. Recent studies have shown that NRPs are multifunctional coreceptors with the ability to bind different protein families, including vascular endothelial growth factor (VEGF), transforming growth factor-β1 (TGF-β1) and other growth factors[10-13]. This has led to new areas of research into NRPs in diverse biological functions, such as angiogenesis, immune system regulation, and tumor growth and progression[13-20]. Many studies of NRPs and their ligands have increased awareness of their pathological roles and their potential as therapeutic targets[15,16,20-22]. This paper briefly presents our current knowledge of NRP structure, NRPs’ interactions with their various ligands and associated cell surface receptors, and their role in liver diseases.
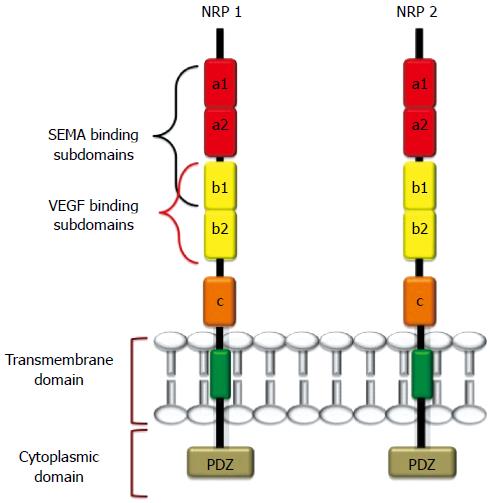
NRP1 and NRP2 are 120-140 kDa multifunctional transmembrane glycoproteins composed of 923 and 926 amino acids, respectively[1,23]. NRP1 and NRP2 genes are located in two different chromosomes (NPR1 in chromosome 10p12 and NRP2 in chromosome 2q34), but they share approximately 44% sequence homology at the amino acid level[1,14,17-19,20,23,24]. NRPs comprise a large N-terminal extracellular domain, a very short transmembrane domain and a small cytoplasmic tail (Figure 1). The extracellular domain is further divided into three subdomains: (1) the a1-a2 subdomains, which are homologous to the C1r, and C1s complement components [complement binding motifs (CUBs)][25]; (2) the B subdomain, possessing two b-domain repeats (b1 and b2) that are homologous to coagulation factors V and VIII[26]; and (3) the C domain, which contains an MAM (meprin, A5/NRP, protein tyrosine phosphatase μ) domain that is likely involved in NRP oligomerization with the transmembrane domain, which in turn possesses a conserved GXXXG repeat[27-29]. The intracellular cytoplasmic domain interacts and binds with several proteins and PDZ-motif-containing proteins (e.g., GIPC, synectin)[28,29]. The latter proteins play important roles in signaling complexes and in maintaining the structural integrity of transmembrane proteins.
NRP2 also has two splice variants, NRP2A and NRP2B, that exhibit varying levels of sequence homology with NRP1[24,25]. Several soluble isoforms of NRPs (sNRPs) that lack transmembrane or cytoplasmic domains also exist[22].
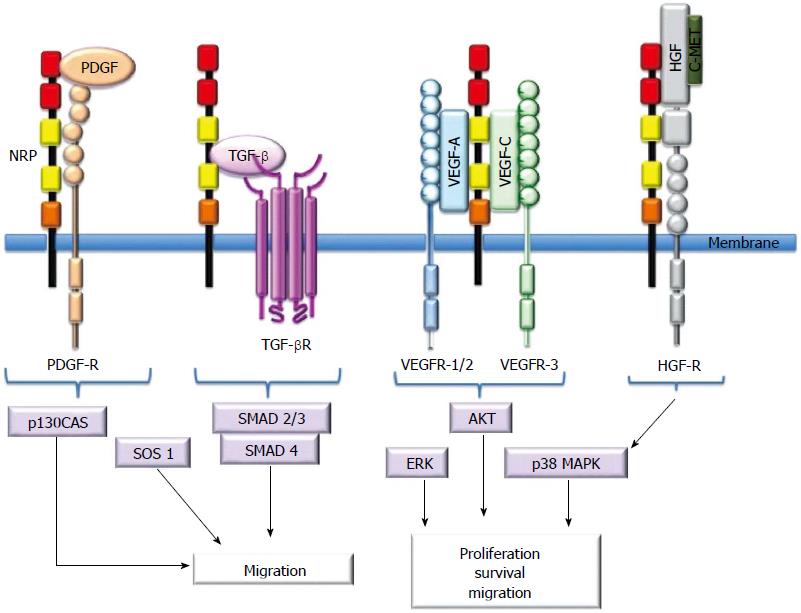
NRPs bind several ligand families, including class 3 semaphorins (SEMA3s) and heparin-binding members of the VEGF family[5,10,11,13]. Although NRPs lack a signaling role, they are thought to mediate functional responses by forming complexes with other transmembrane receptors to generate holoreceptors[1]. NRPs may act as receptors for other growth factors as well[23]. Figure 2 presents a short description of NRP interactions with ligands and coreceptors and their signaling pathways.
SEMA3s are members of the large family of SEMAs that were first identified as axon-guidance molecules[30]. However, accumulated evidence indicates that SEMAs are involved in cell apoptosis, cell migration, tumor behavior, angiogenesis and immune dysregulation[30,31-34]. In contrast to other SEMAs, SEMA3s bind NRPs as cell surface receptors[32]. The transduction of the SEMA3 signal necessitates the interaction with plexins, which form complexes with NRPs[32,33]. There are seven SEMA3s (A to G), and most of these subtypes, with the exception of SEMA3E, bind NRP1, NRP2 or both[30,32,34]. NRP1 is a receptor for SEMA3A (collapsin-1), 3C and 3F, and NRP2 preferentially binds SEMA3B, 3C, 3D and 3F[20,30,32]. SEMA3s exert considerable effects in endothelial cells[14,29,30]. By blocking the VEGF-NRP interaction (see below), SEMA3A and SEMA3F inhibit VEGF-related cell proliferation and migration[30,31]. SEMA3A and SEMA3F influence vascular remodeling by inhibiting the adhesion of endothelial cells (ECs) to the extracellular matrix (ECM) via integrins and contribute to vascular development by inducing EC apoptosis[35-37]. Therefore, these peculiarities of NRPs with SEMA3s make them potential targets for anti-angiogenic therapies.
