Published online May 14, 2015. doi: 10.3748/wjg.v21.i18.5473
Peer-review started: November 8, 2014
First decision: December 11, 2014
Revised: December 31, 2014
Accepted: February 12, 2015
Article in press: February 13, 2015
Published online: May 14, 2015
AIM: To assess the effects of dihydromyricetin (DHM) as a hepatoprotective candidate in reducing hepatic injury and accelerating hepatocyte proliferation after carbon tetrachloride (CCl4) treatment.
METHODS: C57 BL/6 mice were used in this study. Mice were orally administered with DHM (150 mg/kg) for 4 d after CCl4 treatment. Serum and liver tissue samples were collected on days 1, 2, 3, 5 and 7 after CCl4 treatment. The anti-inflammatory effect of DHM was assessed directly by hepatic histology detection and indirectly by serum levels of aspartate aminotransferase (AST), alanine aminotransferase (ALT), albumin, and superoxide dismutase (SOD). Inflammatory cytokines, such as interleukin (IL)-1β, IL-6 and tumor necrosis factor-α (TNF-α), were detected using ELISA kits. Proliferating cell nuclear antigen (PCNA) staining was used to evaluate the role of DHM in promoting hepatocyte proliferation. Hepatocyte apoptosis was measured by TUNEL assay. Furthermore, apoptosis proteins Caspases-3, 6, 8, and 9 were detected by Western blot. SP600125 were used to confirm whether DHM regulated liver regeneration through JNK/TNF-α pathways.
RESULTS: DHM showed a strong anti-inflammatory effect on CCl4-induced liver injury in mice. DHM could significantly decrease serum ALT, AST, IL-1β, IL-6 and TNF-α and increase serum albumin, SOD and liver SOD compared to the control group after CCl4 treatment (P < 0.05). PCNA results indicated that DHM could significantly increase the number of PCNA positive cells compared to the control (348.9 ± 56.0 vs 107.1 ± 31.4, P < 0.01). TUNEL assay showed that DHM dramatically reduced the number of apoptotic cells after CCl4 treatment compared to the control (365.4 ± 99.4 vs 90.5±13.8, P < 0.01). Caspase activity detection showed that DHM could reduce the activities of Caspases- 8, 3, 6 and 9 compared to the control (P < 0.05). The results of Western blot showed that DHM increased the expression of JNK and decreased TNF-α expression. However, DHM could not affect TNF-α expression after SP600125 treatment. Furthermore, DHM could significantly improve the survival rate of acute liver failure (ALF) mice (73.3% vs 20.0%, P < 0.0001), and SP600125 could inhibit the effect of DHM.
CONCLUSION: These findings demonstrate that DHM alleviates CCl4-induced liver injury, suggesting that DHM is a promising candidate for reversing liver injury and ALF.
Core tip: Our research confirmed that dihydromyricetin (DHM) plays an anti-inflammatory role in the carbon tetrachloride (CCl4) induced acute liver injury mice. It was shown that DHM could alleviate CCl4-induced acute liver injury by reducing apoptosis and accelerating proliferation of hepatocytes. Furthermore, DHM treatment up-regulated Jun kinase expression in liver tissue, and the mice which were injected with SP600125 could not survive in the acute liver failure induced by lethal dose CCl4 injection.
-
Citation: Xie J, Liu J, Chen TM, Lan Q, Zhang QY, Liu B, Dai D, Zhang WD, Hu LP, Zhu RZ. Dihydromyricetin alleviates carbon tetrachloride-induced acute liver injury
via JNK-dependent mechanism in mice. World J Gastroenterol 2015; 21(18): 5473-5481 - URL: https://www.wjgnet.com/1007-9327/full/v21/i18/5473.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i18.5473
The liver plays a crucial role in the regulation of body metabolism as well as in detoxification. Due to these essential functions, injuries to this organ need to be rapidly and efficiently remedied. Hepatotoxins, such as alcohol, acetaminophen, and carbon tetrachloride (CCl4), induced liver injury, which is characterized by hepatocyte necrosis and dysfunction of the liver. Since the mechanism of CCl4-induced liver injury is very similar to liver diseases, it is commonly used as a hepatotoxin in experimental hepatopathy[1-3].
Dihydromyricetin was isolated from the tender stem and leaves of the Ampelopsis grossedentata species. DHM has numerous pharmacological activities, such as anti-inflammatory, antioxidation and anticarcinogenic effects[4]. In the present study, we aimed to assess the effects of DHM as a hepatoprotective candidate in reducing hepatic injury and accelerating hepatocyte proliferation following CCl4 treatment. The present findings indicate that DHM shows a potent antihepatotoxic activity in recovery of hepatocellular apoptosis and acceleration of liver regeneration during liver injury. A better understanding of DHM-regulated liver regeneration will be important to develop effective interventions to prevent or treat liver disease.
