Published online Apr 28, 2015. doi: 10.3748/wjg.v21.i16.5002
Peer-review started: October 4, 2014
First decision: October 29, 2014
Revised: November 7, 2014
Accepted: January 8, 2015
Article in press: January 8, 2015
Published online: April 28, 2015
Processing time: 206 Days and 20.1 Hours
AIM: To study the ability of endocytoscopy to identify normal gastric mucosa and to exclude Helicobacter pylori (H. pylori) infection.
METHODS: Endocytoscopic examination of the gastric corpus and antrum was performed in 70 consecutive patients. Target biopsy specimens were also obtained from the assessed region and multiple H. pylori tests were performed. The normal endocytoscopy patterns of the corpus and antrum were divided into the normal pit-dominant type (n-Pit) or the normal papilla-dominant type (n-Pap), respectively characterized as either regular pits with capillary networks or round, smooth papillary structures with spiral capillaries. On the other hand, normal mucosa was defined as mucosa not demonstrating histological abnormalities, including inflammation and atrophy.
RESULTS: The sensitivity and specificity of n-Pit for normal mucosa in the gastric corpus were 94.4% and 97.1%, respectively, whereas those of n-Pap for normal mucosa in the antrum were 92.0% and 86.7%, respectively. The positive predictive values of n-Pit and n-Pap for H. pylori-negative tissue were 88.6% and 93.1%, respectively, and their negative predictive values for H. pylori-negative tissues were 42.9% and 41.5%, respectively. The inter-observer agreement for determining n-Pit and n-Pap for normal mucosa were 0.857 and 0.769, respectively, which is considered reliable.
CONCLUSION: N-Pit and n-Pap, seen using EC, are considered useful predictors of normal mucosa and the absence of H. pylori infection.
Core tip: The identification of minute inflammatory or atrophic changes is very difficult using conventional endoscopy. This is because these changes are usually predicted using non-specific endoscopic findings, such as superficial color, edema and erosions. However, endocytoscopy enables real-time histology, which corresponds well with conventional histopathology. The procedure is also simple (the endocytoscope only has to make contact with the gastric wall) and is therefore expected to be used regularly globally in the future.
-
Citation: Sato H, Inoue H, Ikeda H, Sato C, Phlanusittepha C, Hayee B, Santi EGR, Kobayashi Y, Kudo SE.
In vivo gastric mucosal histopathology using endocytoscopy. World J Gastroenterol 2015; 21(16): 5002-5008 - URL: https://www.wjgnet.com/1007-9327/full/v21/i16/5002.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i16.5002
Endocytoscopy (EC) was developed as an ultra-magnifying technology for in vivo pathological diagnoses[1,2]. As a result of in vivo staining, EC enables detailed examinations, with images comparable to those obtained using microscopy. In our practice, EC is primarily used for the differential diagnosis of neoplastic and non-neoplastic lesions because the atypical cellular and nuclear structures can be clearly identified[3-10].
Additionally, EC can be expected to definitively distinguish between normal mucosa and pathological gastritis [most commonly induced by Helicobacter pylori (H. pylori) infection]. Conventional endoscopy allows the recognition of gastritis dependent upon non-specific and indirect findings, such as changing patterns of superficial colors, edema and erosions. However, EC is expected to be able to more accurately identify minute changes than any other endoscopic modality. EC should also be able to differentiate clearly between the fundic and antral glands, similar to the capability of narrow band imaging (NBI).
Therefore, the purpose of the present study was to assess the ability of EC to identify normal gastric patterns, corresponding to histopathologically normal mucosa, as well as to exclude cells with possible H. pylori infections.
This study was performed at Showa University Northern Yokohama Hospital, a tertiary referral center in Japan, between December 2013 and February 2014. Study participants were prospectively and consecutively recruited from the cohort of patients undergoing endoscopic examination for any indication. Patients who had undergone gastric surgery, were receiving anticoagulant therapy, or had other significant co-morbidities that might affect endoscopic examination were excluded from the study.
The study was approved by our Institutional Review Board and conducted as part of a large study registered in the University Hospital Medical Information Network in Japan (UMIN000007745). Written informed consent was obtained from all patients.
All EC examinations were performed using an integrated-type endocytoscope (GIF-Y0002, a prototype from Olympus, Tokyo, Japan). The endocytoscope used has one lens that can increase the image magnification from that of a conventional endoscope to 380 × magnification (tissue field of view, 700 μm × 600 μm). A hand-operated lever is used to allow gradual magnification at the center of the monitor, ensuring that the area of interest is accurately located (Figure 1). The clinical use of the prototype endoscope was also approved by the hospital’s ethics committee.