The VEGF family and their tyrosine kinase receptors [VEGF-receptor-1 (VEGFR-1), VEGFR-2 and VEGFR-3] play pivotal roles in physiological and pathological angiogenesis[38-40]. There are numerous isoforms of VEGF, and VEGF165 is the most active and studied[38-40]. In particular, NRP1 is a high-affinity coreceptor for VEGF165[39,40]. NRP1 also binds VEGFR2, and their coexpression enhances VEGF165-mediated angiogenesis and vascular development[40]. VEGF165 also contributes to VEGFR2-NRP1 complex formation through its own binding activity[39-41].
NRP2 is a coreceptor for VEGFR-3 as a result of stimulation by VEGF-C and VEGF-D[42-44]. In experimental models, VEGF-C and VEGF-D have induced lymphovascular development and stimulated lymph node metastasis via VEGFR-3[43,44]. Taken together, these findings delineate the role of NRP2 in lymphangiogenesis.
Given their role in promoting angiogenesis, NRPs, particularly NRP1, has been identified as potential targets for anti-angiogenic therapy[15,16,38,40,43] Blockade of NRP1-VEGF coupling, by enhancing the efficacy of anti-VEGF therapy, has been demonstrated to be an effective therapeutic approach in cancer[16,20-22,31].
NRPs may also interact with other heparin-binding proteins, such as the fibroblast growth factor family (FGF-1, FGF-2, FGF-4), galectin-1, hepatocyte growth factor (HGF), TGF-β1, epidermal growth factor (EGF) and platelet-derived growth factor (PDGF).
FGF-2 binds NRP1 and stimulates the growth of ECs in human umbilical veins[36,45]. Galectin-1 selectively binds NRP1, and Gal-NRP1 interactions mediate EC migration and adhesion and enhance VEGFR-2 phosphorylation[36,45].
HGF may regulate EC functions[45,46-48]. NRP1 and NRP2 are functional coreceptors for HGF[47]. Phosphorylation of c-Met, a tyrosine kinase receptor that binds HGF, is also enhanced by NRPs because they can activate the c-Met receptor and consequently play important roles in the regeneration of certain organs and the regulation of proliferation, migration and survival of ECs[46,48].
NRPs may also interact with other cellular receptors. Experimental studies demonstrated that NRP1 forms a complex with β1 integrin in cancer cell lines[49-51].
The coreceptor function of NRP1 in TGF-β signaling was recently identified. NRP1 activates both the latent (LAP-TGF-β) and active forms of TGF-β[52-54], and it improves the affinity of TGF-β for its receptors, TGF-βRI/II/III. NRP2, like NRP1, is also a coreceptor for active TGF-β1[52-54]. Important roles for NRPs in the regulation of the immune system, epithelial mesenchymal transition (EMT) and fibrosis have been proposed because of the close association of NRPs with growth factors; these roles are supported by previous observations[22,34,52-54].
The roles of EGF and its high-affinity receptor EGFR in cellular differentiation and proliferation are well described. Dysregulation of NRP1 has been observed to inhibit EGFR signaling activity and consequently impair its function[55].
Like EGF, PDGF plays important roles in cellular proliferation[56]. PDGFR-α/β promotes angiogenesis and vascular smooth muscle cell (VSMC) mobilization[57]. NPR-1 acts a coreceptor to enhance the affinity of PDGFR to PDGF[58,59]. It has been demonstrated that PDGF from tumor cells interacts with NRP1 to promote motility in VSMCs[56-60].
The hedgehog (Hh) signaling system is recognized for its fundamental role in cellular differentiation, proliferation, and tissue polarity during embryonic development and the maintenance of a stem cell phenotype[22,61-69]. Previous studies have indicated that an aberrant activation of this pathway in adult life is associated with cancer progression, aggressive tumor behavior and metastasis[22,61-64]. The regulatory roles of NRPs in the Hh signaling system were observed in recent studies[63,68,69]. In an elegant study, Hillman et al[68] demonstrated that NRP1 and NRP2 are positive regulators of Hh signal transduction. They observed coexpression of NRPs and Hh at similar times and locations during development. A positive feedback circuit has also been suggested, based on the induction of NRPs by Hh signaling and an increase in Hh target gene activation due to overexpression of NRPs. Cao et al[69] observed that NRP1 knockdown in a renal carcinoma model resulted in a more differentiated phenotype and the inhibition of sonic Hh, leading to the conclusion that NRPs promote an undifferentiated phenotype in cancer cells.
More recently, Hh pathway involvement has been studied in the pathogenesis of non-neoplastic diseases, including liver diseases, and the involvement of Hh signaling in liver regeneration and fibrogenesis has been documented[65-67]. Accumulated evidence indicates that the Hh signaling system contributes to many processes, including transformation of quiescent HSCs into a more fibroblastic phenotype, angiogenesis, EMT, accumulation of inflammatory cells and multipotent progenitor cells[65-67]. However, the relationship between NRPs and the Hh signaling pathway has not been studied in non-neoplastic liver diseases.
The additive effect of blockade of NRP1-VEGF coupling in anti-angiogenic therapy has been documented, but nebulous data exist regarding the expression of NRPs in many cancer types and their association with tumor-related angiogenesis and tumor progression[15,16,19,20-22,31,38,43]. Neutralizing antibodies to NRP1 are currently under investigation in phase I trials[70-76]. Despite the significant improvements in diagnosis and treatment, the outcome of patients with liver cancer remains dismal, and novel strategies are necessary. However, the role of NRPs in liver tumors has been investigated in a limited number of studies, and experimental models exceed the number of clinicopathological studies.