Tumor necrosis factor-α (TNF-α) is a pro-inflammatory cytokine. Activation of TNF-receptor family members is considered to play an important role in the pathogenesis and progression of liver disease[5,6]. TNF-α is implicated in hepatocyte apoptosis, but the pathways contributing to initiation and progression of acute liver injury are presently vague[7]. The JNK signaling pathway plays an important role in TNF-dependent acute liver damage[8,9]. JNK has been shown to be involved in liver carcinogenesis and be required for hepatocellular carcinoma (HCC) cell proliferation and hepatocyte proliferation in liver regeneration[10]. In a previous study, we found that CCl4 could increase TNF-α expression in serum and liver tissue, which results in acute liver injury[11]. Furthermore, we found that DHM could up-regulate the activation of JNK, and then decreased the expression of TNF-α in CCl4-induced liver injury mice. In addition, the hepatoprotective role of DHM could be inhibited after blocking the activation of JNK. These results suggest that DHM could be a treatment option for liver injury. We thus assess its therapeutic potential in clinically relevant models of TNF-mediated liver damage and acute liver failure (ALF).
The animal protocol was designed to minimize pain or discomfort to the animals. The animals were acclimatized to laboratory conditions (23 °C, 12 h/12 h light/dark, 50% humidity, ad libitum access to food and water) for two weeks prior to experimentation. Intragastric gavage administration was carried out with conscious animals, using straight gavage needles appropriate for the animal size (15-17 g body weight: 22 gauge, 1 inch length, 1.25 mm ball diameter). All animals were euthanized by barbiturate overdose (intravenous injection, 150 mg/kg pentobarbital sodium) for tissue collection.
This research was implemented following the recommendations in the Guide for the Care and Use of Laboratory Animals of the Ministry of Health of the People’s Republic of China. The protocol was approved by the Committee on the Ethics of Animal Experiments of Guangdong Medical College (Permit Number: SYXK 2008-0007). Male C57BL/6 mice which were 8 wk old (purchased from Shanghai Slac Laboratory Animal Corporation and kept in SPF environment) were used in this research.
Main materials used in this research included DHM and CCl4 (Sigma-aldrich, St Louis, United States), assay kits for detection of serum ALT, AST, albumin, superoxide dismutase (SOD) and tissue SOD (Jiancheng, Nanjing, China), assay kits for detection of serum IL-1β, IL-6 and TNF-α (RD corporation, Minneapolis, United States), mouse monoclonal antibody to proliferating cell nuclear antigen (PCNA) and SABC staining kit (Boster, Wuhan, China), primary antibodies to JNK, phosphorylation-JNK, TNF-α, Cytochrome C, Bax and β-actin (Cell signaling, Beverly, MA), colorimetric assay kits of Caspases-3, 6, 8, and 9 activities (Calbiochem, La Jolla, CA), In Suit Cell Death Detection kit-POD (Roche, Basal, Switzerland), and SP600125 (Selleckchem, Houston, United States).
The preparation of the animal model was done as previously described[11,12]. Briefly, acute liver injury was induced in mice by intraperitoneal injection of CCl4 (1 mL/kg) to test the hepatoprotective role of DHM. Meanwhile, ALF mice were prepared by intraperitoneal injection of a lethal dose of CCl4 (2.6 mL/kg) to test the effect of DHM on the survival rate of mice[11]. DHM was dissolved in 0.5% sodium carboxymethylcellulose (CMC-Na) diluted in ultrapure water, to a final concentration of 37.5 mg/mL. Then, mice were treated orally with DHM (150 mg/kg per mouse; once per day, for 4 d) 2 h after CCl4 treatment. JNK inhibitor SP600125 (50 mg/kg) was administered by intraperitoneal injection 1 h prior to DHM treatment[13]. Mice were dedicated on days 1, 2, 3, 5 and 7 after CCl4 treatment to examine a series of indicators of liver injury and regeneration.
Serum AST, ALT, albumin and SOD levels were determined with the commercial assay kits. Serum IL-6, IL-1β and TNF-α levels were determined with ELISA kits according to the manufacturer’s instructions.
Histology and injury grading were performed as previously described[11]. The degree of necrosis after acute liver injury was evaluated by the degree of necrotic lesions in the parenchyma.
The hepatocyte proliferation was evaluated by PCNA staining. Liver tissues were fixed in neutral buffered formalin for at last 24 h, and then embedded in paraffin to make pathological section. The sections were stained using PCNA antibody and the SABC staining kit according to manufacturer’s protocol. After that, sections were observed under a light microscope and the PCNA positive cells were counted in at least 5 fields.
Cell apoptosis rate was detected using In Suit Cell Death Detection kit-POD according to the manufacturer’s instructions. Briefly, liver tissues were dewaxed and rehydrated by using xylene and a graded series of ethanol, and then incubated for 15 min at 37 °C with proteinase K working solution. After that, the samples were incubated at 37 °C in dark for 1 h with 50 μL Converter-POD per sample for 30 min. Hematoxylin was used to stain the nuclei and the stained cells was analyzed under a light microscope.
One hundred and twenty mice were divided randomly into four groups. Group 1 was administered with a lethal dose of CCl4 (2.6 mg/kg) and served as a control; group 2 was administered with 2.6 mg/kg CCl4, and 2 h later, each mouse was orally treated with 150 mg/kg per day DHM; group 3 was injected intraperitoneally with SP600125, one hour before CCl4 administration, and 2 h later, each mouse was orally treated with 150 mg/kg per day DHM. Survival rates in these groups were recorded every 12 h.
