Published online Jun 14, 2015. doi: 10.3748/wjg.v21.i22.6820
Peer-review started: January 22, 2015
First decision: March 10, 2015
Revised: March 31, 2015
Accepted: May 7, 2015
Article in press: May 7, 2015
Published online: June 14, 2015
Processing time: 148 Days and 0.9 Hours
Non-alcoholic fatty liver disease (NAFLD) is considered to be an independent cardiovascular disease (CVD) risk factor. However, simple steatosis has a benign clinical course without excess mortality. In contrast, the advanced form of NAFLD, non-alcoholic steatohepatitis (NASH) with liver fibrosis increases mortality by approximately 70%, due to an increase in CVD mortality by approximately 300%. Chronic kidney disease (CKD) may be caused by NAFLD/NASH and it substantially increases CVD risk, especially in the presence of type 2 diabetes mellitus. Moreover, CKD may trigger NAFLD/NASH deterioration in a vicious cycle. NAFLD/NASH is also related to increased arterial stiffness (AS), an independent CVD risk factor that further raises CVD risk. Diagnosis of advanced liver fibrosis (mainly by simple non-invasive tests), CKD, and increased AS should be made early in the course of NAFLD and treated appropriately. Lifestyle measures and statin treatment may help resolve NAFLD/NASH and beneficially affect the CVD risk factors mentioned above.
Core tip: Non-alcoholic fatty liver disease (NAFLD) is an independent cardiovascular disease (CVD) risk factor. However, simple steatosis has a rather benign clinical course, while its advanced form, non-alcoholic steatohepatitis (NASH) substantially increases total mortality, mainly due to increased CVD events. In this review we propose the use of statin treatment for NASH, given its beneficial effect on NAFLD/NASH and CVD risk. There are data suggesting biopsy proven amelioration of NASH and normalization in liver ultrasonography and enzyme values as well as improvement of chronic kidney disease and arterial stiffness that usually accompany NASH and exacerbate CVD risk.
- Citation: Athyros VG, Tziomalos K, Katsiki N, Doumas M, Karagiannis A, Mikhailidis DP. Cardiovascular risk across the histological spectrum and the clinical manifestations of non-alcoholic fatty liver disease: An update. World J Gastroenterol 2015; 21(22): 6820-6834
- URL: https://www.wjgnet.com/1007-9327/full/v21/i22/6820.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i22.6820
Non-alcoholic fatty liver disease (NAFLD), a term describing the most common liver disease (affects approximately 15%-30% of the general population in Western Countries), is characterized by accumulation of fat (> 5%) in liver cells in the absence of excessive alcohol intake, chronic viral hepatitis or other liver disease[1,2].
NAFLD has a high prevalence (75%-100%) in populations with pre-existing metabolic conditions characterized by insulin resistance (IR) such as obesity, metabolic syndrome (MetS) or type 2 diabetes mellitus (T2DM)[1,2]. NAFLD prevalence continues to increase due to the obesity and T2DM pandemic[1].
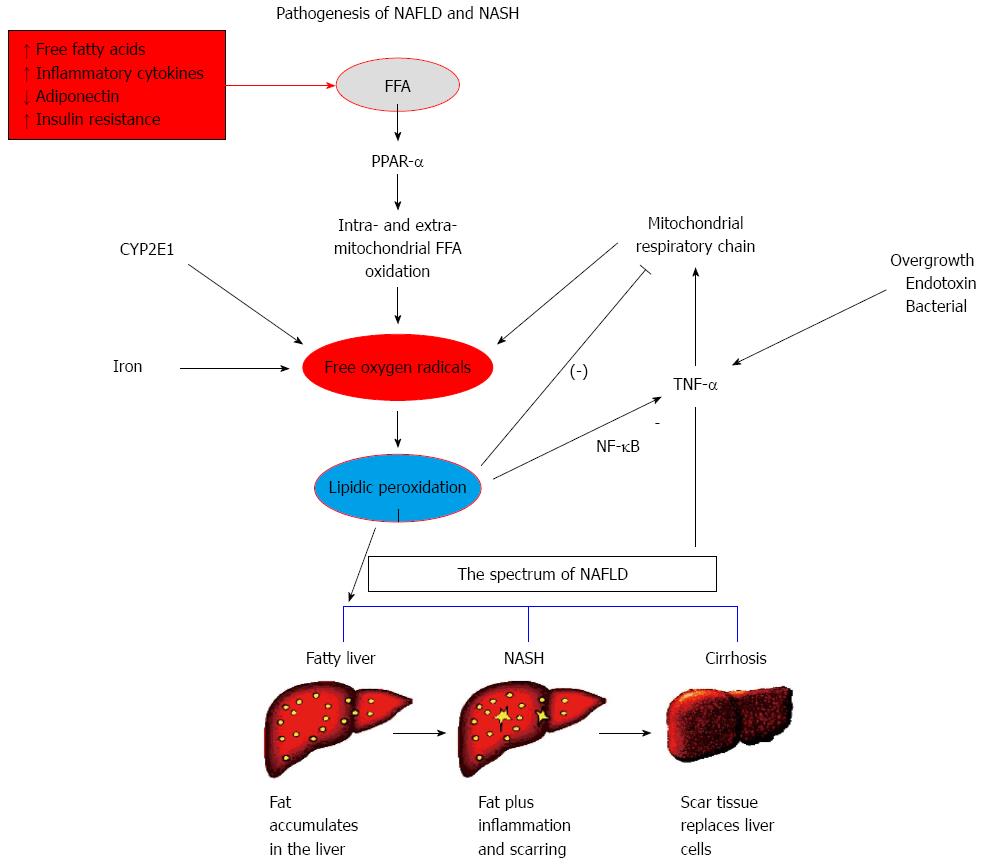
Histological manifestations of NAFLD range from simple steatosis, non-alcoholic steatohepatitis (NASH; characterized by hepatocellular necroinflammation and ballooning), liver fibrosis and cirrhosis, which in some cases may progress to hepatocellular carcinoma[3]. Despite its high prevalence, the pathogenesis of steatosis and the progression to NASH with fibrosis⁄cirrhosis, and the natural history of NAFLD are not yet entirely clear (Figure 1)[4]. The evidence[3] suggests that NASH prevalence ranges from 3%-5% (> 20% of NAFLD cases) in the general population, however this rises to 37% in the morbidly obese[5].
NAFLD/NASH is considered as the hepatic manifestation of MetS, and are closely related to cardiovascular disease (CVD)[6], to the extent that NAFLD/NASH and CVD are viewed as two aspects of a shared disease[6]. More patients with NASH die from CVD than from liver disease[6,7]. Nevertheless, CVD risk level is not the same across the entire histological and clinical spectrum of NAFLD[8]. It seems that simple steatosis and NASH are considered disease states of different CVD risk involvement, each with different consequences, linked to environmental and genetic factors[9,10]. The multiple parallel hit theory suggests that NASH may occur directly in many individuals without previous simple steatosis[9,10]. It is possible that NASH can happen in the absence of simple steatosis because inflammation related to NASH pathogenesis might originate in the gut microbiota, in response to the prime neutrophil chemokines and macrophage-inflammatory protein-2, inflamed adipose tissue and to circulating inflammatory cells. In any case, the identification and management of high CVD risk patients with NAFLD/NASH remains a clinical challenge[8]. This narrative review considers this key issue, referring only to recent advances in diagnosis, risk stratification and treatment of NAFLD/NASH[9,10].
The United States National Health and Nutrition Examination Survey (NHANES) was conducted in 1988-1994 and followed-up 11154 participants until the end of 2006 (mean follow up: 14.5 years). The findings suggest that the degree of liver fibrosis is related to clinical outcome in NAFLD/NASH patients[7]. The diagnosis of NAFLD was based on liver ultrasonography and the degree of liver fibrosis in NAFLD patients was determined without a biopsy by the NAFLD fibrosis score (NFS), the AST-platelet ratio index (APRI) and the FIB-4 score[7]. The ultrasonographic prevalence of NAFLD was 34% (if projected to the entire United States adult population this represents 43.2 million Americans), however, if only people with moderate to severe steatosis were included in NAFLD diagnosis its prevalence falls to 20.2%, projecting to 25.6 million United States adults[7]. The majority of NAFLD patients (71.7%) had simple steatosis, while 28.3% had NFS values suggesting an intermediate (25.1%) or high (3.2%) level of liver fibrosis[7]. These data project to 10.8 million United States adults with NAFLD with some evidence of advanced fibrosis, 9.4 million with an intermediate probability and 1.4 million with a high probability of fibrosis[7]. The 15 years follow-up showed that NAFLD in the form of steatosis was not related with higher total mortality compared with those without NAFLD (adjusted HR = 1.05, 95%CI: 0.93-1.19, P = NS)[7]. In contrast, there was a progressive increase in total mortality with increasing levels of liver fibrosis scores [adjusted HR = 1.69, 95%CI: 1.09-2.63 for the NFS, 1.85 (1.02-3.37) for the APRI, and 1.66 (0.98-2.82) for the FIB-4], as compared with subjects without fibrosis[7]. The increase in mortality was mainly due to increased CVD mortality (HR = 3.46, 95%CI: 1.91-6.25 for NFS; 2.53, 1.33-3.83 for APRI; 2.68, 1.44-4.99 for FIB-4)[7]. Thus, prospective data from this US representative cohort suggest that steatosis per se, does not increase total and CVD mortality, while NASH, with advanced fibrosis as evaluated by non-invasive markers, was related to increased total and (mainly) CVD mortality[7].
The fact that NAFLD at the stage of simple steatosis has a benign clinical course without excess mortality has been reported 10 years ago[11]. Also, the fact that NASH with liver fibrosis was related to increased overall and CVD mortality was established by studies with a long-term follow-up (up to 28 years)[12,13]. Moreover, fibrosis or even cirrhosis was found in 15%-50% of NASH patients on their index biopsy, suggesting that a good portion of NASH patients develop progressive liver disease that could lead to liver-related mortality[14,15]. A study reporting survival of biopsy proven NAFLD or alcoholic fatty liver disease (AFLD) after 24 and 20 years of follow-up, respectively, showed that CVD was the most common cause of death (48%) in NAFLD patients, while liver-related death was recorded in 7% of these patients[16]. In contrast, AFLD patients had a liver-related death rate of 36% and CVD death rate of 32%[16]. NASH patients with moderate or severe fibrosis at baseline showed a worse survival rate than patients with none or mild fibrosis at baseline (adjusted HR = 2.09, P = 0.01)[16].
