Published online Jan 14, 2015. doi: 10.3748/wjg.v21.i2.517
Peer-review started: May 2, 2014
First decision: June 10, 2014
Revised: August 4, 2014
Accepted: October 15, 2014
Article in press: October 15, 2014
Published online: January 14, 2015
AIM: To investigate the feasibility and accuracy of cone beam computed tomography (CBCT) in assessing the ablation zone after liver tumor ablation.
METHODS: Twenty-three patients (17 men and 6 women, range: 45-85 years old, mean age 65 years) with malignant liver tumors underwent ultrasound-guided percutaneous tumor ablation [radiofrequency (n = 14), microwave (n = 9)] followed by intravenous contrast-enhanced CBCT. Baseline multidetector computed tomography (MDCT) and peri-procedural CBCT images were compared. CBCT image quality was assessed as poor, good, or excellent. Image fusion was performed to assess tumor coverage, and quality of fusion was rated as bad, good, or excellent. Ablation zone volumes on peri-procedural CBCT and post-procedural MDCT were compared using the non-parametric paired Wilcoxon t-test.
RESULTS: Rate of primary ablation effectiveness was 100%. There were no complications related to ablation. Local tumor recurrence and new liver tumors were found 3 mo after initial treatment in one patient (4%). The ablation zone was identified in 21/23 (91.3%) patients on CBCT. The fusion of baseline MDCT and peri-procedural CBCT images was feasible in all patients and showed satisfactory tumor coverage (at least 5-mm margin). CBCT image quality was poor, good, and excellent in 2 (9%), 8 (35%), and 13 (56%), patients respectively. Registration quality between peri-procedural CBCT and post-procedural MDCT images was good to excellent in 17/23 (74%) patients. The median ablation volume on peri-procedural CBCT and post-procedural MDCT was 30 cm3 (range: 4-95 cm3) and 30 cm3 (range: 4-124 cm3), respectively (P-value > 0.2). There was a good correlation (r = 0.79) between the volumes of the two techniques.
CONCLUSION: Contrast-enhanced CBCT after tumor ablation of the liver allows early assessment of the ablation zone.
Core tip: Immediate intravenous contrast-enhanced cone beam computed tomography after percutaneous tumor ablation of the liver provides early assessment of the ablation zone and may provide the same information as multidetector computed tomography (MDCT) performed 1-2 mo after ablation. This is particularly interesting for centers that do not have MDCT in interventional rooms.
- Citation: Abdel-Rehim M, Ronot M, Sibert A, Vilgrain V. Assessment of liver ablation using cone beam computed tomography. World J Gastroenterol 2015; 21(2): 517-524
- URL: https://www.wjgnet.com/1007-9327/full/v21/i2/517.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i2.517
Local ablative techniques have become increasingly popular for the treatment of small malignant tumors of the liver, especially hepatocellular carcinoma (HCC) and liver metastases. Radiofrequency ablation (RFA) is the most widely used of these methods[1]. Microwave ablation (MWA) is another technique that has become increasingly popular since it does not require the conduction of electricity into tissue and it is not limited by charring[2]. Its efficacy has been shown to be similar to RFA in local tumor control[3,4].
One of the main drawbacks of local ablation is local intrahepatic recurrence. The most common risk factors for local tumor progression are tumor size, proximity to a major vessel, subcapsular location, and an insufficient ablative safety margin[5-7]. The latter factor seems to play a major role because significant agreement (86.8%) was found between the exact sites of local tumor progression and an insufficient ablative margin[8].
Procedure guidance methods include ultrasound, computed tomography (CT) scan and, more rarely, magnetic resonance imaging[9-11]. Depending on the institution, the patient characteristics, and the experience of the interventional radiologists, percutaneous tumor ablation can be guided by ultrasound only, CT scan only, or both. In the latter case, ultrasound is usually used for initial probe placement and CT to assess tumor coverage.
Cone beam computed tomography (CBCT) has been mainly evaluated for vascular applications such as transarterial chemoembolization[12-14], but also for non-vascular applications, such as image-guided procedures of the spine and pelvis[15]. Morimoto et al[16] described their initial experience in the liver in five patients who underwent radiofrequency ablation for malignant tumors, in which CBCT was used for needle placement but not to assess tumor coverage. More recently, Iwazawa et al[17,18] compared the effectiveness of CBCT and contrast-enhanced CT in assessing ablation margins. The authors concluded that the techniques were nearly equivalent. Because of the advantages of US-guided procedures and limitations of these techniques in monitoring tumor coverage, we hypothesized that CBCT could be used to assess effective ablation, especially in centers without CT dedicated to interventional radiology.