Liver tissues were homogenized and lysed with RIPA buffer (Beyotime, Jiangsu, China), and the protein concentration in each sample was detected with a BSA assay kit (Beyotime, Jiangsu, China). Proteins were extracted to detect cytochrome C by using the Mitochondria/Cytosol Fractionation Kit. Proteins were electrophoresed on an SDS-PAGE gel, and then transferred onto PVDF membranes. The membranes were then incubated with primary and secondary antibodies. Signal detection was performed by using enhanced ECL chemiluminescence reagents (GE-Healthcare, Pittsburgh, United States).
Caspases-3, 6, 8 and 9 activities were determined in liver extracts by measuring proteolytic cleavage of the proluminescent substrates Z-IETDAFC, AC-DEVD-AMC, AC-VEID-AFC and AC-LEHD-AFC (Calbiochem, La Jolla, CA). The fluorescence was determined based on the amount of released AFC (caspase-8, -6, -9, λex = 400, λem = 505) or AMC (caspase-3, λex = 380, λem = 460). The results are expressed as arbitrary units of fluorescence (AUF) per milligram of protein.
The data between the CCl4 group and DHM group were compared by t-test, and the survival results were analyzed by log-rank test and presented as Kaplan-Meier survival curves. The statistical methods of this study were reviewed by Qingyu Zhang from Affiliated Hospital of Guangdong Medical College.
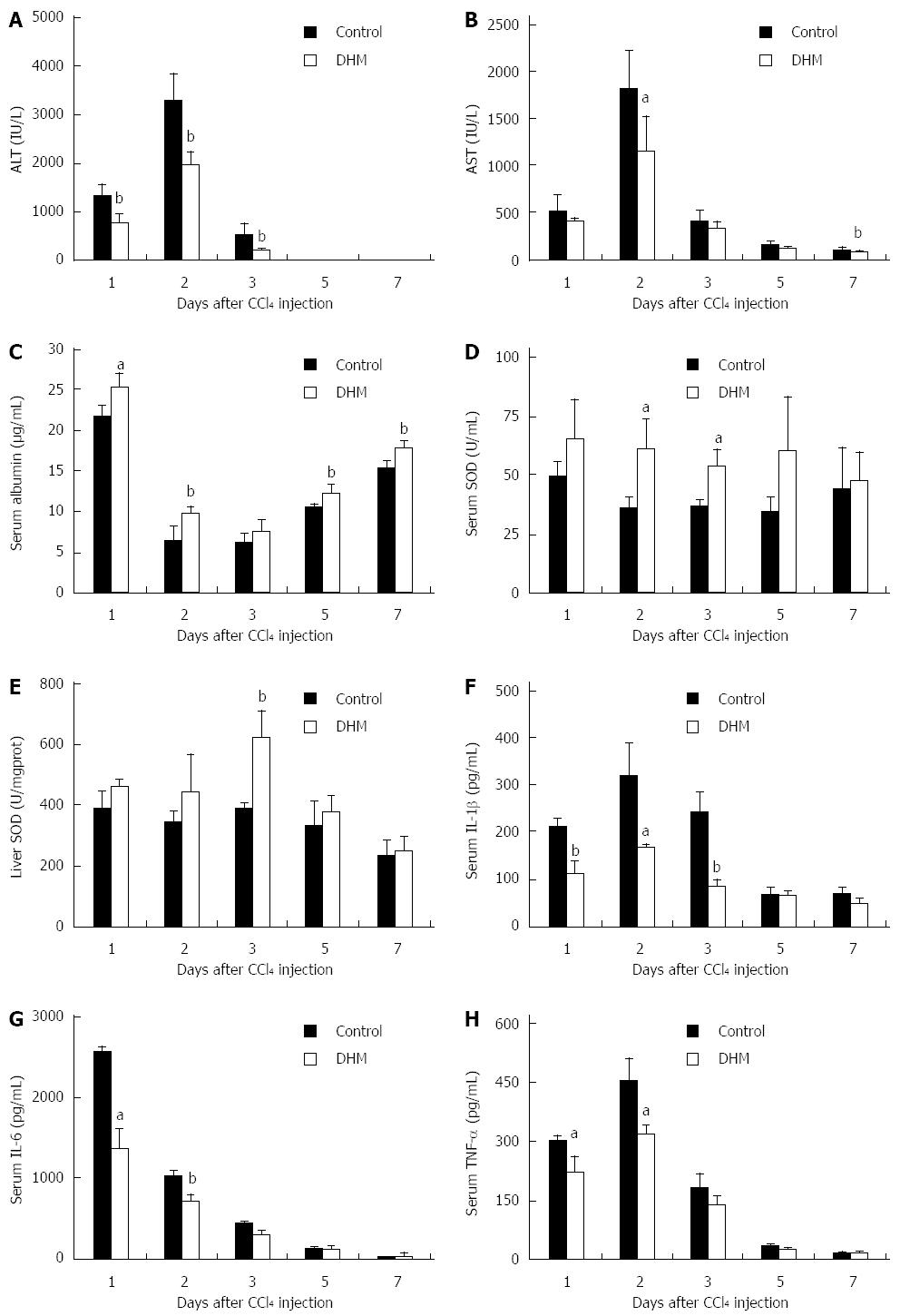
To confirm the role of DHM in protecting mice against acute liver injury, we employed serum ALT, AST, albumin and SOD as indicators. After CCl4 injection, serum ALT and AST were rapidly increased to peak levels on day 2, and then decreased thereafter, while DHM could significantly decrease serum ALT and AST from day 1 to day 5 (Figure 1A and B). The attenuation of serum AST and ALT indicated that DHM possesses a good protective effect on liver function. Serum albumin level is also considered an important indicator for evaluating functional recovery of acutely injured liver. Serum albumin was significantly raised after DHM administration compared to the control (Figure 1C). SOD belongs to active oxygen scavenging enzyme systems, which is regarded as a biomarker to judge the anti-oxidative ability of the liver. We found that the activities of SOD in both serum and liver tissue were enhanced markedly compared to the control after DHM treatment in mice with CCl4-induced liver injury (Figure 1D and E). Furthermore, serum IL-1β was found to be rapidly increased after CCl4 treatment, which was also supported in previous studies[14], whereas DHM administration resulted in significant attenuation from day 1 to day 5 after CCl4 injection (Figure 1F). IL-6 and TNF-α have been identified as attractive targets for liver regeneration. The data showed that DHM could markedly down-regulate IL-6 and TNF-α (Figure 1G and H).