The first finding related to the role of NRPs in liver cancer was obtained in an experimental study that investigated the role of SOX4 in cell migration of intrahepatic metastasis of hepatocellular carcinoma (HCC)[77]. High levels of two SOX4 target genes, NRP1 and SEMA3C, correlated with an increase in cell migration, which was reduced by the silencing of NRP1 and SEMA3C[77]. Subsequent experimental studies in tumor cell lines of the role of NRPs in tumor angiogenesis revealed that inhibition of NRP1 using RNA-interference (RNAi) has an angiostatic potential that might be promising in the treatment of HCC[78]. Administration of small interfering RNAs (siRNAs) specific for NRP1 inhibited tumor angiogenesis and vascular remodeling in a mouse model of HCC[79,80]. An increase of NRP1 expression in both vascular and tumor compartments parallel to disease progression has been observed in transgenic mice developing HCC[79]. Blockade of NRP1 function with peptide N (an NRP1-binding recombinant protein and competitive inhibitor of VEGF-A165/NRP1 interaction) leads to the inhibition of vascular remodeling and growth, which supports NRP1 as a new target for increasing the effectiveness of anti-angiogenic therapies in HCC[79-81]. More recently, it was demonstrated in experimental models that NRPs not only affect angiogenesis in tumor growth and progression but also contribute to matrix stiffness via regulation of tumor matrix maturation in experimental models[82]. However, the significance of this finding in liver cancer requires further investigation.
A few studies have investigated the role of NRPs in patients with liver cancer[81,83,84]. NRP1 expression has been observed in hepatic ECs in both human biopsies and in HCC samples but not in normal hepatocytes[81-84]. One study found that the increased NRP1 expression in hepatocytes was significantly associated with HCC, supporting its role in tumor progression[81]. From the pathological point of view, it would be interesting to investigate whether the presence of NRP1 expression in hepatocytes might be useful for the discrimination of HCC from other neoplastic lesions, including borderline dysplastic nodules, especially in needle biopsies from patients with suspicious liver lesions.
A more recent study in 638 HCC patients highlighted that VEGFR-2 and peritumoral NRP1 expression were associated with prolonged recurrence time and extended overall survival[83]. It is speculated that, after curative hepatectomy, peritumoral hepatocytes expressing abundant of NRP1 or VEGFR-2 may exert a decoy effect and compete for VEGFA binding with ECs, providing a hostile peritumoral environment for recurrence and metastasis[83]. Up-regulation of NRP1 in cholangiocarcinomas was also noted, with higher NRP1 expression in invasive tumors than in dysplastic lesions[84].
The findings of these studies are not sufficiently conclusive to define the exact role of NRP1 in tumor progression and behavior. Therefore, further studies in patients with liver cancer are warranted.
As noted above, NRPs serve pleiotropic functions by integrating distinct critical pathways that are principal to physiological and pathological function. NRP1 and NRP2 expression has not been detected in normal hepatocytes[81,83], but NRP1 has been detected in hepatic stellate cells (HSCs) and liver sinusoidal ECs (LSECs)[81,83,85-89]. One study investigated the role of hemodynamic forces in morphology and gene expression of LSECs during early phases of liver regeneration (LR) and found that stress imposed on LSECs was associated with the translocation of VEGFR-2 and NRP1 from the perinuclear to the plasma membrane and with cytoskeletal localization[86]. These results suggested that a VEGFR-2/NRP1 complex plays a role in the early signal that leads to LR[86]. More recently, another experimental model of LR investigated the expression of NRP1 and SEMA3A during sinusoidal remodeling and found that NRP1 and SEMA3A were constitutively expressed in hepatocytes and LSECs, respectively[88]. NRP1 expression transiently increased after partial hepatectomy and returned to the basal level at the termination of LR, in contrast to SEMA3A[88,89]. These data suggest that NRP1 contributes to sinusoidal remodeling during LR, but further studies are needed to better understand the interplay of NRP1 and other coreceptors in LSECs and other parenchymal cells.
Numerous interactions between the ECM, HSCs, ECs and immune cells occur during liver fibrogenesis in chronic liver diseases (CLDs)[90,91]. In these interplays, a central role has been attributed to HSC activation, which is a complex event that provides multiple potential targets for therapeutic intervention. A vast number of signaling pathways contribute to the activation of HSCs, including Toll-like receptor 4, hedgehog, adiponectin, TGF-β1, and PDGF[90,91]. Because NRP1 has been reported to bind and activate the latent form of TGF-β1, it is hypothesized that it might also serve as a TGF-β1 coreceptor that regulates TGF-β1 signaling during the transition from a quiescent to an activated stage in fibroblasts, including HSCs[54,59]. Indeed, an experimental study demonstrated that NRP1 can control two aspects of TGF-β signaling during fibroblast activation[54]. In cell cultures, NRP1 was observed to down-regulate Smad1/5 signaling, which inhibits fibrosis progression by maintaining collagen-producing cells in a quiescent in state, and it up-regulated Smad2/3 signaling, which promotes fibrosis by activating fibroblasts[54]. In the same study, NRP1 mRNA levels gradually increased during the activation of HSC cultures. The mechanism of NRP1 control of the Smad1/5 and Smad2/3 counterbalance has not been completely delineated, but these findings suggest that NRP1 plays an important role in the activation of HSCs during the evolution of liver fibrosis[54,92].
Angiogenesis has a key role in the wound-healing response to chronic liver injury, and HSCs have emerged as the main ECM-producing fibrogenic cell type in this process. HSCs may also act as proangiogenic cells during the evolution of liver fibrosis[90-92]. Indeed, activated HSCs may respond to hypoxia using an HIF-1α-related pathway through increases in VEGF and Ang-1 and their receptors VEGFR-2 and Tie-2, respectively[90,91,93,94]. VEGF and Tie-2 may also activate HSCs[90,91]. VEGF induces HSC migration and proliferation as well[90,91,93,94]. HSCs may also modulate angiogenesis independently of hypoxia by responding to several mediators, including PDGF[93]. In an elegant study, Cao et al[59] investigated PDGF-dependent HSC recruitment and the accompanying sinusoidal vascular remodeling during liver fibrosis in both experimental models and humans. They demonstrated that NRP1 was up-regulated in activated HSCs and colocalized with PDGF-receptor β (PDGFRβ) in the injury models as well as in human and rat HSC cell lines. In human HSCs, inhibition of NRP1 using siRNA reduced PDGF-induced motility independently of VEGF receptor and SEMA3. By contrast, NRP1 overexpression increased cell motility and TGF-β-dependent collagen production. Similarly, HSCs from mice lacking NRP1 exhibited reduced migration in response to PDGF treatment. More importantly, an NRP1-neutralizing antibody improved recruitment of HSCs and inhibited liver fibrosis in a rat model, and it also diminished VEGF responses in cultured liver ECs. In addition, this antibody decreased VEGF-induced angiogenesis, which implicates NRP1 as a regulatory target of angiogenesis. Notably, the influence of the NRP1-neutralizing antibody was more prominent in CCL4-induced liver injury than in bile duct-ligated liver damage, warranting further studies of the transcriptional regulation of NRP1 in different models of liver fibrosis. NRP1 overexpression was also observed in liver specimens from patients with cirrhosis related to hepatitis C and steatohepatitis. All of these findings indicate that NRP1 contributes to the progression of liver fibrosis through either its influence on angiogenesis or its effects on PDGF and TGF-β signaling pathways.