Liver biopsy is the gold standard for the diagnosis of NAFLD or NASH[16]. The classification in liver biopsy is as follow: Steatosis should have a > 5% fat content in the liver biopsy. Fibrosis stage was defined as follows: 0 = none, 1 = perisinusoidal or periportal fibrosis, 1A = mild perisinusoidal fibrosis, 1B = moderate perisinusoidal fibrosis, 1C = portal/periportal fibrosis, 2 = perisinusoidal - portal/periportal fibrosis, 3 = bridging fibrosis, and, 4 = cirrhosis[17]. However, it is not possible to perform a liver biopsy in all patients with NAFLD (a considerable proportion of the general population); not all patients need a liver biopsy and many will not consent. Thus, non-invasive tests can be used instead to evaluate the stage of fibrosis and consequently the overall risk of these patients[18]. A recent study evaluated the usefulness of 4 validated non-invasive scoring systems that were originally designed to distinguish patients from those without advanced liver fibrosis in comparison with the results of liver biopsy[18]. Thus, 310 biopsy proven NAFLD/NASH patients were followed for median period of 105 mo. The 4 tests were the 3 used in the United States National Health and Nutrition Examination Survey[7], NFS, APRI, and FIB-4[19,20], plus the more recently introduced BARD score[21]. These are calculated using the original published formulas[19-21]. The score of these simple non-invasive tests were useful for the identification of NAFLD patients in this study who are at increased risk for total and liver-related mortality and predicted clinical outcome successfully[18]. These results were confirmed by a meta-analysis of 32 studies evaluating the diagnostic accuracy of these 4 non-invasive tests in comparison with the results of liver biopsy[22]. Results of this evaluation suggested an excellent sensitivity and specificity of these tests[22]. Thus, with the use of these 4 simple tests NAFLD patients can be evaluated for both CVD risk and progressive liver disease risk[22]. These tests can also divide NASH patients into those with and those without advanced fibrosis and therefore increased overall risk, indicating which patients need more intensive therapy[22]. It seems that these data were lost in the large number of papers on NAFLD, which has dramatically increased during the last 5 years. The vast majority of the papers or the majority of studies analyzed in a review on the NAFLD and CVD association, include NAFLD patients diagnosed by ultrasonography or alanine aminotransferase (ALT) levels[23,24]. These usually report an increased CVD risk in NAFLD patients, however it is not clear which NAFLD patients shape this risk: all NAFLD patients or those with (advanced) fibrosis? Only a few studies identify this association and also indicate NAFLD patients at risk of systemic complications, based on the 4 simple tests mentioned above to distinguish NAFLD patient with liver fibrosis[25]. One of these studies used the NFS and the FIB-4 scores to separate 1559 NAFLD patients that included those with high possibility of liver fibrosis (group 1) and those with a low probability (group 2)[25]. In group 1 the prevalence of CVD at baseline was 7.7% vs 2.3% (NFS, P = 0.002) and 9.0% vs 2.3% (FIB-4, P = 0.0012)[25]. The prevalence of T2DM at baseline was in group 1 in comparison with group 2, 31.5% vs 3.1% (NFS, P < 0.0001) and 17.0% vs 4.7% (FIB-4, P < 0.0001)[25]. New onset diabetes (NOD) prevalence was 4.5% vs 1.2% (NFS, P = 0.034) and 3.6% vs 1.2% (FIB-4, P = 0.11), and of CVD in these patients was 5.0% vs 0.9% (NFS, P = 0.0019) and 5.4% vs 0.9% (FIB-4, P = 0.0034)[25]. This study brings forward another issue: that of pre-existing T2DM and the risk of NOD according to the degree of liver fibrosis in NAFLD patients; diabetes further increases the risk of CVD morbidity and mortality in NAFLD and MetS patients[26]. This can also be seen from the other way around: in T2DM patients the prevalence of NAFLD is 75%-100%, of NASH 63%-87%, liver fibrosis 22%-60%, and advanced liver fibrosis (4%-9%)[27], according to the number of MetS components, visceral obesity, older age, increased duration of T2DM, and the presence of family history of T2DM[27]. This is a vicious cycle leading from NAFLD/NASH to T2DM and vice versa. In any case the presence of NAFLD, and especially with (advanced) liver fibrosis, in T2DM patients is associated with increased overall (CVD and liver-related) mortality[27]. Given that an increase in the adherence to multiple interventions in patients with T2DM is feasible and effective in better controlling the disease, as shown in a best practice study[28], and that similar measures may improve NAFLD/NASH[26] this is an interesting implication for the treatment of both diseases[28-31].
We need to abandon the idea that elevated liver enzymes and steatosis on ultrasonography alone could define CVD and liver-related risk; we need to calculate the overall risk of NAFLD/NASH patients with the use of the 4 simple tests, routinely available in clinical practice[32]. We need a pragmatic approach to diagnosis and staging of NAFLD so that patients at risk of complications can be identified[32]. This approach has implications for both diagnosis and treatment[33].
The prevalence of chronic kidney disease (CKD) among the general population in the United States is 13% (that of early stages of CKD, including stage 3, is 11%)[34], in Europe it is similar[35], while in Japan it is much higher; 20% of the adult population is estimated to have CKD stage 3-5 [glomerular filtration rate (GFR) < 60 mL/min per 1.73 m2][36]. In total, there seem to be more than 1.1 million patients with end-stage-renal disease (ESRD) worldwide[37]. CKD is an independent CVD risk factor substantially increasing its morbidity and mortality, to the degree that it is considered as a coronary heart disease (CHD) risk equivalent[38]. CVD is the leading cause of morbidity (40% of hospitalizations) and mortality (50% of deaths) in CKD patients[37,38]. Less than a half of CKD patients have develop ESRD, because most of them die from CVD before they develop ESRD[37,38].
NAFLD is considered to be a risk factor for CKD and is associated with an increased prevalence and incidence of CKD[37,39]. In a community-based study involving 2103 patients with T2DM the prevalence of stage 3 or higher of CKD was 15% among patients with ultrasound-diagnosed NAFLD vs 9% (P < 0.001) among T2DM patients without NAFLD, after adjustment for numerous baseline confounding factors and independent traditional CKD risk factors[40]. Another study evaluated 1361 subjects with an abnormal oral glucose tolerance test (OGTT) on routine screening[41]. Participants with ultrasound-diagnosed NAFLD had a higher prevalence of microalbuminuria compared with patients with impaired glucose tolerance who did not have NAFLD (19% vs 6.3% in abnormal OGTT subjects; 32.6% vs 4.5% in newly diagnosed T2DM patients; P < 0.0001) after adjusting for several classical risk factors[41]. These results suggest that NAFLD is a predictor of another CKD manifestation, microalbuminuria, in patients with prediabetes or T2DM[41].
Two studies from the NHANES 2001 through 2006 investigated the association between serum surrogate markers of NAFLD, gamma-glutamyl-transpeptidase (GGT) and bilirubin concentrations, and CKD in a (United States) nation-wide representative sample of 13188 adults[42,43]. Serum GGT elevation was associated with an increased odds of CKD (OR = 2.38, 95%CI: 2.02-2.80, P < 0.0001)[42], while total bilirubin levels were independently related with both decreasing estimated-GFR and increasing albuminuria in United States adults[43].
A recent review suggests that NAFLD, and mainly NASH, is related with an increased and independent risk of developing CVD, T2DM, CKD, and colorectal cancers[44]. Finally, in a meta-analysis, which included 33 studies with 63902 participants, NAFLD was associated with an increased risk of prevalent (OR = 2.12, 95%CI: 1.69-2.66) and incident (HR = 1.79, 95%CI: 1.65-1.95) CKD[45]. NASH was related to a higher prevalence (OR = 2.53, 95%CI: 1.58-4.05) and incidence (HR = 2.12, 95%CI: 1.42-3.17) of CKD than simple steatosis[45], and advanced fibrosis in NASH was associated with an even higher prevalence (OR = 5.20, 95%CI: 3.14-8.61) and incidence (HR = 3.29, 95%CI: 2.30-4.71) of CKD than NASH without advanced fibrosis[45]. These data suggest that the presence and the severity of NAFLD/NASH and advanced fibrosis are clearly related with an increased risk and the severity of CKD[45].
Recent studies suggest the NAFLD/NASH is characterized by inflammation of the liver which may secrete proinflammatory, pro-fibrogenic, and anti-fibrinolytic substances, including fetuin-A, tumor necrosis factor-alpha (TNF-α), and plasminogen activator inhibitor-1 (PAI-1), all causing kidney injury[46]. The above mentioned meta-analysis also found that even mild renal impairment may promote NAFLD generation, within a vicious cycle, with unfavourable CVD and metabolic consequences[45], suggesting that CKD per se may contribute to the pathogenesis of NAFLD[45,47]. It has been shown in male Sprague-Dawley rats that nephrectomy results in a substantial dysregulation of hepatic fatty acid metabolism, steatohepatitis, IR, glucose and lipid metabolism aberrations, early even prior to glomerulosclerosis and CKD development[47].
Moreover, in liver transplant recipients the odds of developing CKD is high, while the risk of death in these patients increases exponentially when GFR is < 30 mL/min per 1.73 m2 (HR = 2.67 and 5.47; 95%CI: 1.80-9.65, for stage 4 and 5, respectively)[48]. Given that available therapeutic options to reverse CKD are restricted, it should be emphasized that there is a need for hepatologists to diagnose CKD early in patients with chronic liver disease, mainly NAFLD, to optimize management aimed at delaying CKD progression[49]. In a large registry including 1120295 adults it was estimated that the adjusted HR for hospitalizations is high and for death in stage 5 CKD patients reaches 5.9 (95%CI: 5.4-6.5), while that for overt atherosclerotic CVD is 3.4 (95%CI: 3.1-3.8) compared with subjects with normal renal function[50], suggesting a low quality of life and a reduced life expectancy[50]. It has also been shown that stage 3 or higher diabetic nephropathy is related to an annual mortality rate of 20%, similar only to that of cancer[51]. If a patient has T2DM and CKD plus NAFLD/NASH (clinical practice suggests that almost all patients with diabetic nephropathy have NAFLD) the CVD burden is even greater[45,52,53].