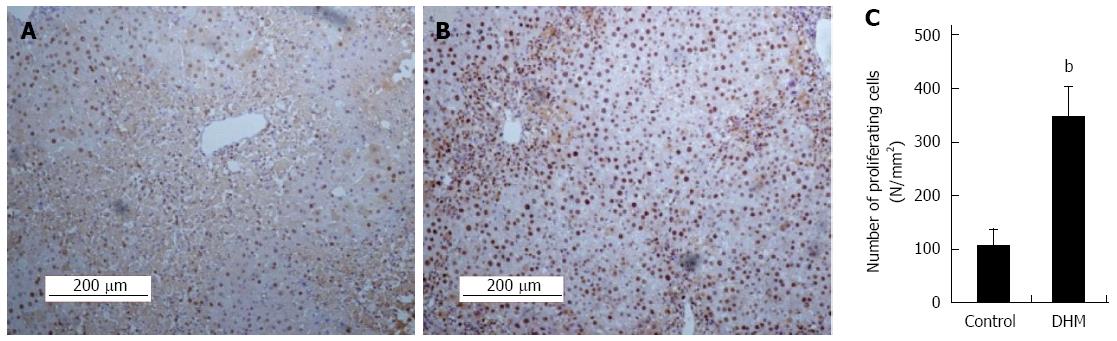
To determine whether DHM could accelerate hepatocyte proliferation from acute phase during liver regeneration, we evaluated the proliferation of hepatocytes by using immunostaining of PCNA in liver sections on day 2 after CCl4 treatment. Compared with the control, DHM significantly increased the number of PCNA positive cells. A great number of PCNA+ hepatocytes were found surrounding the portal area (Figure 2A and B). PCNA+ cells in at least 12 mm2 tissue sections were counted for each mouse, and the data showed that DHM could markedly accelerate hepatocyte proliferation from an acute phase (Figure 2C).
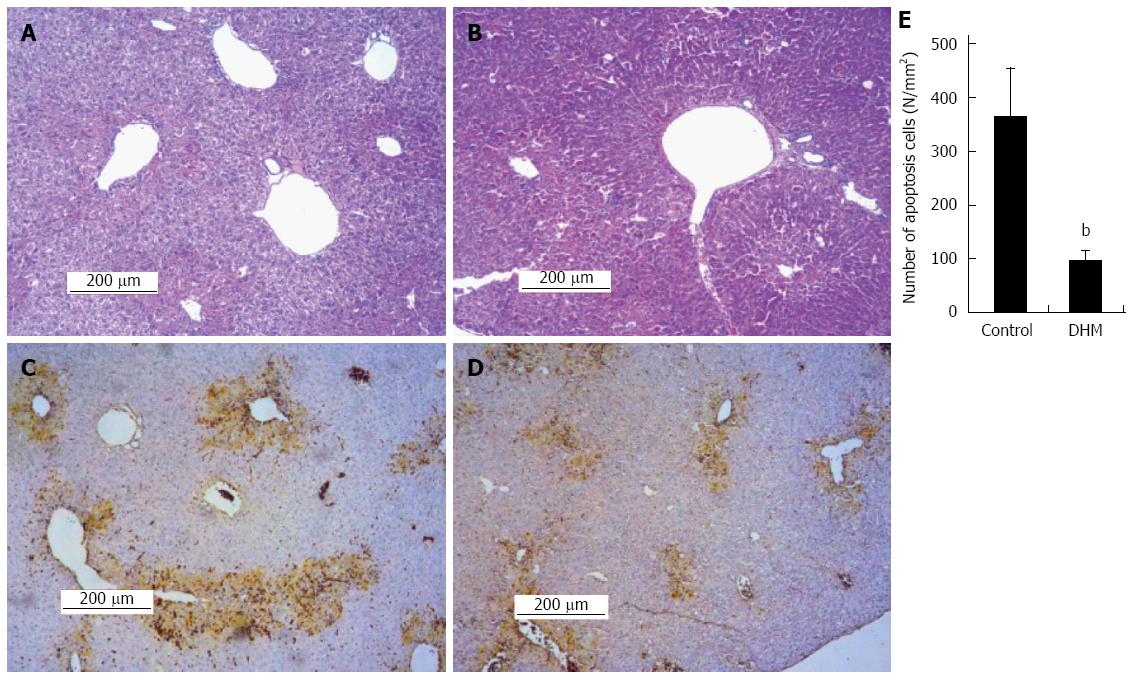
The H&E staining and TUNEL assay were used to investigate the effect of DHM on hepatocellular necrosis, inflammation and the apoptosis. Liver sections stained with HE showed that the DHM administrated group demonstrated only moderate necrosis, maintaining normal architecture; the necrotic areas were significantly diminished around the central vein and centrilobular regions compared with the control on day 2 after CCl4 treatment (Figure 3A and B). The