For EC examination, a previously reported mixture of 0.1% methylene blue and 0.05% crystal violet was used to stain in vivo tissues in a manner approximating conventional hematoxylin/eosin-stained histopathological specimens. Crystal violet effectively dyes the cytoplasm, while methylene blue staining reveals cell structure details, including the nucleus and cytoplasm[11].
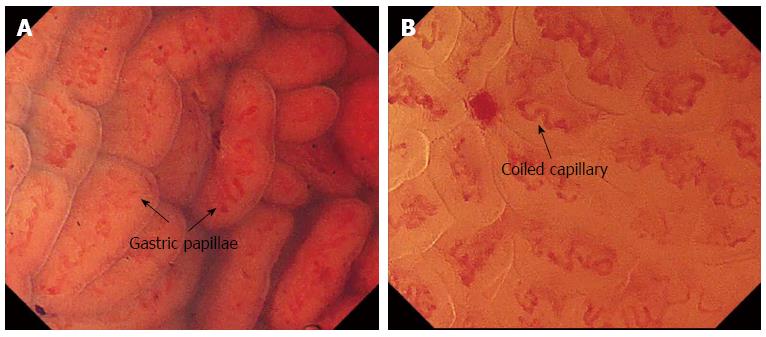
The normal EC pattern was divided into two types: the normal pit-dominant type (n-Pit) and the normal papilla-dominant type (n-Pap). In the n-Pit type, regular pits are observed as “pin-holes” or short linear structures with capillary networks present around the pit, corresponding to a normal fundus gland (Figure 2). In the n-Pap type, each papillary structure has a round, smooth surface with a spiral capillary contained within the pit; these structures are regularly and closely arranged. The finding is considered to be indicative of a normal antral gland (Figure 3).
There are also some key points common to the both types. Normal gastric epithelium does not consist of intestinal epithelium and does not have an absorptive function; hence, dye uptake is poor. During EC of normal mucosa, the staining solution is observed to pool or accumulate within the gastric pits. Moreover, active mucus production also prevents staining. These characteristics are affected by pathological changes. Atrophic epithelium is superficially stained owing to the opening of the pit and to the decreased mucus production. In the presence of atrophic changes, the gastric pits widen due to shortening of the secretory duct (the duct forming the pit) and shrinkage of the stroma (the stroma forms the papilla), allowing dye uptake. In the presence of intestinal metaplasia, the superficial epithelium is completely stained, allowing goblet cell identification. During active gastritis, necrotic tissue and bacterial infiltration can be appreciated following adequate removal of the surface mucus.
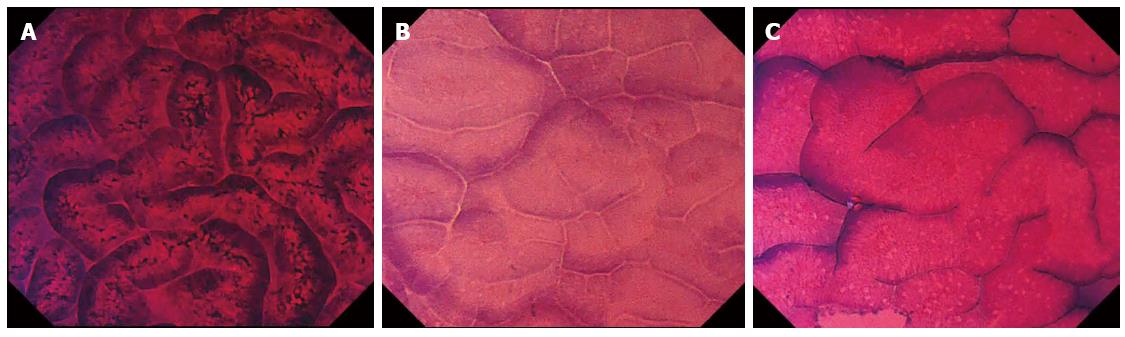
Abnormal EC images were classified as having irregular EC patterns: i-Pit or i-Pap, respectively (Figure 4).
All endoscopic procedures were carried out by one endoscopist with 2 years of experience involving more than 100 EC procedures. Conventional upper gastrointestinal tract endoscopy, using an H260Z (Olympus, Tokyo, Japan), was performed prior to EC under intravenous anesthesia. The surface mucus was cleared using dimethicone/water irrigation (Gascon, Kissei Medical, Tokyo, Japan).