Thus, the purpose of this study was to evaluate the feasibility and accuracy of CBCT in assessing the ablation zone immediately after US-guided percutaneous tumor ablation with either RFA or MWA compared to pre-procedural CT and post-procedural CT 1-2 mo after ablation.
Institutional review board approval was obtained for this retrospective study and informed consent was waived. Inclusion criteria were: (1) the presence of a solitary malignant liver tumor treated percutaneously < 40 mm in diameter; (2) RFA or MWA performed in the interventional radiology room with CBCT acquisition immediately after tumor ablation; and (3) available pre- and 1-2 mo post-procedural contrast-enhanced CT scan examinations.
Between June 2009 and September 2012, 29 patients were identified. Six of these patients were excluded: three were lost to follow-up and another three could not receive iodine intravenous contrast agents. Thus, the final patient population included 23 patients (17 men and 6 women, range 45-85 years old, mean age 65 years old). There were 17 patients with HCC. Twelve patients presented with treatment-naïve HCC while five others had new tumors following either liver resection (n = 2), or transarterial chemoembolization (n = 3). Four patients had recurrent liver metastases following liver resection (3 colorectal and 1 breast). Two patients had recurrent intrahepatic cholangiocarcinoma following liver resection. The median tumor size was 20 mm (range: 8-40 mm). The median follow-up was 10.8 mo (range: 2-33 mo).
The routine protocol included a baseline multidetector CT (MDCT) scan examination performed before percutaneous tumor ablation for future comparison. A CT scan examination was performed between 1-2 mo after tumor ablation, every 3 mo for 1 year, and every 6 mo thereafter.
Helical multiphasic CT examinations were performed using a 64-section MDCT scan (CT Light-Speed Ultra; GE Healthcare, Milwaukee, WI, United States). All examinations included an unenhanced liver acquisition, and arterial, portal venous, and delayed phase images at 30-35, 70, and 180 s, respectively, after an intravenous injection of a 2 mL/kg dose of non-ionic contrast material (Iobitridol; Xenetix®, Guerbet, Aulnay-sous-bois, France), administered at a rate of 4 mL/s with a mechanical power injector (Medrad; Pittsburgh, PA, United States). Reconstruction slice thickness was 1.25 mm.
For each procedure, coagulation parameters were within the normal values. All RF (n = 14) and microwave (n = 9) ablations were performed percutaneously by one of two radiologists (8 and 10 years of experience) in the angiography room using real-time ultrasound guidance on an inpatient basis. Patients received conscious sedation with fentanyl citrate and propofol. Local anesthesia was achieved by injecting 5 mL of 1% lidocaine hydrochloride.
RF procedures were performed with a 15-gauge expandable electrode (Talon-Star-Burst; RITA Medical System, Mountain View, CA, United States) for monopolar (n = 5), and with internally cooled electrodes (Prosurge; Celon/Olympus, Berlin, Germany) for multiprobe (n = 9) procedures. Microwave procedures were performed with a 14-gauge cooled-shaft antenna (Aculis; Microsulis Medical Limited, Denmead, Hampshire, United Kingdom) in the remaining patients. Single-session ablations were performed depending on the tumor size and manufacturer’s recommendations, and included a 5-mm margin of normal-appearing hepatic tissue surrounding the tumor.
CBCT was performed at the end of the tumor ablation procedure. Electrodes were retracted to avoid metallic artifacts. If tumor coverage was satisfactory on 3D images, electrodes were removed and the needle track was treated by thermocoagulation.
CBCT was performed during breath holding, after elevating the patients’ arms above the head on a large format digital flat-panel angiographic system (GE-Healthcare Innova™ 4100IQ; Chalfont St Giles, United Kingdom). In the first 14 patients, the CBCT acquisitions took 10 seconds (10 s group) while in the remaining 9 patients CBCT rotation speed was decreased and acquisitions were obtained during a 20-s rotation protocol (20 s group). Acquisition started 70 s after intravenous administration of 90 mL of a non-ionic contrast agent (Iobitridol; Xenetix®, Guerbet, Aulnay-sous-bois, France), administered at a rate of 3 mL/s with a mechanical power injector (Medrad; Pittsburgh, PA, United States). No arterial phase acquisitions were obtained.
Reconstructed images were automatically forwarded to a specific workstation (Innova3DXR software and Advantage Workstation; GE Healthcare, Chalfont St Giles, United Kingdom) through the local network for analysis using various renderings of 3D images, such as volume rendering, maximum intensity projection, and multi-planar reformations.