results of TUNEL assay demonstrated that DHM significantly decreased the number of apoptotic cells in the section of liver tissue compared with the control on day 2 after CCl4 injection, and only a fewer apoptotic cells were detected in the visual field (Figure 3C and D). At least 12 mm2 tissue sections were counted for each mouse, and the data showed that DHM could significantly reduce hepatocellular apoptosis (Figure 3E).
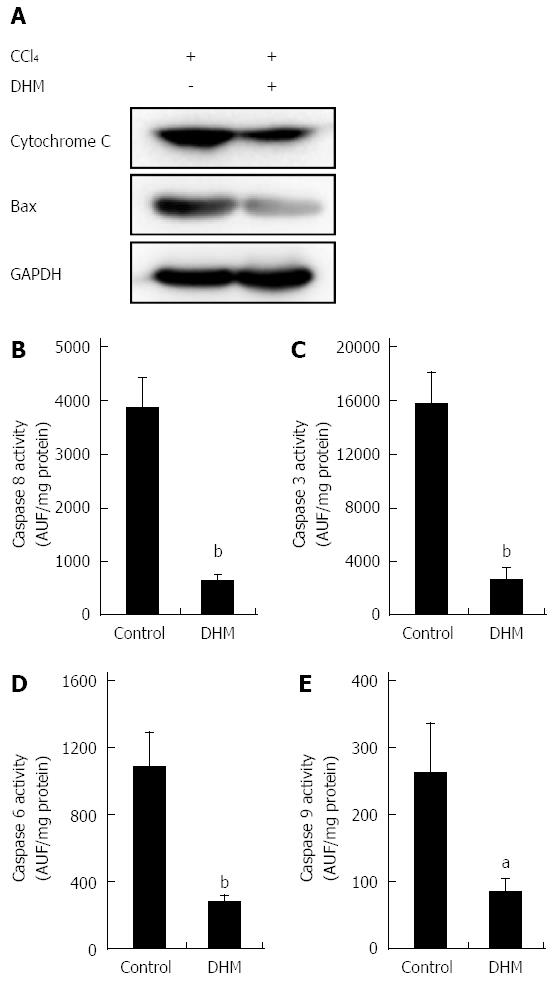
After displaying the effect of DHM in protecting hepatocytes from hepatotoxicity induced by CCl4, we investigated the relationship between DHM and hepatoprotective pathways. As shown in Figure 4A, the release of Cytochrome c and Bax expression in the DHM groups were significantly inhibited compared to the control on day 2 after CCl4 treatment. Similarly, the activities of caspases-8, 3, 6 and 9 in the liver were significantly lower in the DHM group than in the control group on day 2 after CCl4 treatment (Figure 4B-E). These results indicated that DHM could efficiently reduce hepatic injury by inhibiting the activities of Cytochrome C and Caspases-mediated apoptosis pathways.