Staining was then achieved using 2 mL of the methylene blue and crystal violet mixture, described above. The time interval between staining and EC examination was 5 s. EC of the antrum and corpus (greater curvature side) was performed at maximum magnification (380 ×) and recorded as high-definition video and image files. EC assessment was performed in real time, with the corpus images being classified as demonstrating either n-Pit or i-Pit; images from the antral region were similarly classified as n-Pap or i-Pap.
EC assessment was performed in real-time by one reviewer. To calculate inter-observer agreement in image classification, the EC image files, excluding conventional endoscopy, were divided according to the area of observation (gastric corpus or antral region), with 2 EC images in each case. An independent external reviewer, blinded to the patient information, conventional endoscopy results and histopathological diagnosis, reviewed and reclassified the EC images (n-Pit/i-Pit or n-Pap/i-Pap in the corpus or antral regions, respectively).
After EC examination, two forceps biopsy specimens were obtained from each region, corresponding to the area of EC examination, for pathological assessment and for microscopic H. pylori examination. Two random biopsies of the antrum and corpus were also taken for use in the rapid urease test (Pyloritek, Eidia, Tokyo Japan). A total of four biopsies were performed in each case. A serum pepsinogen test[12,13] was concomitantly performed. A positive test result in any H. pylori test was interpreted as indicating the presence of an H. pylori infection.
To calculate inter-observer agreement, the EC image files not including conventional endoscopy were divided into two parts (gastric corpus and antral region), with two EC images in each. A second, independent external reviewer, blinded to the patient information, results of conventional endoscopy and histopathological diagnosis, reviewed the EC images, classifying them as n-Pit or i-Pit in the gastric corpus or as n-Pap or i-Pap in the antral region.
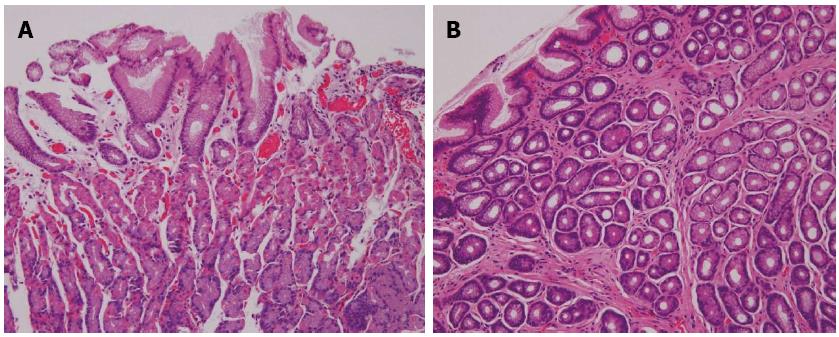
Fixed biopsy specimens were assessed for the presence or absence of active inflammation and atrophic changes. H. pylori infection was evaluated according to the updated Sydney system[14]. Regular arrangement of the surface mucosal epithelium, with absent or few inflammatory cells, were the criteria for normal mucosal assessment of the histopathology specimens (Figure 5).
All analyses were performed using STATA, version 11.2 (Stata, College Station, TX, United States). Continuous variables are expressed as mean ± SD and confidence intervals (CI) are given as 95%CI in parentheses.
Inter-observer agreement was assessed using kappa statistics and interpreted as proposed by Landis and Koch[15]. A kappa value = 0 demonstrated the absence of agreement; < 0.20, slight agreement; 0.21-0.40, fair; 0.41-0.60, moderate; 0.61-0.80, substantial; and > 0.81, almost perfect agreement.
A total of 70 consecutive patients were enrolled in this study (mean age 66.5 ± 12.8 years, 45 males). Six participants had esophageal cancer and 15 had gastric cancer not involving the examined areas of the corpus and antrum; these individuals were referred to our hospital for treatment. The other indications for EC were achalasia (n = 6); screening for other diseases, e.g., liver cirrhosis and preoperative testing due to colon cancer (n = 13), endoscopic surveillance following endoscopic treatment (n = 13), medical examinations (n = 8) and upper endoscopy due to non-specific abdominal symptoms (n = 9). Each EC procedure, including biopsy, was performed within 7 min; adverse events were not observed.