Thus, NAFLD and CKD share some key cardiometabolic risk factors and it is suggested that they have common pathophysiological mechanisms; one can lead to the other and their co-existence leads to a geometrically increased CVD risk in patients that have both conditions, especially in those with metabolic disorders such as T2DM[37,39,45,52,53]. Given that there is available treatment of NAFLD[54-56] and CKD (up to stage 3)[57], that also prevents CVD or its complications[58,59], especially in patients with T2DM with increased CVD risk[60,61], hepatologists, nephrologists, and cardiologists should place additional emphasis on the early diagnosis of both NAFLD and CKD[62,63].
Another issue is that elevated serum uric acid (SUA) levels, related to renal function and T2DM[64-66], may play a role in the pathogenesis of NAFLD. There seems to be a link between elevated SUA levels and MetS/NAFLD[67-69]. Given that NAFLD, CKD, and elevated SUA levels are implicated in increased CVD risk, attention should be given to SUA within the treatment of NAFLD[68-71]. It has been reported that some lipid and blood pressure lowering treatments that decrease CVD risk and improve/preserve renal function, while improving NAFLD, reduce SUA levels with their off-target effects[68-73]. These specific drugs should be preferred for the treatment of NAFLD risk factors, such as arterial hypertension or dyslipidaemia.
NAFLD was found to be an independent predictor of faster progression of arterial stiffness (AS), even after adjusting for other CVD risk factors, thus further increasing CVD risk. Pulse Wave Velocity (PWV), a measure of AS, is independently associated with increased CVD risk across several patient groups and even in the general population, in both genders[74-76]. NAFLD is associated with AS, as evaluated by PWV, even in a non-obese, non-hypertensive, and non-diabetic young and middle-aged (Chinese) population[77]. NAFLD patients with severe liver fibrosis have the higher increase in AS[78]. Moreover, in patients with stage 3-5 CKD, PWV is much higher than in patients with CKD stage 1-2 and constitutes a major clinical determinant of CVD event rate and severity, independently of traditional CVD risk factors[79,80]. The pathogenesis of AS in NAFLD is not clear. One mechanism might be related to the systemic inflammation linked to NAFLD, and mainly NASH with fibrosis[78], which seems to have an adverse effect on arterial compliance; high-sensitivity C-reactive protein (hsCRP) and pro-inflammatory cytokines may have adverse effects on the elastic properties of the wall of large arteries[81]. Arterial compliance was low in NAFLD patients with elevated hsCRP, while NAFLD with low hsCRP had no effect on arterial compliance[81]. Central obesity was a vital determinant for both increased AS and elevated hsCRP levels in NAFLD patients[81]. Another issue is whole blood viscosity (WBV), a predictor of CVD events[82]. WBV was shown to be increased in NAFLD and to be independently related with AS, even after adjusting for other CVD risk factors[82]. This association between WBV and AS has been shown in T2DM patients also, which comprise a great portion of NAFLD patients[83]. Thus, detection of abnormal WBV and AS should be performed early within risk stratification in NAFLD patients[82].
There are data suggesting that the older theory on AS pathogenesis (AS is a result of large artery atherosclerosis) is not correct[84]. Stiff arteries suffer from arteriosclerosis, which is different from atherosclerosis, and this is also verified by the fact that there is little or no association between PWV and traditional CVD risk factors, except for age and arterial hypertension[84]. Additionally, PWV does not increase during the early stages of large artery atherosclerosis, but it is increased during the advanced atherosclerotic plaque period, probably due to arterial (aortic) calcification (AC)[84]. AC is a common complication of CKD and ESRD, and the extent of AC in the general population and in patients with CKD is predictive of subsequent CVD mortality beyond traditional CVD risk factors[85]. Thus, late atherosclerosis and CKD both promote AC and increase AS[84,85].
There is evidence that lifestyle changes, antidiabetic drugs, inducible nitric oxide synthesis, antihypertensive agents [mainly inhibitors of the renin-angiotensin-aldosterone system (RAAS)], and statins improve arterial elasticity in patients with a wide spectrum of diseases, such as CVD, CKD, T2DM, MetS, obesity, primary biliary cirrhosis, NAFLD, heart failure with preserved ejection fraction, arterial hypertension and dyslipidaemia[86-100]. Moreover, anti-inflammatory drugs, such as corticosteroids and anti-TNF-α therapy have been shown to improve arterial compliance in patients with chronic inflammatory conditions[101]. Overall a multifactorial approach appears to be the optimal solution for the management of increased AS due to NAFLD[26-31,89,90,93,97]. However, differences appear to exist within classes of agents, with some statins and RAAS inhibitors having a more favourable effects on AS[94-96,99,100].
Data from the Third NHANES (1988-1994) that included 15676 subjects from the United States suggest that the prevalence of ALT elevation was high in the United States, ranging from 7%-15% according to race[102]. In the majority of those with increased ALT levels this could be attributed to NAFLD, in the absence of alcohol abuse, viral hepatitis or hemochromatosis[102]. Moreover, unexplained ALT elevation (61% of cases) was strongly associated with adiposity and other features of MetS[102]. NHANES data collected from 1988 to 2008 showed that the prevalence of major causes of chronic liver disease remained stable, except for NAFLD, which increased steadily, alongside with the increase of the prevalence of metabolic diseases (MetS, T2DM)[103]. Given the increasing rates of obesity, NAFLD prevalence is expected to contribute substantially to the increased burden of chronic liver disease (and CVD) in the United States[103].
An observational study included 342 hyperlipidaemic patients with elevated transaminases who were prescribed a statin, 1437 hyperlipidaemic patients with normal transaminases who were prescribed a statin and 2245 patients with elevated liver enzymes but who were not prescribed a statin[104]. Patients with elevated transaminase levels were given atorvastatin or simvastatin at a median dose of 10 and 20 mg/d[104]. The incidence of further elevation in transaminase levels was similar in the two groups[104]. Among patients treated with a statin, the incidence of mild-moderate elevation in transaminase levels [< 10 times the upper limit of normal (ULN) or < 10 times the baseline transaminase levels] was higher in patients with elevated transaminases at baseline than in those with normal transaminase levels (n = 1437)[104]. However, the incidence of more marked elevation in transaminase levels (> 10 times the ULN) did not differ between the two groups[104]. Another observational study (n = 3399) in patients with elevated transaminase levels who were treated with lovastatin reported similar findings[105]. Moreover, > 112000 person-years of 5 year-exposure in double-blind randomized trials comparing placebo and pravastatin (40 mg once daily) showed low risk for hepatotoxicity[106].
In clinical practice up to 10 years ago it was “forbidden” to prescribe statins, even in high CVD risk patients, if they had a chronic liver disease, including NAFLD, and modestly elevated serum transaminases.
Eight years ago, given that there was no proven effective therapy for NAFLD, atorvastatin (10-80 mg/d) was tested in 25 NAFLD patients for the treatment of their dyslipidaemia; 22 patients completed the study[107]. After 6 mo of atorvastatin treatment, 8 patients (36.3%) had normal transaminase levels, while the remaining patients continued treatment for 12 more months[107]. During that period 20% of patients presented with normal transaminase levels, while the rest of the patients demonstrated a 10% reduction in baseline levels[107]. These results suggested that treatment with atorvastatin in NAFLD patients with dyslipidaemia resulted in a normalization of lipid profile and a significant reduction in serum ALT and this treatment was both effective and safe[107].
Seven years ago a study evaluated the efficacy of atorvastatin (10 mg daily) for 24 mo in the treatment of 31 patients with biopsy proven NASH and dyslipidaemia[108]. Follow-up liver biopsy was performed in 17 patients[108]. After treatment, 23 patients (74.2%) had normal transaminase levels, adiponectin levels were noticeably increased, and the levels of TNF-α were significantly reduced[108]. These results suggest that atorvastatin may have acted via a reduction in markers of systemic inflammation, such as TNF-α, as well as increased adiponectin levels[108], while another study suggested a beneficial effect of atorvastatin on NASH due to the reduction in serum levels of advanced glycosylated end products (AGEs), implicated in the pathogenesis of NASH[109]. However, 4/17 patients had progression of fibrosis in the repeat biopsy after 2-years and 3 of them progressed to stage 3[108]. The authors could not explain these divergent results and attributed them to sampling error, heterogeneity of the population, or untreated postprandial rise in triglyceride (TG) levels[108].
Six years ago a pilot study with 16 NASH patients showed that monotherapy with simvastatin does not seem to be an effective treatment for NASH[110]. From the 16 patients with biopsy proven NASH, 14 completed the study and 10 underwent follow-up biopsy after one year. Although there was a 26% low-density lipoprotein cholesterol (LDL-C) reduction in the simvastatin group as compared with placebo, there was no significant improvement in serum transaminases, hepatic steatosis, necroinflammatory activity or stage of fibrosis[110].
Five years ago, the post hoc analysis of the Greek Atorvastatin and Coronary Heart Disease Evaluation (GREACE) survival study (n = 1600; 437 patients had moderately abnormal liver tests at baseline probably due to NAFLD as indicated by liver ultrasonography and after exclusion of other liver diseases)[111] showed that 227 participants who were treated with statins (mainly atorvastatin, mean dose 24 mg/d) had a substantial improvement in liver tests, ALT, aspartate aminotransferase (AST), and GGT (P < 0.0001), whereas 210 not treated with a statin had a further increase of liver enzyme concentrations[111]. Statin treatment was safe in patients with CVD and NAFLD; only 1% discontinued treatment[110]. Thus, atorvastatin did not have any adverse effect on liver enzymes; on the contrary, it reduced them substantially and improved liver ultrasonography within the 3-year duration of the study[111].
Four years ago a study with pitavastatin (2 mg/d for 12 mo) in 20 patients with biopsy-proven NASH with dyslipidaemia was reported[112]. Liver enzymes and lipid profile were significantly improved, however NAFLD/NASH activity score and fibrosis stage did not change significantly in all patients (they improved in 54% and 42%, respectively) and 3 of the 13 patients with a repeat biopsy had progression of fibrosis during the treatment[112].