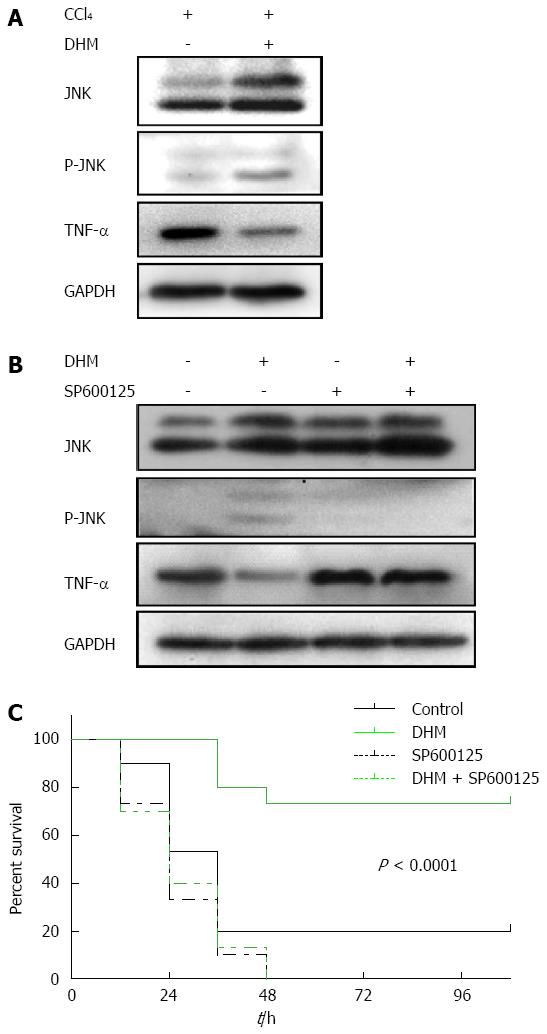
In this part of experiment, the proteins in liver tissues collected both from liver injury mice and ALF mice were detected by Western blot. The result showed that the content of TNF-α was markedly higher in the control compared to DHM-administered groups after CCl4 treatment, but the level of JNK was promoted significantly in DHM-administered groups (Figure 5A and B). Moreover, TNF-α was not reduced in the ALF mice pretreated with JNK-inhibitor SP600125 (Figure 5B). These data demonstrated that TNF-α mediated liver regeneration is regulated by DHM via the JNK pathway.
The data demonstrated that oral administration of DHM offered a strong survival benefit for CCl4-induced ALF mice, and the survival significantly improved probably from the early phase after CCl4 injection (Figure 5C). However, after JNK inhibitor SP600125 (50 mg/kg) was administered by intraperitoneal injection, DHM could not increase the survival of C57BL/6 mice after a lethal dose of CCl4 (Figure 5C), which indicated that DHM may protect against CCl4-induced ALF through the JNK pathway.
CCl4-induced acute liver injury has been used as an ideal model for many years because the mechanism of this hepatotoxin replicates the most cases of human liver disease. Previous studies demonstrated that the pathological roles of CCl4 are restricted to the liver, while lethality of high-dose CCl4 is mostly related with ALF. In the present study, we confirmed the protective role of DHM against CCl4-induced acute liver injury. Serum ALT and AST have been utilized as the bio-markers of liver damage, which were recognized as important indicators to judge the severity of acute liver injury. The results indicated that DHM could markedly attenuate the elevation of serum ALT and AST from an early phase of liver damage. Furthermore, DHM could significantly improve serum albumin, which demonstrated that DHM could promote liver functional recovery. With regard to the role of ROS production in liver disease and pathology, antioxidants might prevent hepatic damage through scavenger activity and increase the activity of intracellular antioxidant enzymes including SOD. Many studies reported that natural antioxidants are efficacious in preventing oxidative stress-related liver injury[15]. In this study, we found that DHM could markedly enhance the activity of SOD both in serum and in the liver, which indicated it as an effective antioxidant. Meanwhile, we used histological methods to reveal the severity of liver necrosis and inflammation. The results also demonstrated that DHM could significantly suppress CCl4-induced inflammation, necrosis, and destruction of liver architecture.
Liver regeneration is shown as hepatocyte proliferation. In the present study, PCNA results definitely demonstrated the positive role of DHM in hepatocyte proliferation. DHM contributes to faster functional recovery by promoting hepatocyte proliferation during the whole liver damage process.
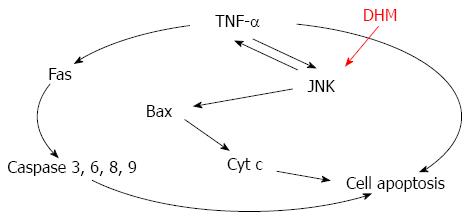
TUNEL staining results demonstrated that DHM could reduce apoptosis of hepatocytes. Serious hepatocyte apoptosis is also the major cause of the death of ALF mice, and the effect of DHM is mediated partly by inhibiting apoptosis pathways, including reducing the release of cytochrome c from the mitochondria, down-regulating Bax and markedly decreasing the activities of caspases-3, 6, 8 and 9 in the liver tissue. TNF-α is able to induce apoptosis via caspase activation pathways[16,17]. DHM protects from CCl4-induced ALF by inhibiting activation of caspases via TNF-α mediated pathway.
To investigate the underlying mechanism of the role of DHM in liver regeneration, we evaluated the effects of DHM on certain key cytokines tightly related with inflammation and cell proliferation. IL-1β, IL-6 and TNF-α, as acute-phase proteins, are commonly considered to be biomarkers that reflect inflammatory conditions. IL-1β plays a vital role in inflammation, usually leading to tissue destruction. A previous study demonstrated that IL-1β has been shown to prevent hepatocyte proliferation[18]. Serum IL-1β increases markedly during most inflammatory processes[19,20]. In this study, we found that DHM could reduce the serum level of IL-1β compared to the control during the progression of liver regeneration.
