Copyright
©The Author(s) 2015.
World J Gastroenterol. Apr 28, 2015; 21(16): 4779-4787
Published online Apr 28, 2015. doi: 10.3748/wjg.v21.i16.4779
Published online Apr 28, 2015. doi: 10.3748/wjg.v21.i16.4779
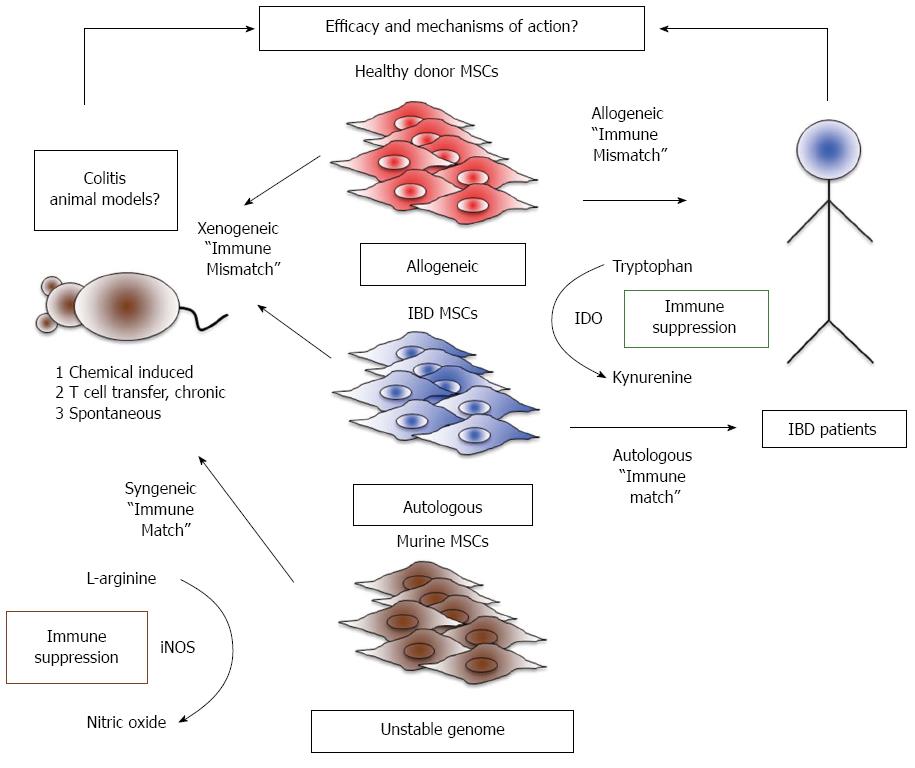
Figure 1 Challenges in modelling efficacy and mechanisms of action of mesenchymal stromal cells between preclinical and clinical studies.
Right: Current clinical trials fall into two major groups: (1) allogeneic studies where inflammatory bowel diseases patients receive mesenchymal stromal cells (MSCs) from a random MHC unmatched randomdonor; and (2) autologous studies where patients are given their own MSCs. In human MSCs, immunosuppression is dependent on Indoleamine 2, 3 dioxygenase (IDO) activity, an enzyme that converts the essential amino acid tryptophan into the immunosuppressive catabolite, kynurenine. Left: Murine MSCs differ from human MSCs in their primary mechanism of immune suppression, utilizing inducible nitric oxide synthase (iNOS) to create nitric oxide as a product of L-arginine catabolism. The therapeutic effects of murine MSCs can be tested by engraftment into a colitic mouse, and is syngeneic if the MSCs are derived from a mouse with the same strain/background as the colitic mouse. However, murine MSCs may potentially transform after prolonged in vitro culture due to genomic instability. IBD: Inflammatory bowel diseases.
- Citation: Chinnadurai R, Ng S, Velu V, Galipeau J. Challenges in animal modelling of mesenchymal stromal cell therapy for inflammatory bowel disease. World J Gastroenterol 2015; 21(16): 4779-4787
- URL: https://www.wjgnet.com/1007-9327/full/v21/i16/4779.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i16.4779