Anti-angiogenic therapy may be promising in CLDs, but large gaps remain in our understanding and ability to treat angiogenesis during the progression of liver fibrosis[90,91,93,94]. Similar efforts have been directed toward the development of new therapies, particularly the growth factor pathways involved in the activation of HSCs, especially the PDGF and TGF-β pathways, in the prevention of fibrosis in CLDs[90,91,94]. However, the targeting of these molecules on an individual basis has only limited therapeutic effect, necessitating the identification of new molecules that can simultaneously target multiple growth factor signaling pathways[90-92]. Thus, NRP1, with its considerable synergistic effects on PDGF, TGF-β and VEGF in liver fibrosis, appears to be a promising therapeutic target for future antifibrotic therapies.
In conclusion, NRPs are transmembrane glycoproteins that utilize several general mechanisms to exert pleiotropic functions in the integration of different critical pathways under physiological and pathological conditions. In addition to the central roles of NRPs in angiogenesis and axonal guidance, their contribution to the regulation of the immune system and fibrosis renders them attractive therapeutic targets for many non-neoplastic diseases and cancers.
The number of studies in liver is too small to determine the precise role of NRPs, but evidence supports an association with tumor behavior, LR and the progression of fibrosis in CLDs. Increased knowledge of the role of NRPs and their signaling pathways in primary liver tumors could promote the development of new therapeutic treatment strategies. Moreover, the expression of NRPs in HCC might constitute a useful tool in the histopathological differential diagnosis of this disease as well as a valuable prognostic parameter in disease monitoring. Therefore, further experimental and clinical studies in a large number of patients are necessary to clarify whether NRPs, especially NRP1, may help to address the overwhelming challenges in current therapy for primary liver tumors, such as resistance and metastasis.
Current findings regarding the interplay between NRPs and VEGF and between PDGF and TGF-β support NRPs as potential targets in the prevention of fibrogenesis in CLDs. Because NRPs act as coreceptors for many growth factors in liver, blockade of NRPs might make it possible to simultaneously inhibit multiple growth factors, such as VEGF, PDGF, and TGF-β. Therefore, the association of NRPs with growth factor signaling pathways in the liver requires further investigation to provide useful information for the targeting of NRPs in the prevention of fibrosis in CLDs.
In conclusion although limited data exist about the role of NRPs in liver diseases, our current understandings suggest that they might contribute to sinusoidal remodeling during LR, warranting further studies to better clarify the interplay of NRP1 and other coreceptors in LSECs and other parenchymal cells. The finding about the association of NRPs with malignant transformation and aggressive behavior of liver tumors should be supported by large scaled studies. Finally, besides its role in angiogenesis, especially NRP1 with its considerable synergistic effects on PDGF, TGF-β and VEGF appears to be a promising therapeutic target for future antifibrotic therapies in CLDs. However more studies are needed to conclude if blocking of NRPs will present a new approach to target multiple growth factor pathways in the prevention of liver fibrosis.
P- Reviewer: Lu WY, Peltec A S- Editor: Qi Y L- Editor: A E- Editor: Liu XM
| 1. | Narayanan N, Su N, Bedard P. Inhibitory and stimulatory effects of fluoride on the calcium pump of cardiac sarcoplasmic reticulum. Biochim Biophys Acta. 1991;1070:83-91. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 265] [Cited by in F6Publishing: 281] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 2. | Takagi S, Tsuji T, Amagai T, Takamatsu T, Fujisawa H. Specific cell surface labels in the visual centers of Xenopus laevis tadpole identified using monoclonal antibodies. Dev Biol. 1987;122:90-100. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 119] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 3. | Giger RJ, Urquhart ER, Gillespie SK, Levengood DV, Ginty DD, Kolodkin AL. Neuropilin-2 is a receptor for semaphorin IV: insight into the structural basis of receptor function and specificity. Neuron. 1998;21:1079-1092. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 270] [Cited by in F6Publishing: 293] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 4. | Giger RJ, Cloutier JF, Sahay A, Prinjha RK, Levengood DV, Moore SE, Pickering S, Simmons D, Rastan S, Walsh FS. Neuropilin-2 is required in vivo for selective axon guidance responses to secreted semaphorins. Neuron. 2000;25:29-41. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 319] [Cited by in F6Publishing: 353] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 5. | Kolodkin AL, Ginty DD. Steering clear of semaphorins: neuropilins sound the retreat. Neuron. 1997;19:1159-1162. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 891] [Cited by in F6Publishing: 916] [Article Influence: 33.9] [Reference Citation Analysis (0)] |
| 6. | He Z, Tessier-Lavigne M. Neuropilin is a receptor for the axonal chemorepellent Semaphorin III. Cell. 1997;90:739-751. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 873] [Cited by in F6Publishing: 888] [Article Influence: 32.9] [Reference Citation Analysis (0)] |
| 7. | Takagi S, Kasuya Y, Shimizu M, Matsuura T, Tsuboi M, Kawakami A, Fujisawa H. Expression of a cell adhesion molecule, neuropilin, in the developing chick nervous system. Dev Biol. 1995;170:207-222. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 153] [Cited by in F6Publishing: 160] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 8. | Fujisawa H. Discovery of semaphorin receptors, neuropilin and plexin, and their functions in neural development. J Neurobiol. 2004;59:24-33. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 129] [Cited by in F6Publishing: 137] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 9. | Kitsukawa T, Shimono A, Kawakami A, Kondoh H, Fujisawa H. Overexpression of a membrane protein, neuropilin, in chimeric mice causes anomalies in the cardiovascular system, nervous system and limbs. Development. 1995;121:4309-4318. [PubMed] [Cited in This Article: ] |