TNF-α activates intracellular pathways to regulate inflammation and proliferation. In the liver, TNF-α is a mediator of hepatotoxicity, which has been shown to be critical for liver injury and ALF[21]. TNF-α also has been identified as an attractive target for liver regeneration[22]. TNF-α is a pro-inflammatory mediator in hepatocyte apoptosis, which has close relationship with cytotoxicity induced by CCl4[11]. DHM could significantly reduce serum and liver TNF-α. Moreover, DHM-mediated protection on the liver could be inhibited in JNK-inhibitor SP600125 pretreated mice. While JNK was partly blocked by its inhibitor, TNF-α was rapidly up-regulated, which resulted in even more severe liver damage. In this study, oral administration of DHM could significantly decrease the mortality rate of C57 BL/6 mice treated with an LD50 dosage of CCl4. It is reasonable to hypothesize that DHM could protect against CCl4-induced ALF mainly through attenuating hepatotoxicity and facilitating the restoration of liver functions. JNK inhibitor SP600125 was administered prior to DHM[23], and DHM administration could not improve survival of CCl4-treated mice, which indicated that DHM protects against CCl4-induced ALF directly through regulating JNK pathway.
Together, these data suggest that DHM prevents TNF-mediated hepatotoxicity by inhibiting TNF-α expression via the JNK signaling pathway (Figure 6). Importantly, a previous study demonstrated that mice lacking JNK displayed decreased hepatocyte proliferation in a mouse model of liver regeneration[10]. Meanwhile, pretreatment with JNK inhibitor did not provide protection against CCl4 hepatotoxicity[23]. Our findings also confirmed that JNK is critically involved in DHM triggered acceleration of liver regeneration. However, JNK may enhance c-Jun-mediated cell death in HCC[24,25], which seems to be inconsistent with our results. In our opinion, the function of c-Jun seems to be more complex, since c-Jun could also mediate hepatocyte survival in TNF-α-dependent acute liver injury[26]. Since activation of JNK is believed to be important in various liver diseases, a clear characterization of the functions of JNK and its targets among other signaling pathways, such as p38 and NF-κB[9,27], will provide novel and important insights into the molecular links between inflammation and liver injury.
In conclusion, we demonstrated that DHM has strong beneficial effects against CCl4-induced acute liver injury and ALF. The protective effect of DHM makes it be a novel therapeutic candidate for acute liver injury and ALF.
The liver plays a crucial role in the regulation of body metabolism as well as in detoxification. Due to these essential functions, injuries to this organ need to be rapidly and efficiently remedied.
Dihydromyricetin (DHM) has numerous pharmacological activities, such as anti-inflammatory, antioxidation and anticarcinogenic effects. However, whether DHM could reduce hepatic injury and accelerate hepatocyte proliferation or not is not yet known.
This is the first study to demonstrate that DHM can accelerate liver regeneration and protect liver from the death of acute liver failure (ALF) via Jun kinase dependent mechanism.
DHM may serve as a potential effective candidate agent for the therapy of chemical liver injury and ALF
The study is well conceived and performed, and the results are quite interesting and potentially helpful for clinical application.
P- Reviewer: Ricci-Vitiani L S- Editor: Qi Y L- Editor: Wang TQ E- Editor: Ma S
| 1. | Taniguchi M, Takeuchi T, Nakatsuka R, Watanabe T, Sato K. Molecular process in acute liver injury and regeneration induced by carbon tetrachloride. Life Sci. 2004;75:1539-1549. [PubMed] [Cited in This Article: ] |
| 2. | Badger DA, Sauer JM, Hoglen NC, Jolley CS, Sipes IG. The role of inflammatory cells and cytochrome P450 in the potentiation of CCl4-induced liver injury by a single dose of retinol. Toxicol Appl Pharmacol. 1996;141:507-519. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 59] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 3. | Masuda Y. [Learning toxicology from carbon tetrachloride-induced hepatotoxicity]. Yakugaku Zasshi. 2006;126:885-899. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 36] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 4. | Wu S, Liu B, Zhang Q, Liu J, Zhou W, Wang C, Li M, Bao S, Zhu R. Dihydromyricetin reduced Bcl-2 expression via p53 in human hepatoma HepG2 cells. PLoS One. 2013;8:e76886. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 5. | Ding WX, Yin XM. Dissection of the multiple mechanisms of TNF-alpha-induced apoptosis in liver injury. J Cell Mol Med. 2004;8:445-454. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 6. | Fausto N, Campbell JS, Riehle KJ. Liver regeneration. Hepatology. 2006;43:S45-S53. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1126] [Cited by in F6Publishing: 1152] [Article Influence: 64.0] [Reference Citation Analysis (0)] |
| 7. | Luedde T, Trautwein C. Intracellular survival pathways in the liver. Liver Int. 2006;26:1163-1174. [PubMed] [Cited in This Article: ] |