The following analysis of images was retrospectively performed by two experienced radiologists blinded to the results, and a consensus was obtained: (1) evaluation of CBCT image quality; (2) assessment of technical success, i.e., tumor coverage at the end of tumor ablation; and (3) comparison of ablation zones with CBCT with 1-2 mo post-procedural MDCT data.
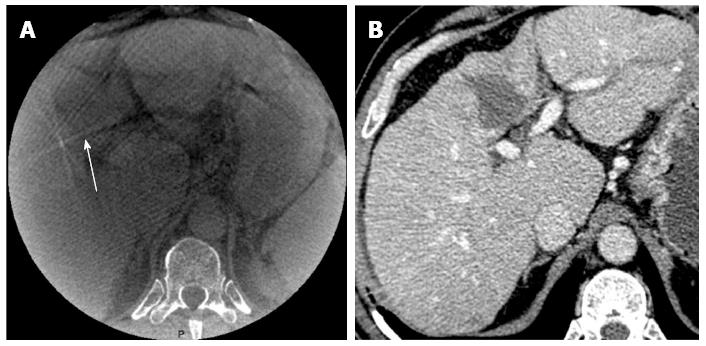
Image quality of cone beam CT: The quality of CBCT images was evaluated on a semi-quantitative scale ranging from 1-3 based on detection of the ablation zone and the presence of artifacts as follows: (1) poor: no visible ablation; (2) good: detection of ablation but the presence of artifacts preventing a clear definition of the zone; and (3) excellent: detection of the ablation zone and no artifacts. Figure 1 illustrates a poor image quality.
Assessment of tumor coverage: Baseline MDCT and peri-procedural CBCT images were compared and tumor coverage was assessed using multimodality image fusion performed with commercially available software (Integrated Registration; GE Healthcare, Chalfont St Giles, United Kingdom). The quality of image fusion was rated in relation to the contours of the liver and vessels by an experienced radiologist as follows: (1) bad registration (> 20 mm for liver contours); (2) good registration (between 5-20 mm for both liver vessels and contours); and (3) excellent registration for both liver vessels and contours (< 5 mm for both liver vessels and contours). Tumor coverage was defined as partial if portions of the tumors could be seen outside the hypo-attenuating ablation zone, complete if the entire tumor was located within the hypo-attenuating ablation zone, and satisfactory if at least 5 mm margin of normal-appearing hepatic tissue surrounded the tumor in the hypo-attenuating ablation area. The margins were assessed in the three spatial dimensions by measuring the distance between the border of the treated lesion and the outer border of the ablation zone.
Comparison of ablation zones with cone beam CT and 1-2 mo MDCT: The effectiveness of the technique was evaluated 1-2 mo after treatment with contrast-enhanced MDCT using standard, previously described terminology[19]. Peri-procedural CBCT images were compared to MDCT images. The ablation zone volume was calculated and compared for both sets of images using specific semi-automatic software (Volume Viewer; GE Healthcare, Waukesha, WI, United States).
Radiation dose: Dose area product (DAP) of each 3D acquisition performed on CBCT was initially stored and the mean DAP was calculated. We also recorded the radiation dose of 1-2 mo post-procedural multiphasic MDCT. In order to compare the radiation dose between CBCT and CT, conversion factors were used to obtain effective dose estimation in milliSievert (mSv). For CT, we used the conversion factor of 0.015 mSv/mGy per centimeter which is commonly used. The issue of patient dose in C-arm CBCT is complex. No conversion factor has been yet broadly adopted for 3D acquisitions with CBCT. Researchers estimated a DAP to effective dose coefficient for hepatic interventions of 0.16 mSv/mGy per square centimeter. We extrapolated this result to calculate a mean effective dose.
Numerical variables were expressed as medians and ranges. Categorical variables were summarized as numbers and percentages. Ablation zone volumes obtained from peri-procedural CBCT and from 1-2 mo post-procedural MDCT were compared using the non-parametric paired Wilcoxon t-test (P < 0.05 was considered to be significant). Ablation zone volumes measured on peri-procedural CBCT and 1-2 mo post-procedural MDCT were compared with the Bland and Altman plot and Pearson’s correlation coefficient. Statistical analyses were performed using SAS software (SAS Institute, Cary, NC, United States).
All tumors were treated according to protocol and covered completely as confirmed by ultrasound. Complete ablation was confirmed 1-2 mo post-CBCT on MDCT. Thus the rate of primary effectiveness was 100%. There were no complications related to ablation. Local tumor recurrence and new liver tumors were found 3 mo after initial treatment in one patient (4%).
In this study, the delay between preoperative CT and tumor ablation ranged from 1 to 58 d (median, 7 d) and the delay between tumor ablation and post-operative CT ranged from 28 to 63 d (median, 47 d).