| 10. | Plein A, Fantin A, Ruhrberg C. Neuropilin regulation of angiogenesis, arteriogenesis, and vascular permeability. Microcirculation. 2014;21:315-323. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 92] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 11. | Kawasaki T, Kitsukawa T, Bekku Y, Matsuda Y, Sanbo M, Yagi T, Fujisawa H. A requirement for neuropilin-1 in embryonic vessel formation. Development. 1999;126:4895-4902. [PubMed] [Cited in This Article: ] |
| 12. | Eichmann A, Makinen T, Alitalo K. Neural guidance molecules regulate vascular remodeling and vessel navigation. Genes Dev. 2005;19:1013-1021. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 193] [Cited by in F6Publishing: 203] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 13. | Staton CA, Kumar I, Reed MW, Brown NJ. Neuropilins in physiological and pathological angiogenesis. J Pathol. 2007;212:237-248. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 165] [Cited by in F6Publishing: 176] [Article Influence: 10.4] [Reference Citation Analysis (1)] |
| 14. | Uniewicz KA, Fernig DG. Neuropilins: a versatile partner of extracellular molecules that regulate development and disease. Front Biosci. 2008;13:4339-4360. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 47] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 15. | Rizzolio S, Tamagnone L. Multifaceted role of neuropilins in cancer. Curr Med Chem. 2011;18:3563-3575. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 65] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 16. | Grandclement C, Borg C. Neuropilins: a new target for cancer therapy. Cancers (Basel). 2011;3:1899-1928. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 69] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 17. | Jubb AM, Strickland LA, Liu SD, Mak J, Schmidt M, Koeppen H. Neuropilin-1 expression in cancer and development. J Pathol. 2012;226:50-60. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 136] [Cited by in F6Publishing: 152] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 18. | Zachary IC. How neuropilin-1 regulates receptor tyrosine kinase signalling: the knowns and known unknowns. Biochem Soc Trans. 2011;39:1583-1591. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 19. | Wild JR, Staton CA, Chapple K, Corfe BM. Neuropilins: expression and roles in the epithelium. Int J Exp Pathol. 2012;93:81-103. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 96] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 20. | Raimondi C, Ruhrberg C. Neuropilin signalling in vessels, neurons and tumours. Semin Cell Dev Biol. 2013;24:172-178. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 21. | Chaudhary B, Khaled YS, Ammori BJ, Elkord E. Neuropilin 1: function and therapeutic potential in cancer. Cancer Immunol Immunother. 2014;63:81-99. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 167] [Cited by in F6Publishing: 152] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 22. | Prud’homme GJ, Glinka Y. Neuropilins are multifunctional coreceptors involved in tumor initiation, growth, metastasis and immunity. Oncotarget. 2012;3:921-939. [PubMed] [Cited in This Article: ] |
| 23. | Nakamura F, Goshima Y. Structural and functional relation of neuropilins. Adv Exp Med Biol. 2002;515:55-69. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 62] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 24. | Rossignol M, Gagnon ML, Klagsbrun M. Genomic organization of human neuropilin-1 and neuropilin-2 genes: identification and distribution of splice variants and soluble isoforms. Genomics. 2000;70:211-222. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 137] [Cited by in F6Publishing: 145] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 25. | Gaboriaud C, Gregory-Pauron L, Teillet F, Thielens NM, Bally I, Arlaud GJ. Structure and properties of the Ca(2+)-binding CUB domain, a widespread ligand-recognition unit involved in major biological functions. Biochem J. 2011;439:185-193. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 26. | Fuentes-Prior P, Fujikawa K, Pratt KP. New insights into binding interfaces of coagulation factors V and VIII and their homologues lessons from high resolution crystal structures. Curr Protein Pept Sci. 2002;3:313-339. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 58] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 27. | Kiedzierska A, Smietana K, Czepczynska H, Otlewski J. Structural similarities and functional diversity of eukaryotic discoidin-like domains. Biochim Biophys Acta. 2007;1774:1069-1078. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 70] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 28. | Gu C, Limberg BJ, Whitaker GB, Perman B, Leahy DJ, Rosenbaum JS, Ginty DD, Kolodkin AL. Characterization of neuropilin-1 structural features that confer binding to semaphorin 3A and vascular endothelial growth factor 165. J Biol Chem. 2002;277:18069-18076. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 180] [Cited by in F6Publishing: 186] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 29. | Roth L, Nasarre C, Dirrig-Grosch S, Aunis D, Crémel G, Hubert P, Bagnard D. Transmembrane domain interactions control biological functions of neuropilin-1. Mol Biol Cell. 2008;19:646-654. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 75] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 30. | Yazdani U, Terman JR. The semaphorins. Genome Biol. 2006;7:211. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 303] [Cited by in F6Publishing: 324] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 31. | Schuch G, Machluf M, Bartsch G, Nomi M, Richard H, Atala A, Soker S. In vivo administration of vascular endothelial growth factor (VEGF) and its antagonist, soluble neuropilin-1, predicts a role of VEGF in the progression of acute myeloid leukemia in vivo. Blood. 2002;100:4622-4628. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 112] [Cited by in F6Publishing: 118] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 32. | Janssen BJ, Malinauskas T, Weir GA, Cader MZ, Siebold C, Jones EY. Neuropilins lock secreted semaphorins onto plexins in a ternary signaling complex. Nat Struct Mol Biol. 2012;19:1293-1299. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 149] [Cited by in F6Publishing: 124] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 33. | Lepelletier Y, Moura IC, Hadj-Slimane R, Renand A, Fiorentino S, Baude C, Shirvan A, Barzilai A, Hermine O. Immunosuppressive role of semaphorin-3A on T cell proliferation is mediated by inhibition of actin cytoskeleton reorganization. Eur J Immunol. 2006;36:1782-1793. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 123] [Cited by in F6Publishing: 138] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 34. | Delgoffe GM, Woo SR, Turnis ME, Gravano DM, Guy C, Overacre AE, Bettini ML, Vogel P, Finkelstein D, Bonnevier J. Stability and function of regulatory T cells is maintained by a neuropilin-1-semaphorin-4a axis. Nature. 2013;501:252-256. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 382] [Cited by in F6Publishing: 423] [Article Influence: 38.5] [Reference Citation Analysis (0)] |