Three years ago a study included 42 biopsy-proven NASH patients treated with atorvastatin 10 mg/d for 12 mo. Atorvastatin significantly decreased liver transaminase, GGT, LDL-C, TGs, type IV collagen, and TNF-α levels, while it improved NAFLD activity score and increased liver to spleen density ratio[113].
During the same year a prospective study investigated the effect 2.5 mg/d rosuvastatin for 24 mo in 19 patients with biopsy-proven NASH with dyslipidaemia[114]. Transaminase levels, relatively low at the beginning, were not significantly changed during the treatment, while the lipid profile was significantly improved[114]. At the same time NAFLD activity score and fibrotic stage did not change significantly in all patients; they were improved in 33% and 33% of patients, and remained stable in 33% and 56% of patients, respectively, while 1 of 9 patients had progression of fibrosis during rosuvastatin treatment[114]. This result was attributed to the very low dose of rosuvastatin administered (2.5 mg/d)[114]. During the same year a post hoc analysis of the survival study Assessing the Treatment Effect in Metabolic Syndrome Without Perceptible Diabetes (ATTEMPT)[115] with an atorvastatin- (34 or 24 mg/d) based multifactorial treatment approach in patients and MetS and NAFLD showed that attaining multiple treatment targets is safe and beneficial in primary prevention patients with MetS and NAFLD[115]. Lipid levels and liver enzymes were normalized and ultrasonographic evidence of NAFLD resolved during the 42 mo duration of the study in both intensive (mean dose 34 mg/d) and standard (mean dose 24 mg/d) atorvastatin treatment groups[115].
In 2013 the post hoc analysis of the Incremental Decrease in End Points Through Aggressive Lipid Lowering (IDEAL) trial (n = 8863) showed that high dose atorvastatin treatment (80 mg) in 1081 (12.2%) patients, who had an ALT ≥ ULN resulted in normalisation of ALT values[116]. The higher the atorvastatin dose (80 mg/d) the greater the reduction in liver enzyme levels[116].
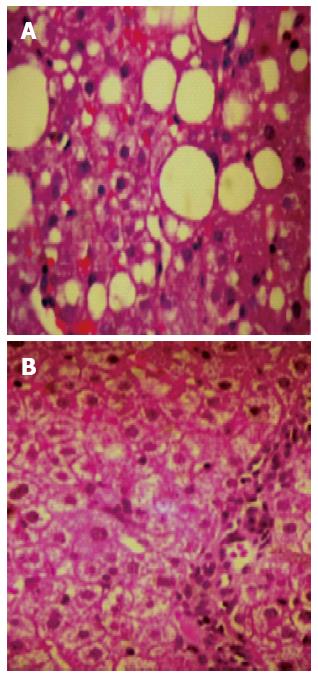
In 2014 a pilot study (n = 6) with 10 mg/d of rosuvastatin monotherapy in biopsy proven NASH patients with MetS, showed within one year of treatment a normalisation of lipid profile, all liver enzymes, and complete resolution of NASH in the repeat biopsy (fibrosis, necroinflammation, ballooning, and steatosis were totally absent and histology revealed a normal liver tissue) in 5 out of 6 patients[117]. In 2015 we completed the study, for which the pilot was designed (unpublished data), with 20 biopsy proven (repeat biopsy after 12 mo of treatment) NASH patients with MetS treated with rosuvastatin 10 mg/d as monotherapy. The results remained as impressive as in the pilot study (Figure 2) and this study confirmed that the patients did not have MetS any longer, due to the reduction in TGs, the increase in high density lipoprotein cholesterol (HDL-C), and a paradoxical (substantial by 20 mg/dL) reduction in fasting plasma glucose. Waist circumference and body mass index did not change, thus, the improvement could not be attributed to reduction of (abdominal) obesity.
As stated in the above data there has been a gradual and hesitant attempt during the last 8 years to investigate the efficacy of statins in the treatment NAFLD/NASH. The results of these studies suggest that the effect of statins on NAFLD/NASH is intensity of compound and dose dependent[107-117]. The post hoc analyses of GREACE, ATTEMPT and IDEAL studies are hypothesis generating and there is a need for randomized controlled prospective studies on this issue. However, with all statins, except rosuvastatin, out of patent no pharmaceutical company is eager to finance a prospective study on this issue. This has to be done by independent researchers. We did our part, let others continue. In any case, we all know that several years are needed in order for guidelines to state a new indication for a drug. However, the information provided may help clinicians make decisions for patients with a highly prevalent disease.
From all the above the conclusion with practical implications was described by a Hepatologist: “Yes! Statins can be given to liver patients”[118].
Ezetimibe can be added to statin treatment in patients who cannot achieve LDL-C targets despite treatment with the maximal tolerated dose of a high intensity statin[119]. The effect of ezetimibe on NAFLD was studied in a few, mainly uncontrolled studies with rather small number of patients. Serum ALT levels significantly decreased within 6 mo and in 4 patients levels reached the normal range (< 30 U/L), which was accompanied with at least a 10% decrease in serum total cholesterol and LDL-C. However, ezetimibe had no effect on liver steatosis as assessed with ultrasonography[120]. In another uncontrolled study in 10 patients with NASH, treatment with ezetimibe for 6 mo[121], NASH score and steatosis grade were also significantly improved in the repeat liver biopsy. The fibrosis stage did not change significantly, but 6 of the 10 patients exhibited an improvement in their fibrosis stage[121]. In another uncontrolled study 45 patients with liver biopsy-proven NAFLD were treated with ezetimibe for 24 mo[122]. Histological features of steatosis and necroinflammation improved, but fibrosis stage was not significantly changed[122]. In a recent randomized controlled study in NAFLD patients (16 on ezetimibe and 12 controls), ezetimibe improved hepatic fibrosis but increased hepatic long-chain fatty acids and HbA1c in NAFLD patients[123]. Thus, it is not clear yet which is the long term effect of ezetimibe on NAFLD/NASH (Table 1).
| Ref. | Year | n of all | n with NAFLD/NASH | Method of diagnosis of NAFLD/NASH | Drug | Dose/d | Duration of treatment | Results-main findings |
| Gómez-Domínguez et al[107] | 2006 | 25 | 22 | ALT levels | Atorvastatin | 10-80 mg | 12 mo | Normalization of ALT and lipid profile |
| Hyogo et al[108] | 2008 | 31 | 31 | Biopsy-proven NASH | Atorvastatin | 10 mg | 24 mo | Liver steatosis and NAFLD activity score were significantly improved; in 4 increase in fibrosis stage. |
| Kimura et al[109] | 2010 | 45 | 45 | Biopsy-proven NASH | Atorvastatin | 10 mg | 12 mo | The steatosis grade and NAFLD activity score were significantly improved. |
| Nelson et al[110] | 2009 | 16 | 10 | Biopsy-proven NASH | Simvastatin | 20 mg | 12 mo | No statistically significant improvement in serum ALT, hepatic steatosis, necroinflammatory activity or stage of fibrosis within or between groups. |
| Athyros et al[111] | 2010 | 1600 | 437 | ALT levels -ultrasonography | Atorvastatin | 24 mg | 3 yr | Improved ALT, AST, GGT, AP, and ultrasonography. Reduced cardiovascular events more than those without NAFLD (P = 0.007). |
| Hyogo et al[112] | 2011 | 20 | 13 | Biopsy-proven NASH | Pitavastatin | 2 mg | 12 mo | Improved NAFLD activity score and fibrosis stage In 54% and 42%, respectively, improved |
| histology, however, 3/13 patients progression of fibrosis. | ||||||||
| Hyogo et al[113] | 2012 | 42 | 42 | Biopsy-proven NASH | Atorvastatin | 10 mg | 12 mo | Improved NAFLD activity score, normalization of liver enzymes and TNF-α, and increased liver to spleen density ratio. |
| Nakahara et al[114] | 2012 | 19 | 19 | Biopsy-proven NASH | Rosuvastatin | 2.5 mg | 24 mo | NAFLD activity score and fibrotic stage did not change significantly in all patients, they were improved in 33.3% and 33.3% of patients, and stayed stable in 33.3% and 55.6%, respectively. |
| Athyros et al[115] | 2011 | 1123 | 326 | ALT levels -ultrasonography | Atorvastatin | 20-30 mg | 42 mo | Improved ALT, AST, GGT, AP, and ultrasonography. Eradicated cardiovascular events in high dose group. |
| Tikkanen et al[116] | 2013 | 8863 | 1081 | ALT levels | Atorvastatin | 80 mg | 5 yr | Normalization of liver enzyme values and greater CVD benefit with atorvastatin in the elevated ALT group. |
| Simvastatin | 20-40 mg | |||||||
| Kargiotis et al[117] | 2014 | 6 | 6 | Biopsy-proven NASH | Rosuvastatin | 10 mg | 12 mo | Complete resolution of NASH in 5 patients. Normalization of liver enzyme values in all patients. |
| Monotherapy | ||||||||
| Park et al[122] | 2011 | 45 | 45 | Biopsy-proven NASH | Ezetimibe | 10 mg | 24 mo | Steatosis grade, necroinflammatory grade, ballooning, and NAFLD activity score were significantly improved from baseline. Fibrosis stage was not significantly changed. |
| Zhu et al[124] | 2008 | 72 | 72 | ALT levels -ultrasonography | n-3 PUFA | 6 g | 6 mo | ALT was reduced but other liver enzymes not. Ultrasonography improved in 53% of patients |
Omega-3 fatty acid supplementation has also been used for the treatment of NAFLD/NASH. Omega-3 fatty acid administration is safe and effective for NAFLD patients with dyslipidaemia and can improve their ALT, serum lipid (mainly TG) levels and normalize the ultrasonographic image of the liver[124]. A recent meta-analysis, that included 9 studies (5 randomized controlled trials- RCTs), involving 355 NAFLD patients, tested omega-3 vs control treatment for a period from 8 wk to 12 mo[125]. Results showed that omega-3 fatty acids were beneficial vs placebo in reducing liver fat and decreasing AST, but not ALT activity[125]. Sub-analyses of only the 5 RCTs showed a significant benefit for omega-3 fatty vs control on liver fat, but not for ALT or AST[125]. The pooled data suggest that omega-3 polyunsaturated fatty acid (PUFA) supplementation may decrease liver fat, however, the optimal dose could not be found[125].