Image quality was poor, good, and excellent in 2 (9%), 8 (35%), and 13 (56%), patients respectively (Table 1). The best image quality was observed in the 20 s group [8/9 excellent examinations (89%) vs 5/14 (36%) in the 10 s group, P = 0.04]. In the 2 patients with poor quality CBCT images, the ablation volume could not be visualized because of the very peripheral position of the tumor (n = 1) and artifacts from significant movement during acquisition (n = 1).
| Patient | Sex | Age (yr) | Tumor size (mm) | Tumor volume (cm3) | Ablation volume (cm3) | Registration | CBCT image quality | ||
| CBCT | MDCT | Quality | Time (mo) | ||||||
| 1 | M | 71 | 30 | 13.0 | 40 | 32 | 2 | 3.9 | 3 |
| 2 | M | 63 | 25 | 8.5 | 10 | 12 | 1 | 3.3 | 2 |
| 3 | M | 74 | 32 | 16.0 | 37 | 55 | 3 | 3.4 | 1 |
| 4 | M | 74 | 35 | 22.0 | 77 | 69 | 3 | 3.3 | 3 |
| 5 | M | 55 | 40 | 36.0 | 58.5 | 46 | 2 | 6.3 | 3 |
| 6 | M | 46 | 15 | 3.0 | 12 | 7.5 | 3 | 5 | 3 |
| 7 | M | 46 | 30 | 14.0 | 38 | 30 | 3 | 8 | 2 |
| 8 | M | 46 | 12 | 2.5 | 30 | 32 | 3 | 8 | 2 |
| 9 | M | 68 | 30 | 13.5 | 33 | 59 | 3 | 4.2 | 2 |
| 10 | M | 65 | 14 | 3.0 | 10 | 17 | 1 | 4 | 1 |
| 11 | M | 86 | 8 | 0.5 | 58 | 38 | 1 | 7 | 2 |
| 12 | F | 68 | 20 | 5.0 | 4 | 13 | 1 | 5 | 2 |
| 13 | M | 60 | 15 | 3.5 | 47 | 34 | 1 | 8 | 2 |
| 14 | F | 56 | 30 | 13.0 | 32 | 38 | 3 | 7 | 3 |
| 15 | F | 84 | 22 | 6.0 | 10 | 12 | 3 | 4 | 3 |
| 16 | M | 54 | 35 | 27.0 | 95 | 124 | 3 | 5 | 3 |
| 17 | M | 81 | 24 | 8.5 | 8 | 9 | 3 | 5 | 3 |
| 18 | F | 76 | 13 | 3.0 | 8 | 6 | 3 | 3 | 3 |
| 19 | F | 76 | 14 | 3.5 | 10 | 11 | 2 | 3 | 3 |
| 20 | M | 77 | 18 | 4.0 | 31 | 29 | 3 | 5 | 3 |