| 35. | Serini G, Valdembri D, Zanivan S, Morterra G, Burkhardt C, Caccavari F, Zammataro L, Primo L, Tamagnone L, Logan M. Class 3 semaphorins control vascular morphogenesis by inhibiting integrin function. Nature. 2003;424:391-397. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 467] [Cited by in F6Publishing: 441] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 36. | Guttmann-Raviv N, Shraga-Heled N, Varshavsky A, Guimaraes-Sternberg C, Kessler O, Neufeld G. Semaphorin-3A and semaphorin-3F work together to repel endothelial cells and to inhibit their survival by induction of apoptosis. J Biol Chem. 2007;282:26294-26305. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 165] [Cited by in F6Publishing: 177] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 37. | Mackenzie F, Ruhrberg C. Diverse roles for VEGF-A in the nervous system. Development. 2012;139:1371-1380. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 188] [Cited by in F6Publishing: 202] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 38. | Djordjevic S, Driscoll PC. Targeting VEGF signalling via the neuropilin co-receptor. Drug Discov Today. 2013;18:447-455. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 77] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 39. | Fuh G, Garcia KC, de Vos AM. The interaction of neuropilin-1 with vascular endothelial growth factor and its receptor flt-1. J Biol Chem. 2000;275:26690-26695. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 99] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 40. | Soker S, Miao HQ, Nomi M, Takashima S, Klagsbrun M. VEGF165 mediates formation of complexes containing VEGFR-2 and neuropilin-1 that enhance VEGF165-receptor binding. J Cell Biochem. 2002;85:357-368. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 342] [Cited by in F6Publishing: 337] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 41. | Whitaker GB, Limberg BJ, Rosenbaum JS. Vascular endothelial growth factor receptor-2 and neuropilin-1 form a receptor complex that is responsible for the differential signaling potency of VEGF(165) and VEGF(121). J Biol Chem. 2001;276:25520-25531. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 220] [Cited by in F6Publishing: 226] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 42. | Favier B, Alam A, Barron P, Bonnin J, Laboudie P, Fons P, Mandron M, Herault JP, Neufeld G, Savi P. Neuropilin-2 interacts with VEGFR-2 and VEGFR-3 and promotes human endothelial cell survival and migration. Blood. 2006;108:1243-1250. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 203] [Cited by in F6Publishing: 213] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 43. | Caunt M, Mak J, Liang WC, Stawicki S, Pan Q, Tong RK, Kowalski J, Ho C, Reslan HB, Ross J. Blocking neuropilin-2 function inhibits tumor cell metastasis. Cancer Cell. 2008;13:331-342. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 267] [Cited by in F6Publishing: 277] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 44. | Kärpänen T, Heckman CA, Keskitalo S, Jeltsch M, Ollila H, Neufeld G, Tamagnone L, Alitalo K. Functional interaction of VEGF-C and VEGF-D with neuropilin receptors. FASEB J. 2006;20:1462-1472. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 221] [Cited by in F6Publishing: 214] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 45. | West DC, Rees CG, Duchesne L, Patey SJ, Terry CJ, Turnbull JE, Delehedde M, Heegaard CW, Allain F, Vanpouille C. Interactions of multiple heparin binding growth factors with neuropilin-1 and potentiation of the activity of fibroblast growth factor-2. J Biol Chem. 2005;280:13457-13464. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 123] [Cited by in F6Publishing: 127] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 46. | Matsushita A, Götze T, Korc M. Hepatocyte growth factor-mediated cell invasion in pancreatic cancer cells is dependent on neuropilin-1. Cancer Res. 2007;67:10309-10316. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 100] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 47. | Sulpice E, Plouët J, Bergé M, Allanic D, Tobelem G, Merkulova-Rainon T. Neuropilin-1 and neuropilin-2 act as coreceptors, potentiating proangiogenic activity. Blood. 2008;111:2036-2045. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 126] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 48. | Evans IM, Yamaji M, Britton G, Pellet-Many C, Lockie C, Zachary IC, Frankel P. Neuropilin-1 signaling through p130Cas tyrosine phosphorylation is essential for growth factor-dependent migration of glioma and endothelial cells. Mol Cell Biol. 2011;31:1174-1185. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 87] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 49. | Valdembri D, Caswell PT, Anderson KI, Schwarz JP, König I, Astanina E, Caccavari F, Norman JC, Humphries MJ, Bussolino F. Neuropilin-1/GIPC1 signaling regulates alpha5beta1 integrin traffic and function in endothelial cells. PLoS Biol. 2009;7:e25. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 210] [Cited by in F6Publishing: 226] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 50. | Fukasawa M, Matsushita A, Korc M. Neuropilin-1 interacts with integrin beta1 and modulates pancreatic cancer cell growth, survival and invasion. Cancer Biol Ther. 2007;6:1173-1180. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 90] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 51. | Robinson SD, Reynolds LE, Kostourou V, Reynolds AR, da Silva RG, Tavora B, Baker M, Marshall JF, Hodivala-Dilke KM. Alphav beta3 integrin limits the contribution of neuropilin-1 to vascular endothelial growth factor-induced angiogenesis. J Biol Chem. 2009;284:33966-33981. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 78] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 52. | Glinka Y, Prud’homme GJ. Neuropilin-1 is a receptor for transforming growth factor beta-1, activates its latent form, and promotes regulatory T cell activity. J Leukoc Biol. 2008;84:302-310. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 177] [Cited by in F6Publishing: 195] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 53. | Glinka Y, Stoilova S, Mohammed N, Prud’homme GJ. Neuropilin-1 exerts co-receptor function for TGF-beta-1 on the membrane of cancer cells and enhances responses to both latent and active TGF-beta. Carcinogenesis. 