Recently, several high quality reviews on NAFLD/NASH and CVD risk have been published[126-129]. These recent reviews focused on the relation of NAFLD/NASH or other ectopic fat deposition with CVD risk or even more specific with the risk of arrhythmic complications in these patients, focusing mainly to pathophysiological mechanisms and dedicated a rather small portion to treatment[126-129]. In contrast, our review focused on specific NASH characteristics (degree of liver fibrosis and CVD risk) or confounding factors (NAFLD-chronic kidney disease and CVD risk as well as NAFLD-arterial stiffness and CVD risk) that could all be treated with a single drug, a statin. However, given that improvement of NAFLD/NASH with concomitant reduction in CVD risk may not be a drug class effect, we had to analyze the effect of specific regiments at specific doses for specific treatment periods on the progression, amelioration or even reversal of NAFLD/NASH. This is the main difference between our review and the excellent recent analyses on this issue mentioned above.
There are no prospective RCTs that investigated the effect of statins on CVD morbidity and mortality in NAFLD/NASH patients. Existing data come from post hoc analyses of 3 survival trials: GREACE[111], ATTEMPT[115], and IDEAL[116].
The GREACE post hoc analysis showed that in patients with mild to moderate elevations of serum transaminases, most probably due to NAFLD as indicated by liver ultrasonography, in the absence of alcohol abuse history and the exclusion of other liver diseases, 24 mg/d of atorvastatin induced substantial reductions in CVD events (68% vs usual care) during the 3-year follow-up period compared with the participants with CHD and normal liver enzymes (39% vs usual care); P = 0.007[111]. This confirmed the pattern of the higher the CVD risk, the greater the benefit from effective therapy. Moreover, the study results showed that those patients with overt CVD and NAFLD that were at very high risk for recurrent CVD events or CVD death were those who were deprived of statin therapy, up to 10 years ago, because the fear of a transaminase increase with a statin.
The ATTEMPT study was a primary prevention study in patients with MetS without overt CVD or T2DM and its post hoc analysis investigated the effect of atorvastatin-based multifactorial intervention on ultrasonography verified NAFLD[115]. There were no CVD events in patients with LDL-C levels < 100 mg/dL (atorvastatin dose 34 mg/dL) for the 42 mo of the duration of the study, while there were 5 non-fatal events occurring in the lower atorvastatin dose (24 mg/d) group (log-rank-P = 0.024).
The IDEAL post hoc analysis showed a substantially reduced 5-year CVD event rate with atorvastatin 80 mg/d in patients with elevated transaminases levels (probably due to NAFLD) as compared with 20-40 mg of simvastatin, a less effective dose of a less effective hypolipidaemic agent (11.5% for simvastatin and 6.5% for atorvastatin, HR = 0.556; 95%CI: 0.367-0.842; P = 0.0056)[116]. This totally confirmed the findings of GREACE and ATTEMPT studies.
Thus, it seems that statin treatment is safe in NAFLD/NASH patients[102-105], may contribute to the normalization of liver function and structure[107-117], and reduces CVD morbidity and mortality in these high CVD risk patients[111,115,116].
NAFLD has been considered as an independent CVD risk factor. However, it seems that simple steatosis is not related with higher total, CVD or liver-related mortality compared with the general population. In contrast, increasing values of liver fibrosis scores (in NASH patients) is linked to a progressive increase in total mortality by approximately 70% compared with subjects without fibrosis. The increase in overall mortality was mainly due to a higher CVD mortality. Besides fibrosis, the development of CKD substantially increases total and CVD mortality. NAFLD/NASH is considered to be a risk factor for CKD and CKD contributes to NAFLD pathogenesis within a vicious cycle. Liver and kidney disease progress in parallel substantially reducing life expectancy. NAFLD/NASH increase AS which is independently associated with increased CVD risk across many different patient groups and even in the general population. Early detection of NAFLD/NASH, advanced fibrosis, CKD and increased AS could lead to the treatment of this cluster of CVD risk factors with lifestyle measures and multifactorial drug intervention, mainly based on high intensity statins at moderate to high doses. These seem to be safe, resolve NAFLD/NASH and could contribute to the primary or secondary prevention of CVD[118,119,130,131].
P- Reviewer: Camps J, Fernandez-Rodriguez CM, Gwak GY, Kawaguchi T, Kucera O, Reddy AB S- Editor: Ma YJ L- Editor: A E- Editor: Ma S
| 1. | Baran B, Akyüz F. Non-alcoholic fatty liver disease: what has changed in the treatment since the beginning? World J Gastroenterol. 2014;20:14219-14229. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 31] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (1)] |
| 2. | Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34:274-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2405] [Cited by in RCA: 2287] [Article Influence: 163.4] [Reference Citation Analysis (0)] |
| 3. | Jiang CM, Pu CW, Hou YH, Chen Z, Alanazy M, Hebbard L. Non alcoholic steatohepatitis a precursor for hepatocellular carcinoma development. World J Gastroenterol. 2014;20:16464-16473. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 32] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 4. | Eguchi A, Povero D, Alkhouri N, Feldstein AE. Novel therapeutic targets for nonalcoholic fatty liver disease. Expert Opin Ther Targets. 2013;17:773-779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 5. | Machado M, Marques-Vidal P, Cortez-Pinto H. Hepatic histology in obese patients undergoing bariatric surgery. J Hepatol. 2006;45:600-606. [PubMed] |
| 6. | Fargion S, Porzio M, Fracanzani AL. Nonalcoholic fatty liver disease and vascular disease: state-of-the-art. World J Gastroenterol. 2014;20:13306-13324. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 144] [Cited by in RCA: 166] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 7. | Kim D, Kim WR, Kim HJ, Therneau TM. Association between noninvasive fibrosis markers and mortality among adults with nonalcoholic fatty liver disease in the United States. Hepatology. 2013;57:1357-1365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 624] [Cited by in RCA: 622] [Article Influence: 51.8] [Reference Citation Analysis (0)] |
| 8. | Dowman JK, Tomlinson JW, Newsome PN. Systematic review: the diagnosis and staging of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2011;33:525-540. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 228] [Cited by in RCA: 222] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 9. | Takaki A, Kawai D, Yamamoto K. Multiple hits, including oxidative stress, as pathogenesis and treatment target in non-alcoholic steatohepatitis (NASH). Int J Mol Sci. 2013;14:20704-20728. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 230] [Cited by in RCA: 333] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 10. | Farrell GC, van Rooyen D, Gan L, Chitturi S. NASH is an Inflammatory Disorder: Pathogenic, Prognostic and Therapeutic Implications. Gut Liver. 2012;6:149-171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 281] [Cited by in RCA: 303] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 11. | Dam-Larsen S, Franzmann M, Andersen IB, Christoffersen P, Jensen LB, Sørensen TI, Becker U, Bendtsen F. Long term prognosis of fatty liver: risk of chronic liver disease and death. Gut. 2004;53:750-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 364] [Cited by in RCA: 364] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 12. | Ekstedt M, Franzén LE, Mathiesen UL, Thorelius L, Holmqvist M, Bodemar G, Kechagias S. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44:865-873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1647] [Cited by in RCA: 1704] [Article Influence: 89.7] [Reference Citation Analysis (0)] |
| 13. | Söderberg C, Stål P, Askling J, Glaumann H, Lindberg G, Marmur J, Hultcrantz R. Decreased survival of subjects with elevated liver function tests during a 28-year follow-up. Hepatology. 2010;51:595-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 529] [Cited by in RCA: 560] [Article Influence: 37.3] [Reference Citation Analysis (0)] |
| 14. | Day CP. Non-alcoholic steatohepatitis (NASH): where are we now and where are we going? Gut. 2002;50:585-588. [PubMed] |
| 15. | James OF, Day CP. Non-alcoholic steatohepatitis (NASH): a disease of emerging identity and importance. J Hepatol. 1998;29:495-501. [PubMed] |
| 16. | Haflidadottir S, Jonasson JG, Norland H, Einarsdottir SO, Kleiner DE, Lund SH, Björnsson ES. Long-term follow-up and liver-related death rate in patients with non-alcoholic and alcoholic related fatty liver disease. BMC Gastroenterol. 2014;14:166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 83] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 17. | Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313-1321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6807] [Cited by in RCA: 8189] [Article Influence: 409.5] [Reference Citation Analysis (5)] |
| 18. | Angulo P, Bugianesi E, Bjornsson ES, Charatcharoenwitthaya P, Mills PR, Barrera F, Haflidadottir S, Day CP, George J. Simple noninvasive systems predict long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology. 2013;145:782-9.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 398] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 19. | Angulo P, Hui JM, Marchesini G, Bugianesi E, George J, Farrell GC, Enders F, Saksena S, Burt AD, Bida JP. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology. 2007;45:846-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1917] [Cited by in RCA: 2272] [Article Influence: 126.2] [Reference Citation Analysis (1)] |
| 20. | Shah AG, Lydecker A, Murray K, Tetri BN, Contos MJ, Sanyal AJ; Nash Clinical Research Network. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2009;7:1104-1112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1205] [Cited by in RCA: 1154] [Article Influence: 72.1] [Reference Citation Analysis (1)] |
| 21. | Harrison SA, Oliver D, Arnold HL, Gogia S, Neuschwander-Tetri BA. Development and validation of a simple NAFLD clinical scoring system for identifying patients without advanced disease. Gut. 2008;57:1441-1447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 545] [Cited by in RCA: 629] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 22. | Musso G, Gambino R, Cassader M, Pagano G. Meta-analysis: natural history of non-alcoholic fatty liver disease (NAFLD) and diagnostic accuracy of non-invasive tests for liver disease severity. Ann Med. 2011;43:617-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 886] [Cited by in RCA: 916] [Article Influence: 65.4] [Reference Citation Analysis (0)] |