| 21 | M | 77 | 11 | 2.5 | 13 | 30 | 3 | 5 | 3 |
| 22 | F | 50 | 13 | 3.0 | 27 | 19 | 2 | 6 | 3 |
| 23 | M | 57 | 14 | 3.5 | 7 | 4 | 1 | 8 | 2 |
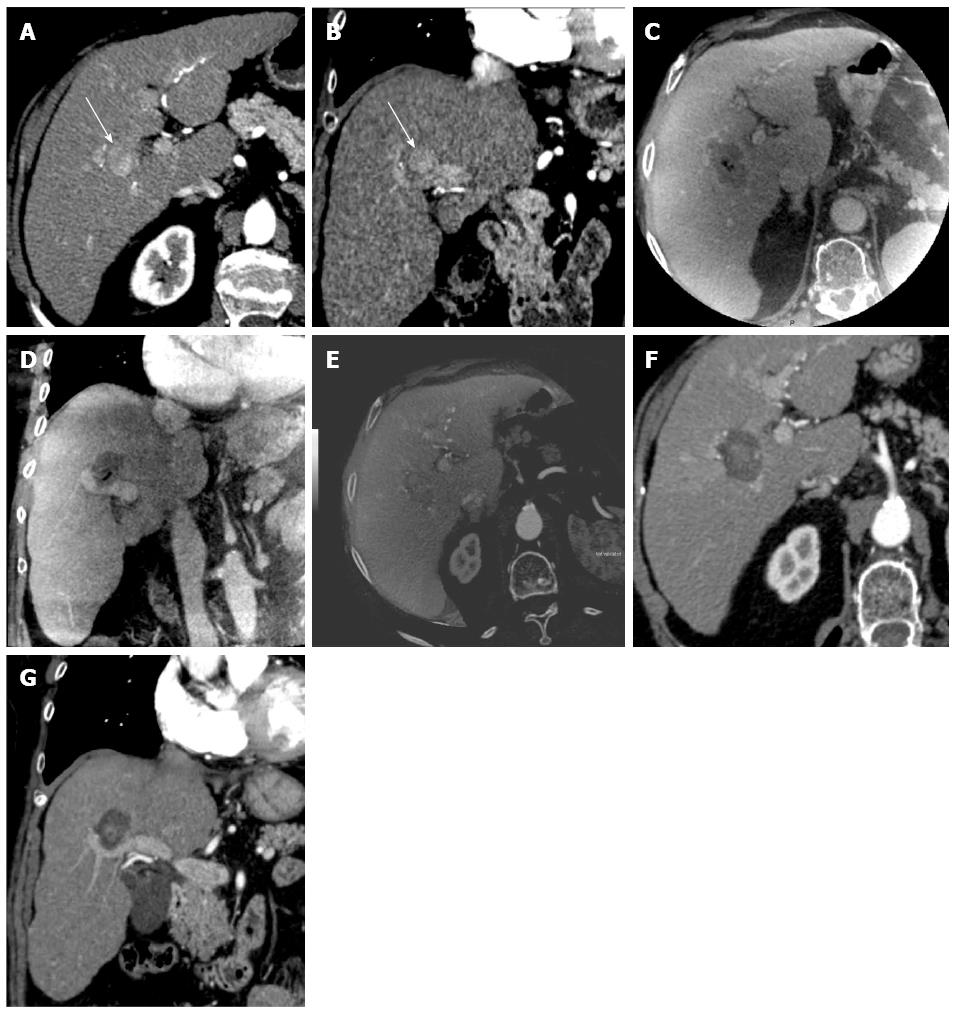
For patients with visible ablation zones (21/23, 91%), the fusion of baseline MDCT and peri-procedural CBCT images showed satisfactory tumor coverage (Figure 2). The minimal margin ablation of 5 mm was achieved in all cases.
Registration quality between peri-procedural CBCT and baseline MDCT images was excellent in 13 (57%) patients, good in 4 (17%) patients, and bad in 6 (26%) patients (Table 1). The median registration time was 5 min (range: 3-8 min). The best registration was observed in the 20 s group [6/9 excellent registrations (67%) vs 6/14 (43%) in the 10 s group, P = 0.04]. Figure 2 shows a comparison between peri-procedural CBCT and both baseline and 1-2 mo post-procedural MDCT images.
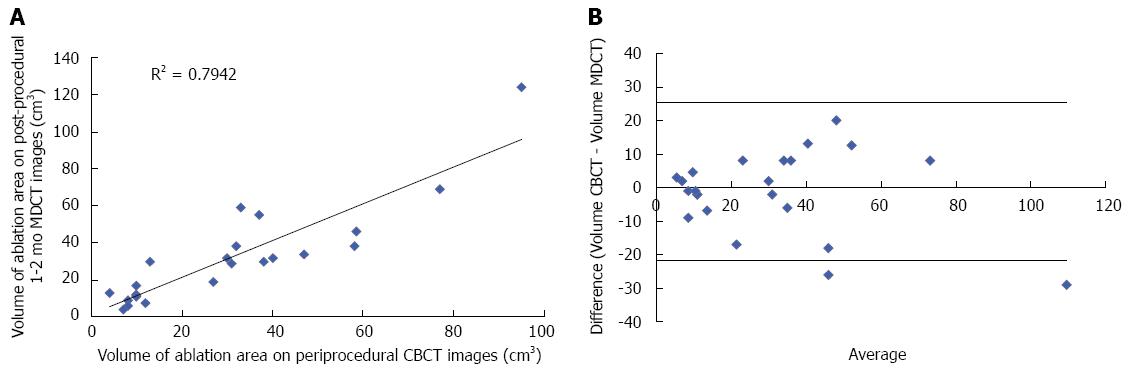
The median ablation zone volumes of peri-procedural CBCT and 1-2 mo post-procedural MDCT images were 30 cm3 (range: 4-95 cm3) and 30 cm3 (range: 4-124 cm3), respectively (P-value > 0.2) (Table 1). There was a good correlation (r = 0.79) between the volumes of the two techniques. Figure 3 shows the distribution of the ablation zone volumes assessed by both CBCT and 1-2 mo post-procedural MDCT images, and the Bland-Altman limits of agreement.
The median DAP of 3D acquisitions performed on CBCT was 43.8 Gy.cm2 (range: 21-74 Gy.cm2). The median effective dose was 7.0 mSv (range: 3.3-11.8 mSv) for CBCT. The median effective dose was 36 mSv (range: 20-52 mSv) for all CT phases and 14.7 mSv (range: 6-27 mSv) for one CT acquisition.