2011;32:613-621. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 125] [Cited by in F6Publishing: 128] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 54. | Cao Y, Szabolcs A, Dutta SK, Yaqoob U, Jagavelu K, Wang L, Leof EB, Urrutia RA, Shah VH, Mukhopadhyay D. Neuropilin-1 mediates divergent R-Smad signaling and the myofibroblast phenotype. J Biol Chem. 2010;285:31840-31848. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 67] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 55. | Rizzolio S, Rabinowicz N, Rainero E, Lanzetti L, Serini G, Norman J, Neufeld G, Tamagnone L. Neuropilin-1-dependent regulation of EGF-receptor signaling. Cancer Res. 2012;72:5801-5811. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 75] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 56. | Banerjee S, Sengupta K, Dhar K, Mehta S, D’Amore PA, Dhar G, Banerjee SK. Breast cancer cells secreted platelet-derived growth factor-induced motility of vascular smooth muscle cells is mediated through neuropilin-1. Mol Carcinog. 2006;45:871-880. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 74] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 57. | Pellet-Many C, Frankel P, Evans IM, Herzog B, Jünemann-Ramírez M, Zachary IC. Neuropilin-1 mediates PDGF stimulation of vascular smooth muscle cell migration and signalling via p130Cas. Biochem J. 2011;435:609-618. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 109] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 58. | Ball SG, Bayley C, Shuttleworth CA, Kielty CM. Neuropilin-1 regulates platelet-derived growth factor receptor signalling in mesenchymal stem cells. Biochem J. 2010;427:29-40. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 66] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 59. | Cao S, Yaqoob U, Das A, Shergill U, Jagavelu K, Huebert RC, Routray C, Abdelmoneim S, Vasdev M, Leof E. Neuropilin-1 promotes cirrhosis of the rodent and human liver by enhancing PDGF/TGF-beta signaling in hepatic stellate cells. J Clin Invest. 2010;120:2379-2394. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 120] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 60. | Dhar K, Dhar G, Majumder M, Haque I, Mehta S, Van Veldhuizen PJ, Banerjee SK, Banerjee S. Tumor cell-derived PDGF-B potentiates mouse mesenchymal stem cells-pericytes transition and recruitment through an interaction with NRP-1. Mol Cancer. 2010;9:209. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 61. | Jia Y, Wang Y, Xie J. The Hedgehog pathway: role in cell differentiation, polarity and proliferation. Arch Toxicol. 2015;89:179-191. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 55] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 62. | Macdonald TJ. Hedgehog Pathway in Pediatric Cancers: They’re Not Just for Brain Tumors Anymore. Am Soc Clin Oncol Educ Book. 2012;605-609. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 63. | Goel HL, Pursell B, Chang C, Shaw LM, Mao J, Simin K, Kumar P, Vander Kooi CW, Shultz LD, Greiner DL. GLI1 regulates a novel neuropilin-2/α6β1 integrin based autocrine pathway that contributes to breast cancer initiation. EMBO Mol Med. 2013;5:488-508. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 111] [Cited by in F6Publishing: 120] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 64. | Tsai CL, Hsu FM, Tzen KY, Liu WL, Cheng AL, Cheng JC. Sonic Hedgehog Inhibition as a Strategy to Augment Radiosensitivity of Hepatocellular Carcinoma. J Gastroenterol Hepatol. 2015;Epub ahead of print. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 65. | Grzelak CA, Martelotto LG, Sigglekow ND, Patkunanathan B, Ajami K, Calabro SR, Dwyer BJ, Tirnitz-Parker JE, Watkins DN, Warner FJ. The intrahepatic signalling niche of hedgehog is defined by primary cilia positive cells during chronic liver injury. J Hepatol. 2014;60:143-151. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 66. | Pereira Tde A, Witek RP, Syn WK, Choi SS, Bradrick S, Karaca GF, Agboola KM, Jung Y, Omenetti A, Moylan CA. Viral factors induce Hedgehog pathway activation in humans with viral hepatitis, cirrhosis, and hepatocellular carcinoma. Lab Invest. 2010;90:1690-1703. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 95] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 67. | Witek RP, Yang L, Liu R, Jung Y, Omenetti A, Syn WK, Choi SS, Cheong Y, Fearing CM, Agboola KM. Liver cell-derived microparticles activate hedgehog signaling and alter gene expression in hepatic endothelial cells. Gastroenterology. 2009;136:320-330.e2. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 153] [Cited by in F6Publishing: 166] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 68. | Hillman RT, Feng BY, Ni J, Woo WM, Milenkovic L, Hayden Gephart MG, Teruel MN, Oro AE, Chen JK, Scott MP. Neuropilins are positive regulators of Hedgehog signal transduction. Genes Dev. 2011;25:2333-2346. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 60] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 69. | Cao Y, Wang L, Nandy D, Zhang Y, Basu A, Radisky D, Mukhopadhyay D. Neuropilin-1 upholds dedifferentiation and propagation phenotypes of renal cell carcinoma cells by activating Akt and sonic hedgehog axes. Cancer Res. 2008;68:8667-8672. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 75] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 70. | Teesalu T, Sugahara KN, Kotamraju VR, Ruoslahti E. C-end rule peptides mediate neuropilin-1-dependent cell, vascular, and tissue penetration. Proc Natl Acad Sci USA. 2009;106:16157-16162. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 555] [Cited by in F6Publishing: 595] [Article Influence: 39.7] [Reference Citation Analysis (0)] |
| 71. | Sugahara KN, Teesalu T, Karmali PP, Kotamraju VR, Agemy L, Girard OM, Hanahan D, Mattrey RF, Ruoslahti E. Tissue-penetrating delivery of compounds and nanoparticles into tumors. Cancer Cell. 2009;16:510-520. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 897] [Cited by in F6Publishing: 815] [Article Influence: 54.3] [Reference Citation Analysis (0)] |
| 72. | Sugahara KN, Teesalu T, Karmali PP, Kotamraju VR, Agemy L, Greenwald DR, Ruoslahti E. Coadministration of a tumor-penetrating peptide enhances the efficacy of cancer drugs. Science. 2010;328:1031-1035. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 845] [Cited by in F6Publishing: 775] [Article Influence: 55.4] [Reference Citation Analysis (0)] |