| 23. | Liu H, Lu HY. Nonalcoholic fatty liver disease and cardiovascular disease. World J Gastroenterol. 2014;20:8407-8415. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 68] [Cited by in RCA: 83] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 24. | Luo J, Xu L, Li J, Zhao S. Nonalcoholic fatty liver disease as a potential risk factor of cardiovascular disease. Eur J Gastroenterol Hepatol. 2015;27:193-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 25. | Takahashi Y, Kurosaki M, Tamaki N, Yasui Y, Hosokawa T, Tsuchiya K, Nakanishi H, Itakura J, Izumi N. Non-alcoholic fatty liver disease fibrosis score and FIB-4 scoring system could identify patients at risk of systemic complications. Hepatol Res. 2014;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 26. | Athyros VG, Elisaf MS, Alexandrides T, Achimastos A, Ganotakis E, Bilianou E, Karagiannis A, Liberopoulos EN, Tziomalos K, Mikhailidis DP; Assessing the Treatment Effect in Metabolic Syndrome Without Perceptible Diabetes (ATTEMPT) Collaborative Group. Long-term impact of multifactorial treatment on new-onset diabetes and related cardiovascular events in metabolic syndrome: a post hoc ATTEMPT analysis. Angiology. 2012;63:358-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 27. | Doycheva I, Patel N, Peterson M, Loomba R. Prognostic implication of liver histology in patients with nonalcoholic fatty liver disease in diabetes. J Diabetes Complications. 2013;27:293-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 28. | Athyros VG, Hatzitolios A, Karagiannis A, Didangelos TP, Iliadis F, Dolgyras S, Vosnakidis T, Vasiliadis P, Malias I, Tziomalos K. Initiative for a new diabetes therapeutic approach in a Mediterranean country: the INDEED study. Curr Med Res Opin. 2009;25:1931-1940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 29. | Katsiki N, Athyros VG, Karagiannis A, Mikhailidis DP. The role of statins in the treatment of type 2 diabetes mellitus: an update. Curr Pharm Des. 2014;20:3665-3674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 30. | Tziomalos K, Athyros VG, Karagiannis A. Non-alcoholic fatty liver disease in type 2 diabetes: pathogenesis and treatment options. Curr Vasc Pharmacol. 2012;10:162-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 31. | Duvnjak M, Lerotić I, Barsić N, Tomasić V, Virović Jukić L, Velagić V. Pathogenesis and management issues for non-alcoholic fatty liver disease. World J Gastroenterol. 2007;13:4539-4550. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 180] [Cited by in RCA: 169] [Article Influence: 9.4] [Reference Citation Analysis (1)] |
| 32. | Dyson JK, Anstee QM, McPherson S. Non-alcoholic fatty liver disease: a practical approach to diagnosis and staging. Frontline Gastroenterol. 2014;5:211-218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 191] [Cited by in RCA: 239] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 33. | Athyros VG, Katsiki N, Karagiannis A. Comment on: Novel therapeutic targets for non-alcoholic fatty liver disease. Expert Opin Ther Targets. 2013;17:861-862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 34. | Levey AS, Coresh J, Balk E, Kausz AT, Levin A, Steffes MW, Hogg RJ, Perrone RD, Lau J, Eknoyan G; National Kidney Foundation. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2003;139:137-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3156] [Cited by in RCA: 3201] [Article Influence: 145.5] [Reference Citation Analysis (0)] |
| 35. | Locatelli F, Pozzoni P, Tentori F, del Vecchio L. Epidemiology of cardiovascular risk in patients with chronic kidney disease. Nephrol Dial Transplant. 2003;18 Suppl 7:vii2-vii9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 49] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 36. | Iseki K. Chronic kidney disease in Japan. Intern Med. 2008;47:681-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 65] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 37. | Orlić L, Mikolasevic I, Bagic Z, Racki S, Stimac D, Milic S. Chronic kidney disease and nonalcoholic Fatty liver disease-is there a link? Gastroenterol Res Pract. 2014;2014:847539. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 38. | Vanholder R, Massy Z, Argiles A, Spasovski G, Verbeke F, Lameire N; European Uremic Toxin Work Group. Chronic kidney disease as cause of cardiovascular morbidity and mortality. Nephrol Dial Transplant. 2005;20:1048-1056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 423] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 39. | Targher G, Chonchol M, Zoppini G, Abaterusso C, Bonora E. Risk of chronic kidney disease in patients with non-alcoholic fatty liver disease: is there a link? J Hepatol. 2011;54:1020-1029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 134] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 40. | Targher G, Bertolini L, Rodella S, Zoppini G, Lippi G, Day C, Muggeo M. Non-alcoholic fatty liver disease is independently associated with an increased prevalence of chronic kidney disease and proliferative/laser-treated retinopathy in type 2 diabetic patients. Diabetologia. 2008;51:444-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 274] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 41. | Hwang ST, Cho YK, Yun JW, Park JH, Kim HJ, Park DI, Sohn CI, Jeon WK, Kim BI, Rhee EJ. Impact of non-alcoholic fatty liver disease on microalbuminuria in patients with prediabetes and diabetes. Intern Med J. 2010;40:437-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 69] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 42. | Targher G, Kendrick J, Smits G, Chonchol M. Relationship between serum gamma-glutamyltransferase and chronic kidney disease in the United States adult population. Findings from the National Health and Nutrition Examination Survey 2001-2006. Nutr Metab Cardiovasc Dis. 2010;20:583-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 43. | Targher G, Bosworth C, Kendrick J, Smits G, Lippi G, Chonchol M. Relationship of serum bilirubin concentrations to kidney function and albuminuria in the United States adult population. Findings from the National Health and Nutrition Examination Survey 2001-2006. Clin Chem Lab Med. 2009;47:1055-1062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 44. | Armstrong MJ, Adams LA, Canbay A, Syn WK. Extrahepatic complications of nonalcoholic fatty liver disease. Hepatology. 2014;59:1174-1197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 387] [Cited by in RCA: 436] [Article Influence: 39.6] [Reference Citation Analysis (0)] |
| 45. | Musso G, Gambino R, Tabibian JH, Ekstedt M, Kechagias S, Hamaguchi M, Hultcrantz R, Hagström H, Yoon SK, Charatcharoenwitthaya P. Association of non-alcoholic fatty liver disease with chronic kidney disease: a systematic review and meta-analysis. PLoS Med. 2014;11:e1001680. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 527] [Cited by in RCA: 519] [Article Influence: 47.2] [Reference Citation Analysis (0)] |
| 46. | Dogru T, Genc H, Tapan S, Aslan F, Ercin CN, Ors F, Kara M, Sertoglu E, Karslioglu Y, Bagci S. Plasma fetuin-A is associated with endothelial dysfunction and subclinical atherosclerosis in subjects with nonalcoholic fatty liver disease. Clin Endocrinol (Oxf). 2013;78:712-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 69] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 47. | Jin K, Norris K, Vaziri ND. Dysregulation of hepatic fatty acid metabolism in chronic kidney disease. Nephrol Dial Transplant. 2013;28:313-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 48. | Allen AM, Kim WR, Therneau TM, Larson JJ, Heimbach JK, Rule AD. Chronic kidney disease and associated mortality after liver transplantation--a time-dependent analysis using measured glomerular filtration rate. J Hepatol. 2014;61:286-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 125] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 49. | Musso G, Tabibian JH, Charlton M. Chronic kidney disease (CKD) and NAFLD: time for awareness and screening. J Hepatol. 2015;62:983-984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 50. | Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296-1305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7995] [Cited by in RCA: 8501] [Article Influence: 404.8] [Reference Citation Analysis (0)] |
| 51. | Foley RN, Murray AM, Li S, Herzog CA, McBean AM, Eggers PW, Collins AJ. Chronic kidney disease and the risk for cardiovascular disease, renal replacement, and death in the United States Medicare population, 1998 to 1999. J Am Soc Nephrol. 2005;16:489-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 677] [Cited by in RCA: 686] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 52. | Lai YC, Cheng BC, Hwang JC, Lee YT, Chiu CH, Kuo LC, Chen JB. Association of fatty liver disease with nonfatal cardiovascular events in patients undergoing maintenance hemodialysis. Nephron Clin Pract. 2013;124:218-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 53. | Targher G, Byrne CD. Clinical Review: Nonalcoholic fatty liver disease: a novel cardiometabolic risk factor for type 2 diabetes and its complications. J Clin Endocrinol Metab. 2013;98:483-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 227] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 54. | Athyros VG, Katsiki N, Karagiannis A, Mikhailidis DP. Statins and nonalcoholic fatty liver disease: a bright future? Expert Opin Investig Drugs. 2013;22:1089-1093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 55. | Athyros VG, Katsiki N, Karagiannis A, Mikhailidis DP. Are statins ‘IDEAL’ for non-alcoholic fatty liver disease? Curr Med Res Opin. 2014;30:229-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 56. | Kostapanos MS, Athyros VG, Karagiannis A, Mikhailidis DP. Mechanisms linking nonalcoholic fatty liver disease with coronary artery disease. Dig Dis Sci. 2012;57:1109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 57. | Athyros VG, Mikhailidis DP, Papageorgiou AA, Symeonidis AN, Pehlivanidis AN, Bouloukos VI, Elisaf M. The effect of statins versus untreated dyslipidaemia on renal function in patients with coronary heart disease. A subgroup analysis of the Greek atorvastatin and coronary heart disease evaluation (GREACE) study. J Clin Pathol. 2004;57:728-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 279] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 58. | Athyros VG, Papageorgiou AA, Mercouris BR, Athyrou VV, Symeonidis AN, Basayannis EO, Demitriadis DS, Kontopoulos AG. Treatment with atorvastatin to the National Cholesterol Educational Program goal versus ‘usual’ care in secondary coronary heart disease prevention. The GREek Atorvastatin and Coronary-heart-disease Evaluation (GREACE) study. Curr Med Res Opin. 2002;18:220-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 354] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 59. | Athyros VG, Papageorgiou AA, Elisaf M, Mikhailidis DP; GREACE Study Collaborative Group. Statins and renal function in patients with diabetes mellitus. Curr Med Res Opin. 2003;19:615-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 53] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 60. | Zoppini G, Fedeli U, Gennaro N, Saugo M, Targher G, Bonora E. Mortality from chronic liver diseases in diabetes. Am J Gastroenterol. 