This study evaluated a combination of ultrasound and contrast-enhanced CBCT for the assessment of hepatic tumor ablation. Results show that this combination is feasible, effective in assessing tumor coverage, and can provide immediate information on the ablation zone, which is well correlated to the reference CT performed one to two months after the procedure.
The goal of monitoring is to identify changes that occur during tumor ablation and determine tumor coverage, which is a key factor in local tumor progression[15]. Ultrasound, which is effective for tumor targeting, is limited for ablation monitoring, mainly due to the production of gas during the procedure. However, during ultrasound guided-ablation, the addition of a contrast agent (CEUS) provides important information for immediate treatment assessment, to detect and remove residual viable tumor areas[20]. The first aim of this study was to determine whether contrast-enhanced CBCT could be a useful tool and an interesting alternative to CEUS or MDCT for assessing the technical success of treatment. The use of intravenous contrast-enhanced acquisitions provided significant attenuation differences between the treated area and the adjacent liver parenchyma. In this study, the reliability of this technique was very good, since the contrast-enhanced CBCT images visualized the targeted area in all patients except two (9%). Moreover, limited results were observed in the first patients and were due to the learning curve.
Protocol optimization including a decrease in the CBCT rotation speed and patient positioning clearly improved the quality of images. Tumor coverage was satisfactory in all cases with at least a 5-mm margin of normal-appearing hepatic tissue around the tumor within the hypo-attenuating ablated area on fusion images. Tumor coverage is even more important in large tumors treated by a multiprobe technique or other tumor ablation techniques requiring careful needle placement. In these cases, immediate visualization of tumor coverage can be used to reposition the needles and perform an additional ablation. In the present series, tumor coverage was satisfactory so that electrode needle replacement was not necessary. Indeed, combining the CBCT technology and integrated navigation software can also help guide needles during percutaneous tumor ablation[16].
The second aim of this study was to determine whether contrast-enhanced CBCT could predict the ablation volume immediately after the ablative procedure. Thus, peri-procedural CBCT images were compared with 1-2 mo post-procedural MDCT used as the reference imaging modality. There was an excellent correlation between ablation zone volumes. Interestingly, besides the quantitative results, image registration of the two approaches matched very well in most cases, confirming that immediate assessment of the ablation zone was highly reliable. These results are similar to those published by Iwazawa et al[18] indicating that the combination of ultrasound and peri-procedural CBCT provides an interesting alternative to MDCT. This combination could become the standard of care in centers in which interventional radiology CT is not available. In these centers, the use of CBCT rather than diagnostic CT could be more cost-effective, and make diagnostic CT available to other patients. Further study is needed on this issue.
Indeed, despite the advantages of CBCT in interventional radiology rooms, the quality of images with this technology is not as good as that of conventional MDCT. The increased scattered radiation generated by CBCT results in image artifacts, decreased contrast-to-noise, and inaccuracies in CT calculations[13]. Although this new technique will not replace MDCT to detect local tumor progression in the near future, it can provide peri-procedural information to identify incomplete treatment.
CBCT requires increased doses of radiation. However, this is difficult to evaluate because there is no standard dose metric. For instance, the CT dose index and the dose length product do not correctly apply to cone beam geometries in a flat panel detector. Recently, Strocchi et al[21] compared the dose of cone beam CT and MDCT in thoracic biopsy procedures. The authors concluded that although the differences were not always statistically significant, the general distribution of organ doses showed that the MDCT-guided doses were higher than CBCT-guided doses.
We also wanted to compare the radiation dose of CBCT acquisition and CT. This task is rather difficult because there is a lack of a universally accepted common dose metric. For instance, CT dose index and the dose length product do not correctly apply to cone beam geometries of a flat panel detector and the dose is non-linear with the central slice getting the highest dose. However imperfect and indirect, we have found that the median effective dose of CBCT acquisition was inferior to that of MDCT acquisition. Our results are in agreement with recent publications[22,23].
Besides its monocentric, retrospective design, the present study has certain limitations. Firstly, CBCT acquisitions were obtained during the portal venous phase to maximize the contrast between the ablation zone and the liver parenchyma. Nevertheless, the value of arterial-phase CBCT acquisitions for detecting residual arterial enhancement was not assessed. Secondly, an acquisition field of view of 40 cm provides a reconstruction field of view of 23.2 cm due to the magnification effect caused by source image distance. Therefore, patient positioning is important, especially in obese patients. Indeed, positioning should be planned before the procedure, so that the ablation zone is in the center of the acquired volume. In addition, the study population was fairly small. Finally, post-procedural MDCT was performed at different times after the ablation (1-2 mo). This might create a potential bias due to the progressive modification of the ablation zone. Nevertheless, a significant correlation was observed between ablation volume on peri-procedural CBCT and post-procedural MDCT. This suggests that these modifications are limited and do not affect assessment of the ablation volume.