| 73. | Haspel N, Zanuy D, Nussinov R, Teesalu T, Ruoslahti E, Aleman C. Binding of a C-end rule peptide to the neuropilin-1 receptor: a molecular modeling approach. Biochemistry. 2011;50:1755-1762. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 61] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 74. | Roth L, Agemy L, Kotamraju VR, Braun G, Teesalu T, Sugahara KN, Hamzah J, Ruoslahti E. Transtumoral targeting enabled by a novel neuropilin-binding peptide. Oncogene. 2012;31:3754-3763. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 165] [Cited by in F6Publishing: 176] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 75. | Hong TM, Chen YL, Wu YY, Yuan A, Chao YC, Chung YC, Wu MH, Yang SC, Pan SH, Shih JY. Targeting neuropilin 1 as an antitumor strategy in lung cancer. Clin Cancer Res. 2007;13:4759-4768. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 133] [Cited by in F6Publishing: 142] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 76. | Jia H, Cheng L, Tickner M, Bagherzadeh A, Selwood D, Zachary I. Neuropilin-1 antagonism in human carcinoma cells inhibits migration and enhances chemosensitivity. Br J Cancer. 2010;102:541-552. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 80] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 77. | Liao YL, Sun YM, Chau GY, Chau YP, Lai TC, Wang JL, Horng JT, Hsiao M, Tsou AP. Identification of SOX4 target genes using phylogenetic footprinting-based prediction from expression microarrays suggests that overexpression of SOX4 potentiates metastasis in hepatocellular carcinoma. Oncogene. 2008;27:5578-5589. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 146] [Cited by in F6Publishing: 160] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 78. | Raskopf E, Vogt A, Decker G, Hirt S, Daskalow K, Cramer T, Standop J, Gonzalez-Carmona MA, Sauerbruch T, Schmitz V. Combination of hypoxia and RNA-interference targeting VEGF induces apoptosis in hepatoma cells via autocrine mechanisms. Curr Pharm Biotechnol. 2012;13:2290-2298. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 79. | Bergé M, Bonnin P, Sulpice E, Vilar J, Allanic D, Silvestre JS, Lévy BI, Tucker GC, Tobelem G, Merkulova-Rainon T. Small interfering RNAs induce target-independent inhibition of tumor growth and vasculature remodeling in a mouse model of hepatocellular carcinoma. Am J Pathol. 2010;177:3192-3201. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 80. | Xu J, Xia J. NRP-1 silencing suppresses hepatocellular carcinoma cell growth in vitro and in vivo. Exp Ther Med. 2013;5:150-154. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 81. | Bergé M, Allanic D, Bonnin P, de Montrion C, Richard J, Suc M, Boivin JF, Contrerès JO, Lockhart BP, Pocard M. Neuropilin-1 is upregulated in hepatocellular carcinoma and contributes to tumour growth and vascular remodelling. J Hepatol. 2011;55:866-875. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 82. | Yaqoob U, Cao S, Shergill U, Jagavelu K, Geng Z, Yin M, de Assuncao TM, Cao Y, Szabolcs A, Thorgeirsson S. Neuropilin-1 stimulates tumor growth by increasing fibronectin fibril assembly in the tumor microenvironment. Cancer Res. 2012;72:4047-4059. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 88] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 83. | Zhuang PY, Wang JD, Tang ZH, Zhou XP, Yang Y, Quan ZW, Liu YB, Shen J. Peritumoral Neuropilin-1 and VEGF receptor-2 expression increases time to recurrence in hepatocellular carcinoma patients undergoing curative hepatectomy. Oncotarget. 2014;5:11121-11132. [PubMed] [Cited in This Article: ] |
| 84. | Hansel DE, Wilentz RE, Yeo CJ, Schulick RD, Montgomery E, Maitra A. Expression of neuropilin-1 in high-grade dysplasia, invasive cancer, and metastases of the human gastrointestinal tract. Am J Surg Pathol. 2004;28:347-356. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 102] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 85. | Kraizer Y, Mawasi N, Seagal J, Paizi M, Assy N, Spira G. Vascular endothelial growth factor and angiopoietin in liver regeneration. Biochem Biophys Res Commun. 2001;287:209-215. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 51] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 86. | Braet F, Shleper M, Paizi M, Brodsky S, Kopeiko N, Resnick N, Spira G. Liver sinusoidal endothelial cell modulation upon resection and shear stress in vitro. Comp Hepatol. 2004;3:7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 40] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 87. | Kumamoto T, Togo S, Ishibe A, Morioka D, Watanabe K, Takahashi T, Shimizu T, Matsuo K, Kubota T, Tanaka K. Role of nitric oxide synthesized by nitric oxide synthase 2 in liver regeneration. Liver Int. 2008;28:865-877. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 88. | Fu L, Kitamura T, Iwabuchi K, Ichinose S, Yanagida M, Ogawa H, Watanabe S, Maruyama T, Suyama M, Takamori K. Interplay of neuropilin-1 and semaphorin 3A after partial hepatectomy in rats. World J Gastroenterol. 2012;18:5034-5041. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 7] [Cited by in F6Publishing: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 89. | Panigrahy D, Adini I, Mamluk R, Levonyak N, Bruns CJ, D’Amore PA, Klagsbrun M, Bielenberg DR. Regulation of soluble neuropilin 1, an endogenous angiogenesis inhibitor, in liver development and regeneration. Pathology. 2014;46:416-423. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 90. | Elpek GÖ. Cellular and molecular mechanisms in the pathogenesis of liver fibrosis: An update. World J Gastroenterol. 2014;20:7260-7276. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 240] [Cited by in F6Publishing: 242] [Article Influence: 24.2] [Reference Citation Analysis (4)] |
| 91. | Kukla M. Angiogenesis: a phenomenon which aggravates chronic liver disease progression. Hepatology Int. 2013;7:4-12. [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 92. | Troeger JS, Schwabe RF. Neuropilin and liver fibrosis: hitting three birds with one stone? Hepatology. 2011;54:1091-1093. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 93. | Cannito S, Paternostro C, Busletta C, Bocca C, Colombatto S, Miglietta A, Novo E, Parola M. Hypoxia, hypoxia-inducible factors and fibrogenesis in chronic liver diseases. Histol Histopathol. 2014;29:33-44. [PubMed] [Cited in This Article: ] |
| 94. | Povero D, Busletta C, Novo E, di Bonzo LV, Cannito S, Paternostro C, Parola M. Liver fibrosis: a dynamic and potentially reversible process. Histol Histopathol. 2010;25:1075-1091. [PubMed] [Cited in This Article: ] |