2014;109:1020-1025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 120] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 61. | Athyros VG, Papageorgiou AA, Symeonidis AN, Didangelos TP, Pehlivanidis AN, Bouloukos VI, Mikhailidis DP; GREACE Study Collaborative Group. Early benefit from structured care with atorvastatin in patients with coronary heart disease and diabetes mellitus. Angiology. 2003;54:679-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 57] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 62. | Lonardo A, Ballestri S, Targher G, Loria P. Diagnosis and management of cardiovascular risk in nonalcoholic fatty liver disease. Expert Rev Gastroenterol Hepatol. 2015;9:629-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 63] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 63. | Targher G, Chonchol MB, Byrne CD. CKD and nonalcoholic fatty liver disease. Am J Kidney Dis. 2014;64:638-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 160] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 64. | Athyros VG, Mikhailidis DP. Uric acid, chronic kidney disease and type 2 diabetes: a cluster of vascular risk factors. J Diabetes Complications. 2014;28:122-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 65. | Katsiki N, Karagiannis A, Athyros VG, Mikhailidis DP. Hyperuricaemia: more than just a cause of gout? J Cardiovasc Med (Hagerstown). 2013;14:397-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 63] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 66. | Athyros VG, Karagiannis A, Ganotakis ES, Paletas K, Nicolaou V, Bacharoudis G, Tziomalos K, Alexandrides T, Liberopoulos EN, Mikhailidis DP; Assessing The Treatment Effect in Metabolic syndrome without Perceptible diabeTes (ATTEMPT) Collaborative Group. Association between the changes in renal function and serum uric acid levels during multifactorial intervention and clinical outcome in patients with metabolic syndrome. A post hoc analysis of the ATTEMPT study. Curr Med Res Opin. 2011;27:1659-1668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 67. | Cardoso AS, Gonzaga NC, Medeiros CC, Carvalho DF. Association of uric acid levels with components of metabolic syndrome and non-alcoholic fatty liver disease in overweight or obese children and adolescents. J Pediatr (Rio J). 2013;89:412-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 68. | Tsouli SG, Liberopoulos EN, Mikhailidis DP, Athyros VG, Elisaf MS. Elevated serum uric acid levels in metabolic syndrome: an active component or an innocent bystander? Metabolism. 2006;55:1293-1301. [PubMed] |
| 69. | Katsiki N, Doumas M, Athyros VG, Karagiannis A. Hyperuricemia as a risk factor for cardiovascular disease. Expert Rev Cardiovasc Ther. 2015;13:19-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 70. | Athyros VG, Mikhailidis DP, Liberopoulos EN, Kakafika AI, Karagiannis A, Papageorgiou AA, Tziomalos K, Ganotakis ES, Elisaf M. Effect of statin treatment on renal function and serum uric acid levels and their relation to vascular events in patients with coronary heart disease and metabolic syndrome: a subgroup analysis of the GREek Atorvastatin and Coronary heart disease Evaluation (GREACE) Study. Nephrol Dial Transplant. 2007;22:118-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 127] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 71. | Daskalopoulou SS, Athyros VG, Elisaf M, Mikhailidis D. The impact of serum uric acid on cardiovascular outcomes in the LIFE study. Kidney Int. 2004;66:1714-1715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 72. | Athyros VG, Elisaf M, Papageorgiou AA, Symeonidis AN, Pehlivanidis AN, Bouloukos VI, Milionis HJ, Mikhailidis DP; GREACE Study Collaborative Group. Effect of statins versus untreated dyslipidemia on serum uric acid levels in patients with coronary heart disease: a subgroup analysis of the GREek Atorvastatin and Coronary-heart-disease Evaluation (GREACE) study. Am J Kidney Dis. 2004;43:589-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 147] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 73. | Daskalopoulou SS, Tzovaras V, Mikhailidis DP, Elisaf M. Effect on serum uric acid levels of drugs prescribed for indications other than treating hyperuricaemia. Curr Pharm Des. 2005;11:4161-4175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 78] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 74. | Li N, Zhang GW, Zhang JR, Jin D, Li Y, Liu T, Wang RT. Non-alcoholic fatty liver disease is associated with progression of arterial stiffness. Nutr Metab Cardiovasc Dis. 2015;25:218-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 75. | Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol. 2010;55:1318-1327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3382] [Cited by in RCA: 3067] [Article Influence: 204.5] [Reference Citation Analysis (0)] |
| 76. | Lee YJ, Shim JY, Moon BS, Shin YH, Jung DH, Lee JH, Lee HR. The relationship between arterial stiffness and nonalcoholic fatty liver disease. Dig Dis Sci. 2012;57:196-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 61] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 77. | Yu XY, Zhao Y, Song XX, Song ZY. Association between non-alcoholic fatty liver disease and arterial stiffness in the non-obese, non-hypertensive, and non-diabetic young and middle-aged Chinese population. J Zhejiang Univ Sci B. 2014;15:879-887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 78. | Sunbul M, Agirbasli M, Durmus E, Kivrak T, Akin H, Aydin Y, Ergelen R, Yilmaz Y. Arterial stiffness in patients with non-alcoholic fatty liver disease is related to fibrosis stage and epicardial adipose tissue thickness. Atherosclerosis. 2014;237:490-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 79. | Wang MC, Tsai WC, Chen JY, Huang JJ. Stepwise increase in arterial stiffness corresponding with the stages of chronic kidney disease. Am J Kidney Dis. 2005;45:494-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 256] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 80. | Haydar AA, Covic A, Colhoun H, Rubens M, Goldsmith DJ. Coronary artery calcification and aortic pulse wave velocity in chronic kidney disease patients. Kidney Int. 2004;65:1790-1794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 126] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 81. | Chen JY, Chou CH, Tsai WC, Wang MC, Ho CS, Li YH, Tsai YS, Tsai LM. Effects of increased systemic inflammation and central obesity on arterial stiffness in patients with nonalcoholic fatty liver disease. J Am Soc Hypertens. 2012;6:253-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 82. | Yu KJ, Zhang MJ, Li Y, Wang RT. Increased whole blood viscosity associated with arterial stiffness in patients with non-alcoholic fatty liver disease. J Gastroenterol Hepatol. 2014;29:540-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 83. | Li Y, Tian XX, Liu T, Wang RT. Association between whole blood viscosity and arterial stiffness in patients with type 2 diabetes mellitus. Endocrine. 2015;49:148-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 84. | Cecelja M, Chowienczyk P. Role of arterial stiffness in cardiovascular disease. JRSM Cardiovasc Dis. 2012;1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 131] [Cited by in RCA: 212] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 85. | London GM. Mechanisms of arterial calcifications and consequences for cardiovascular function. Kidney Int Suppl (2011). 2013;3:442-445. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 86. | Liu B, Che W, Yan H, Zhu W, Wang H. Effects of rosuvastatin vs. simvastatin/ezetimibe on arterial wall stiffness in patients with coronary artery disease. Intern Med. 2013;52:2715-2719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 87. | Georgianos PI, Sarafidis PA, Lasaridis AN. Arterial stiffness: a novel cardiovascular risk factor in kidney disease patients. Curr Vasc Pharmacol. 2015;13:229-238. [PubMed] |
| 88. | Dahlén EM, Bjarnegård N, Länne T, Nystrom FH, Ostgren CJ. Sagittal abdominal diameter is a more independent measure compared with waist circumference to predict arterial stiffness in subjects with type 2 diabetes--a prospective observational cohort study. Cardiovasc Diabetol. 2013;12:55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 89. | Katsiki N, Koumaras C, Athyros VG, Karagiannis A. Thinking beyond traditional cardiovascular risk factors: the role of arterial stiffness in targeting residual risk. Angiology. 2012;63:9-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 90. | Tziomalos K, Athyros VG, Karagiannis A. Treating Arterial Stiffness in Young and Elderly Patients with the Metabolic Syndrome. Curr Pharm Des. 2014;Epub ahead of print. [PubMed] |
| 91. | Katsiki N, Athyros VG, Karagiannis A, Mikhailidis DP. Metabolic syndrome and non-cardiac vascular diseases: an update from human studies. Curr Pharm Des. 2014;20:4944-4952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 92. | Chen TH, Liao FT, Yang YC, Wang JJ. Inhibition of inducible nitric oxide synthesis ameliorates liver ischemia and reperfusion injury induced transient increase in arterial stiffness. Transplant Proc. 2014;46:1112-1116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 93. | Athyros VG, Pagourelias ED, Gossios TD, Vasilikos VG. Treating Heart Failure with Preserved Ejection Fraction Related to Arterial Stiffness. Can we kill Two Birds with One Stone? Curr Vasc Pharmacol. 2014;Epub ahead of print. [PubMed] |