In conclusion, this study shows that immediate intravenous contrast-enhanced CBCT following percutaneous tumor ablation of the liver is feasible and could provide early assessment of the ablation zone and margins.
Ablation is an increasingly used treatment for focal liver lesions, particularly hepatocellular carcinoma and liver metastases. The risk of local tumoral progression after the treatment is strongly associated with the volume of ablation, and especially the presence of peritumoral safety margins. Early assessment of the ablation volume is therefore very important.
Early evaluation of ablation area can be performed with immediate contrast-enhanced cross-sectional imaging such as computed tomography (CT) or magnetic resonance (MR). However, many teams use ultrasound guidance for liver ablation. In this setting, ablation volume is not easily evaluated. The authors show that contrast-enhanced cone beam computed tomography performed immediately after ablation may help in visualizing of the ablation volume.
Recent studies have demonstrated the clinical value of cone beam computed tomography (CBCT) for the guidance, monitoring and evaluation of numerous vascular and non-vascular interventional radiology procedures such as biopsies or intra-arterial therapies. This study enlarges the field of application of contrast-enhanced CBCT to liver ablation procedures.
By showing ablation volume immediately after ablation, these results may lead to a more confident ablation procedure, avoiding the use of CT or MR which can therefore be dedicated to diagnostic purposes, and hopefully decrease the rate of local tumoral progression.
CBCT refers to a recently developed imaging technique using X-ray. During the image acquisition, an X-ray source rotates around the patient and enables the acquisition of a volume of interest. The technology resembles that of CT, but is performed in interventional radiology rooms using a flat panel detector. It provides three-dimensional rendering of tissues and is very useful for catheter vascular and non-vascular procedure guidance, monitoring and evaluation.
The authors examined the feasibility, image quality and immediate interest of contrast-enhanced CBCT for the evaluation of ablation volume after liver ablation. They showed that contrast-enhanced CBCT is feasible and could provide early assessment of the ablation zone and margins. They also showed that CBCT images are well correlated with the reference CT performed one to two months after the procedure. These results are interesting and may indicate that CBCT can provide early additional information for the management of the patients.
P- Reviewer: Phongkitkarun S S- Editor: Gou SX L- Editor: Logan S E- Editor: Ma S
| 1. | Germani G, Pleguezuelo M, Gurusamy K, Meyer T, Isgrò G, Burroughs AK. Clinical outcomes of radiofrequency ablation, percutaneous alcohol and acetic acid injection for hepatocelullar carcinoma: a meta-analysis. J Hepatol. 2010;52:380-388. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 209] [Cited by in F6Publishing: 226] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 2. | Wright AS, Sampson LA, Warner TF, Mahvi DM, Lee FT. Radiofrequency versus microwave ablation in a hepatic porcine model. Radiology. 2005;236:132-139. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 344] [Cited by in F6Publishing: 330] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 3. | Iida H, Aihara T, Ikuta S, Yamanaka N. A comparative study of therapeutic effect between laparoscopic microwave coagulation and laparoscopic radiofrequency ablation. Hepatogastroenterology. 2013;60:662-665. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 4. | Qian GJ, Wang N, Shen Q, Sheng YH, Zhao JQ, Kuang M, Liu GJ, Wu MC. Efficacy of microwave versus radiofrequency ablation for treatment of small hepatocellular carcinoma: experimental and clinical studies. Eur Radiol. 2012;22:1983-1990. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 124] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 5. | Kim YS, Rhim H, Cho OK, Koh BH, Kim Y. Intrahepatic recurrence after percutaneous radiofrequency ablation of hepatocellular carcinoma: analysis of the pattern and risk factors. Eur J Radiol. 2006;59:432-441. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 187] [Cited by in F6Publishing: 196] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 6. | Nakazawa T, Kokubu S, Shibuya A, Ono K, Watanabe M, Hidaka H, Tsuchihashi T, Saigenji K. Radiofrequency ablation of hepatocellular carcinoma: correlation between local tumor progression after ablation and ablative margin. AJR Am J Roentgenol. 2007;188:480-488. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 262] [Cited by in F6Publishing: 261] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 7. | Mulier S, Ni Y, Jamart J, Ruers T, Marchal G, Michel L. Local recurrence after hepatic radiofrequency coagulation: multivariate meta-analysis and review of contributing factors. Ann Surg. 2005;242:158-171. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 530] [Cited by in F6Publishing: 523] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 8. | Kei SK, Rhim H, Choi D, Lee WJ, Lim HK, Kim YS. Local tumor progression after radiofrequency ablation of liver tumors: analysis of morphologic pattern and site of recurrence. AJR Am J Roentgenol. 2008;190:1544-1551. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 71] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 9. | Clasen S, Pereira PL. Magnetic resonance guidance for radiofrequency ablation of liver tumors. J Magn Reson Imaging. 2008;27:421-433. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 87] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 10. | Solomon SB, Silverman SG. Imaging in interventional oncology. Radiology. 2010;257:624-640. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 11. | Crocetti L, Della Pina C, Cioni D, Lencioni R. Peri-intraprocedural imaging: US, CT, and MRI. Abdom Imaging. 2011;36:648-660. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Virmani S, Ryu RK, Sato KT, Lewandowski RJ, Kulik L, Mulcahy MF, Larson AC, Salem R, Omary RA. Effect of C-arm angiographic CT on transcatheter arterial chemoembolization of liver tumors. J Vasc Interv Radiol. 2007;18:1305-1309. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 65] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 13. | Wallace MJ, Kuo MD, Glaiberman C, Binkert CA, Orth RC, Soulez G. Three-dimensional C-arm cone-beam CT: applications in the interventional suite. J Vasc Interv Radiol. 2009;20:S523-S537. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 59] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 14. | Suk Oh J, Jong Chun H, Gil Choi B, Giu Lee H. Transarterial chemoembolization with drug-eluting beads in hepatocellular carcinoma: usefulness of contrast saturation features on cone-beam computed tomography imaging for predicting short-term tumor response. J Vasc Interv Radiol. 2013;24:483-489. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 15. | Leschka SC, Babic D, El Shikh S, Wossmann C, Schumacher M, Taschner CA. C-arm cone beam computed tomography needle path overlay for image-guided procedures of the spine and pelvis. Neuroradiology. 2012;54:215-223. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 16. | Morimoto M, Numata K, Kondo M, Nozaki A, Hamaguchi S, Takebayashi S, Tanaka K. C-arm cone beam CT for hepatic tumor ablation under real-time 3D imaging. AJR Am J Roentgenol. 2010;194:W452-W454. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 60] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 17. | Iwazawa J, Hashimoto N, Mitani T, Ohue S. Fusion of intravenous contrast-enhanced C-arm CT and pretreatment imaging for ablation margin assessment of liver tumors: A preliminary study. Indian J Radiol Imaging. 2012;22:251-253. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 18. | Iwazawa J, Ohue S, Hashimoto N, Mitani T. Ablation margin assessment of liver tumors with intravenous contrast-enhanced C-arm computed tomography. World J Radiol. 2012;4:109-114. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 15] [Cited by in F6Publishing: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Goldberg SN, Grassi CJ, Cardella JF, Charboneau JW, Dodd GD, Dupuy DE, Gervais D, Gillams AR, Kane RA, Lee FT. Image-guided tumor ablation: standardization of terminology and reporting criteria. J Vasc Interv Radiol. 2005;16:765-778. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 232] [Cited by in F6Publishing: 246] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 20. | Guibal A, Bertin C, Egels S, Savier E, Grenier PA, Lucidarme O. Contrast-enhanced ultrasound (CEUS) follow-up after radiofrequency ablation or cryoablation of focal liver lesions: treated-area patterns and their changes over time. Eur Radiol. 2013;23:1392-1400. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 21. | Strocchi S, Colli V, Conte L. Multidetector CT fluoroscopy and cone-beam CT-guided percutaneous transthoracic biopsy: comparison based on patient doses. Radiat Prot Dosimetry. 2012;151:162-165. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 22. | Tselikas L, Joskin J, Roquet F, Farouil G, Dreuil S, Hakimé A, Teriitehau C, Auperin A, de Baere T, Deschamps F. Percutaneous Bone Biopsies: Comparison between Flat-Panel Cone-Beam CT and CT-Scan Guidance. Cardiovasc Intervent Radiol. 2014;Epub ahead of print. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 23. | Hirota S, Nakao N, Yamamoto S, Kobayashi K, Maeda H, Ishikura R, Miura K, Sakamoto K, Ueda K, Baba R. Cone-beam CT with flat-panel-detector digital angiography system: early experience in abdominal interventional procedures. Cardiovasc Intervent Radiol. 2006;29:1034-1038. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 109] [Cited by in F6Publishing: 109] [Article Influence: 6.4] [Reference Citation Analysis (0)] |