| 8. | Maeda S, Chang L, Li ZW, Luo JL, Leffert H, Karin M. IKKbeta is required for prevention of apoptosis mediated by cell-bound but not by circulating TNFalpha. Immunity. 2003;19:725-737. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 243] [Cited by in F6Publishing: 237] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 9. | Seki E, Brenner DA, Karin M. A liver full of JNK: signaling in regulation of cell function and disease pathogenesis, and clinical approaches. Gastroenterology. 2012;143:307-320. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 338] [Cited by in F6Publishing: 375] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 10. | Hui L, Zatloukal K, Scheuch H, Stepniak E, Wagner EF. Proliferation of human HCC cells and chemically induced mouse liver cancers requires JNK1-dependent p21 downregulation. J Clin Invest. 2008;118:3943-3953. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 249] [Cited by in F6Publishing: 249] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 11. | Zhu R, Zeng G, Chen Y, Zhang Q, Liu B, Liu J, Chen H, Li M. Oroxylin A accelerates liver regeneration in CCl4-induced acute liver injury mice. PLoS One. 2013;8:e71612. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 12. | Zhu RZ, Xiang D, Xie C, Li JJ, Hu JJ, He HL, Yuan YS, Gao J, Han W, Yu Y. Protective effect of recombinant human IL-1Ra on CCl4-induced acute liver injury in mice. World J Gastroenterol. 2010;16:2771-2779. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 48] [Cited by in F6Publishing: 51] [Article Influence: 3.6] [Reference Citation Analysis (1)] |
| 13. | Schwabe RF, Bradham CA, Uehara T, Hatano E, Bennett BL, Schoonhoven R, Brenner DA. c-Jun-N-terminal kinase drives cyclin D1 expression and proliferation during liver regeneration. Hepatology. 2003;37:824-832. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 196] [Cited by in F6Publishing: 196] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 14. | Ogiso T, Nagaki M, Takai S, Tsukada Y, Mukai T, Kimura K, Moriwaki H. Granulocyte colony-stimulating factor impairs liver regeneration in mice through the up-regulation of interleukin-1beta. J Hepatol. 2007;47:816-825. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 15. | Zhu R, Wang Y, Zhang L, Guo Q. Oxidative stress and liver disease. Hepatol Res. 2012;42:741-749. [DOI] [Cited in This Article: ] [Cited by in Crossref: 155] [Cited by in F6Publishing: 157] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 16. | Wang L, Du F, Wang X. TNF-alpha induces two distinct caspase-8 activation pathways. Cell. 2008;133:693-703. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 957] [Cited by in F6Publishing: 1113] [Article Influence: 69.6] [Reference Citation Analysis (0)] |
| 17. | Micheau O, Tschopp J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell. 2003;114:181-190. [PubMed] [Cited in This Article: ] |
| 18. | Cosgrove BD, Cheng C, Pritchard JR, Stolz DB, Lauffenburger DA, Griffith LG. An inducible autocrine cascade regulates rat hepatocyte proliferation and apoptosis responses to tumor necrosis factor-alpha. Hepatology. 2008;48:276-288. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 64] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 19. | Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009;27:519-550. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 60] [Reference Citation Analysis (0)] |
| 20. | Dinarello CA. The IL-1 family and inflammatory diseases. Clin Exp Rheumatol. 2002;20:S1-13. [PubMed] [Cited in This Article: ] |
| 21. | Schwabe RF, Brenner DA. Mechanisms of Liver Injury. I. TNF-alpha-induced liver injury: role of IKK, JNK, and ROS pathways. Am J Physiol Gastrointest Liver Physiol. 2006;290:G583-G589. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 494] [Cited by in F6Publishing: 531] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 22. | He G, Karin M. NF-κB and STAT3 - key players in liver inflammation and cancer. Cell Res. 2011;21:159-168. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 749] [Cited by in F6Publishing: 867] [Article Influence: 61.9] [Reference Citation Analysis (0)] |
| 23. | Gunawan BK, Liu ZX, Han D, Hanawa N, Gaarde WA, Kaplowitz N. c-Jun N-terminal kinase plays a major role in murine acetaminophen hepatotoxicity. Gastroenterology. 2006;131:165-178. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 342] [Cited by in F6Publishing: 352] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 24. | Maeda S, Kamata H, Luo JL, Leffert H, Karin M. IKKbeta couples hepatocyte death to cytokine-driven compensatory proliferation that promotes chemical hepatocarcinogenesis. Cell. 2005;121:977-990. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 910] [Cited by in F6Publishing: 906] [Article Influence: 47.7] [Reference Citation Analysis (0)] |
| 25. | Sakurai T, Maeda S, Chang L, Karin M. Loss of hepatic NF-kappa B activity enhances chemical hepatocarcinogenesis through sustained c-Jun N-terminal kinase 1 activation. Proc Natl Acad Sci USA. 2006;103:10544-10551. [PubMed] [Cited in This Article: ] |
| 26. | Hasselblatt P, Rath M, Komnenovic V, Zatloukal K, Wagner EF. Hepatocyte survival in acute hepatitis is due to c-Jun/AP-1-dependent expression of inducible nitric oxide synthase. Proc Natl Acad Sci USA. 2007;104:17105-17110. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 61] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 27. | Hui L, Bakiri L, Mairhorfer A, Schweifer N, Haslinger C, Kenner L, Komnenovic V, Scheuch H, Beug H, Wagner EF. p38alpha suppresses normal and cancer cell proliferation by antagonizing the JNK-c-Jun pathway. Nat Genet. 2007;39:741-749. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 292] [Cited by in F6Publishing: 294] [Article Influence: 17.3] [Reference Citation Analysis (0)] |