| 94. | Kanaki AI, Sarafidis PA, Georgianos PI, Kanavos K, Tziolas IM, Zebekakis PE, Lasaridis AN. Effects of low-dose atorvastatin on arterial stiffness and central aortic pressure augmentation in patients with hypertension and hypercholesterolemia. Am J Hypertens. 2013;26:608-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 76] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 95. | Tousoulis D, Oikonomou E, Siasos G, Chrysohoou C, Zaromitidou M, Kioufis S, Maniatis K, Dilaveris P, Miliou A, Michalea S. Dose-dependent effects of short term atorvastatin treatment on arterial wall properties and on indices of left ventricular remodeling in ischemic heart failure. Atherosclerosis. 2013;227:367-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 96. | Fassett RG, Robertson IK, Ball MJ, Geraghty DP, Sharman JE, Coombes JS. Effects of atorvastatin on arterial stiffness in chronic kidney disease: a randomised controlled trial. J Atheroscler Thromb. 2010;17:235-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 97. | Papademetriou V, Katsiki N, Doumas M, Faselis C. Halting arterial aging in patients with cardiovascular disease: hypolipidemic and antihypertensive therapy. Curr Pharm Des. 2014;20:6339-6349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 98. | Koumaras C, Katsiki N, Athyros VG, Karagiannis A. Metabolic syndrome and arterial stiffness: the past, the present and the future. J Cardiovasc Med (Hagerstown). 2013;14:687-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 99. | Koumaras C, Tziomalos K, Stavrinou E, Katsiki N, Athyros VG, Mikhailidis DP, Karagiannis A. Effects of renin-angiotensin-aldosterone system inhibitors and beta-blockers on markers of arterial stiffness. J Am Soc Hypertens. 2014;8:74-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 100. | Stojakovic T, Claudel T, Putz-Bankuti C, Fauler G, Scharnagl H, Wagner M, Sourij H, Stauber RE, Winkler K, März W. Low-dose atorvastatin improves dyslipidemia and vascular function in patients with primary biliary cirrhosis after one year of treatment. Atherosclerosis. 2010;209:178-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 101. | Mäki-Petäjä KM, Wilkinson IB. Anti-inflammatory drugs and statins for arterial stiffness reduction. Curr Pharm Des. 2009;15:290-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 40] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 102. | Clark JM, Brancati FL, Diehl AM. The prevalence and etiology of elevated aminotransferase levels in the United States. Am J Gastroenterol. 2003;98:960-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 939] [Cited by in RCA: 945] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 103. | Younossi ZM, Stepanova M, Afendy M, Fang Y, Younossi Y, Mir H, Srishord M. Changes in the prevalence of the most common causes of chronic liver diseases in the United States from 1988 to 2008. Clin Gastroenterol Hepatol. 2011;9:524-530.e1; quiz e60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 758] [Cited by in RCA: 790] [Article Influence: 56.4] [Reference Citation Analysis (0)] |
| 104. | Chalasani N, Aljadhey H, Kesterson J, Murray MD, Hall SD. Patients with elevated liver enzymes are not at higher risk for statin hepatotoxicity. Gastroenterology. 2004;126:1287-1292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 284] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 105. | Vuppalanchi R, Teal E, Chalasani N. Patients with elevated baseline liver enzymes do not have higher frequency of hepatotoxicity from lovastatin than those with normal baseline liver enzymes. Am J Med Sci. 2005;329:62-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 90] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 106. | Pfeffer MA, Keech A, Sacks FM, Cobbe SM, Tonkin A, Byington RP, Davis BR, Friedman CP, Braunwald E. Safety and tolerability of pravastatin in long-term clinical trials: prospective Pravastatin Pooling (PPP) Project. Circulation. 2002;105:2341-2346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 177] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 107. | Gómez-Domínguez E, Gisbert JP, Moreno-Monteagudo JA, García-Buey L, Moreno-Otero R. A pilot study of atorvastatin treatment in dyslipemid, non-alcoholic fatty liver patients. Aliment Pharmacol Ther. 2006;23:1643-1647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 141] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 108. | Hyogo H, Tazuma S, Arihiro K, Iwamoto K, Nabeshima Y, Inoue M, Ishitobi T, Nonaka M, Chayama K. Efficacy of atorvastatin for the treatment of nonalcoholic steatohepatitis with dyslipidemia. Metabolism. 2008;57:1711-1718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 169] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 109. | Kimura Y, Hyogo H, Yamagishi S, Takeuchi M, Ishitobi T, Nabeshima Y, Arihiro K, Chayama K. Atorvastatin decreases serum levels of advanced glycation endproducts (AGEs) in nonalcoholic steatohepatitis (NASH) patients with dyslipidemia: clinical usefulness of AGEs as a biomarker for the attenuation of NASH. J Gastroenterol. 2010;45:750-757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 127] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 110. | Nelson A, Torres DM, Morgan AE, Fincke C, Harrison SA. A pilot study using simvastatin in the treatment of nonalcoholic steatohepatitis: A randomized placebo-controlled trial. J Clin Gastroenterol. 2009;43:990-994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 205] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 111. | Athyros VG, Tziomalos K, Gossios TD, Griva T, Anagnostis P, Kargiotis K, Pagourelias ED, Theocharidou E, Karagiannis A, Mikhailidis DP. Safety and efficacy of long-term statin treatment for cardiovascular events in patients with coronary heart disease and abnormal liver tests in the Greek Atorvastatin and Coronary Heart Disease Evaluation (GREACE) Study: a post-hoc analysis. Lancet. 2010;376:1916-1922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 506] [Cited by in RCA: 498] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 112. | Hyogo H, Ikegami T, Tokushige K, Hashimoto E, Inui K, Matsuzaki Y, Tokumo H, Hino F, Tazuma S. Efficacy of pitavastatin for the treatment of non-alcoholic steatohepatitis with dyslipidemia: An open-label, pilot study. Hepatol Res. 2011;41:1057-1065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 69] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 113. | Hyogo H, Yamagishi S, Maeda S, Kimura Y, Ishitobi T, Chayama K. Atorvastatin improves disease activity of nonalcoholic steatohepatitis partly through its tumour necrosis factor-α-lowering property. Dig Liver Dis. 2012;44:492-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 114. | Nakahara T, Hyogo H, Kimura Y, Ishitobi T, Arihiro K, Aikata H, Takahashi S, Chayama K. Efficacy of rosuvastatin for the treatment of non-alcoholic steatohepatitis with dyslipidemia: An open-label, pilot study. Hepatol Res. 2012;42:1065-1072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 115. | Athyros VG, Giouleme O, Ganotakis ES, Elisaf M, Tziomalos K, Vassiliadis T, Liberopoulos EN, Theocharidou E, Karagiannis A, Mikhailidis DP. Safety and impact on cardiovascular events of long-term multifactorial treatment in patients with metabolic syndrome and abnormal liver function tests: a post hoc analysis of the randomised ATTEMPT study. Arch Med Sci. 2011;7:796-805. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 116. | Tikkanen MJ, Fayyad R, Faergeman O, Olsson AG, Wun CC, Laskey R, Kastelein JJ, Holme I, Pedersen TR. Effect of intensive lipid lowering with atorvastatin on cardiovascular outcomes in coronary heart disease patients with mild-to-moderate baseline elevations in alanine aminotransferase levels. Int J Cardiol. 2013;168:3846-3852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 129] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 117. | Kargiotis K, Katsiki N, Athyros VG, Giouleme O, Patsiaoura K, Katsiki E, Mikhailidis DP, Karagiannis A. Effect of rosuvastatin on non-alcoholic steatohepatitis in patients with metabolic syndrome and hypercholesterolaemia: a preliminary report. Curr Vasc Pharmacol. 2014;12:505-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 118. | Bader T. Yes! Statins can be given to liver patients. J Hepatol. 2012;56:305-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 119. | Mikhailidis DP, Sibbring GC, Ballantyne CM, Davies GM, Catapano AL. Meta-analysis of the cholesterol-lowering effect of ezetimibe added to ongoing statin therapy. Curr Med Res Opin. 2007;23:2009-2026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 123] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 120. | Enjoji M, Machida K, Kohjima M, Kato M, Kotoh K, Matsunaga K, Nakashima M, Nakamuta M. NPC1L1 inhibitor ezetimibe is a reliable therapeutic agent for non-obese patients with nonalcoholic fatty liver disease. Lipids Health Dis. 2010;9:29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 121. | Yoneda M, Fujita K, Nozaki Y, Endo H, Takahashi H, Hosono K, Suzuki K, Mawatari H, Kirikoshi H, Inamori M. Efficacy of ezetimibe for the treatment of non-alcoholic steatohepatitis: An open-label, pilot study. Hepatol Res. 2010;40:566-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 101] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 122. | Park H, Shima T, Yamaguchi K, Mitsuyoshi H, Minami M, Yasui K, Itoh Y, Yoshikawa T, Fukui M, Hasegawa G. Efficacy of long-term ezetimibe therapy in patients with nonalcoholic fatty liver disease. J Gastroenterol. 2011;46:101-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 144] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 123. | Takeshita Y, Takamura T, Honda M, Kita Y, Zen Y, Kato K, Misu H, Ota T, Nakamura M, Yamada K. The effects of ezetimibe on non-alcoholic fatty liver disease and glucose metabolism: a randomised controlled trial. Diabetologia. 2014;57:878-890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 97] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 124. | Zhu FS, Liu S, Chen XM, Huang ZG, Zhang DW. Effects of n-3 polyunsaturated fatty acids from seal oils on nonalcoholic fatty liver disease associated with hyperlipidemia. World J Gastroenterol. 2008;14:6395-6400. [PubMed] |
| 125. | Parker HM, Johnson NA, Burdon CA, Cohn JS, O’Connor HT, George J. Omega-3 supplementation and non-alcoholic fatty liver disease: a systematic review and meta-analysis. J Hepatol. 2012;56:944-951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 450] [Cited by in RCA: 406] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 126. | Subasi CF, Aykut UE, Yilmaz Y. Comparison of noninvasive scores for the detection of advanced fibrosis in patients with nonalcoholic fatty liver disease. Eur J Gastroenterol Hepatol. 2015;27:137-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 127. | Karbasi-Afshar R, Saburi A, Khedmat H. Cardiovascular disorders in the context of non-alcoholic Fatty liver disease: a literature review. J Tehran Heart Cent. 2014;9:1-8. [PubMed] |
| 128. | Byrne CD, Targher G. Ectopic fat, insulin resistance, and nonalcoholic fatty liver disease: implications for cardiovascular disease. Arterioscler Thromb Vasc Biol. 2014;34:1155-1161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 125] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 129. | Ballestri S, Lonardo A, Bonapace S, Byrne CD, Loria P, Targher G. Risk of cardiovascular, cardiac and arrhythmic complications in patients with non-alcoholic fatty liver disease. World J Gastroenterol. 2014;20:1724-1745. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 204] [Cited by in RCA: 200] [Article Influence: 18.2] [Reference Citation Analysis (1)] |
| 130. | Cholesterol Treatment Trialists’ (CTT) Collaboration; Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, Bhala N, Peto R, Barnes EH, Keech A, Simes J, Collins R. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376:1670-1681. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5048] [Cited by in RCA: 4652] [Article Influence: 310.1] [Reference Citation Analysis (1)] |
| 131. | Cholesterol Treatment Trialists’ (CTT) Collaborators; Mihaylova B, Emberson J, Blackwell L, Keech A, Simes J, Barnes EH, Voysey M, Gray A, Collins R, Baigent C. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet. 2012;380:581-590. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1891] [Cited by in RCA: 1962] [Article Influence: 150.9] [Reference Citation Analysis (0)] |