The gastric corpus examination results are shown in Table 1. The sensitivity of the n-Pit classifications for determining a histopathologically normal mucosa (fundus gland) was 94.4% (range: 81.3%-99.3%), with a specificity of 97.1% (range: 84.7%-99.9%). The mean inter-observer kappa score between the two reviewers was 0.857 (range: 0.737- 0.978). Although 4 H. pylori-positive patients were included among the patients with n-Pit, they exhibited closed-type atrophic gastritis that was confined to the gastric antrum[16]. The mean sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) for n-Pit classifications corresponding to normal (H. pylori-negative) tissue were 60.8% (range: 46.1%-74.2%), 78.9% (range: 54.4%-94.0%), 88.6% (range: 73.3%-96.8%) and 42.9% (range: 26.3%-60.6%), respectively.
| EC findings (n) | Active inflammation | Atrophic change | Helicobacter pylori infection | |||
| (+) | (-) | (+) | (-) | (+) | (-) | |
| n-Pit (35) | 0 | 35 | 0 | 35 | 4 | 31 |
| i-Pit (35) | 19 | 16 | 16 | 19 | 15 | 20 |
The results of the antral region examination are described in Table 2. The sensitivity and specificity of the n-Pap determinations corresponding to histopathologically normal mucosa were 92.0% (range: 73.4%-99.0%) and 86.7% (range: 73.2%-95.0%), respectively.
| EC findings (n) | Active inflammation | Atrophic change | Helicobacter pylori infection | |||
| (+) | (-) | (+) | (-) | (+) | (-) | |
| n-Pap (29) | 2 | 27 | 4 | 25 | 2 | 27 |
| i-Pap (41) | 19 | 22 | 35 | 6 | 17 | 24 |
The mean kappa score for inter-observer agreement between the two independent reviewers was 0.769 (range: 0.620-0.919). The mean sensitivity, specificity, PPV and NPV for the n-Pap determinations corresponding to normal (H. pylori-negative) histopathology were 52.9% (range: 38.5%-67.1%), 89.5% (range: 66.9%-98.7%), 93.1% (range: 77.2%-99.2%) and 41.5% (range: 26.3%-57.9%), respectively.
Endoscopic observation of microorganisms, including H. pylori, is technically difficult. In an in vivo setting, an EC magnification of 1100 × is reportedly needed to detect H. pylori[3]. Therefore, using microscopy or EC to look for H. pylori is impractical for the diagnosis of an H. pylori infection. Moreover, tissue stains, e.g., Giemsa stain, used for the diagnosis of H. pylori infections have not yet been proven safe for in vivo use and further evaluation is required to address their toxicity and long-term effects prior to their clinical implementation. At present, other diagnostic tests for H. pylori infection are widely available commercially[17] (i.e., urease test, serum pepsinogen, stool antigen test). These tests require that patients not be currently taking any proton pump inhibitors prior to testing. To date, the most widely used test, currently considered the gold standard, is the urea breath test. Although considered to be a non-invasive procedure, the breath test requires additional scheduling. Amongst its other attributes, EC has the potential to be a “one-step” diagnostic tool for diagnosing H. pylori infection.
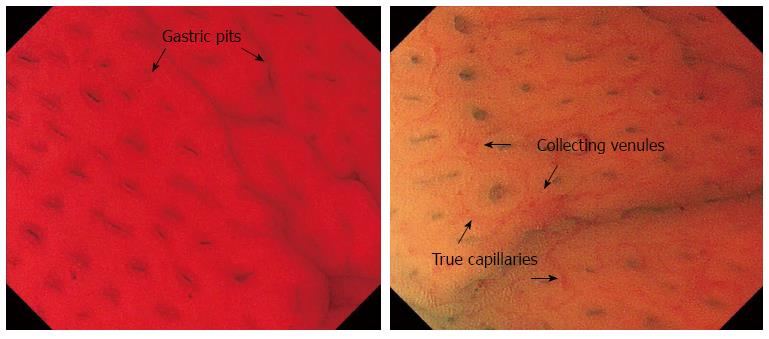
The identification of minute changes in the microstructure of the gastric mucosa during endoscopy is a key for determining the status of an H. pylori infection[18-20]. Yagi et al[21,22] reported that the regular arrangement of collecting venules (RAC) is a practical marker for differentiating normal mucosa from H. pylori-associated gastritis. RAC consists of collecting venules and true capillaries (which form networks) that are visible using conventional endoscopy. The authors also reported that the identification of a “well-defined ridge pattern” (wDRP) in the antral mucosa has 100% specificity for normal (H. pylori-negative) mucosa but a sensitivity of only 54.5%. Recently, a combination of magnifying endoscopy (ME) and NBI has been shown to allow the visualization of small, round pits with a regular arrangement of capillary networks, indicative of normal mucosa[23-26]. The presence of RAC and wDRP, observed using conventional endoscopy, and a normal pit pattern using ME-NBI are considered to be useful markers for identifying normal mucosa in the absence of an H. pylori infection.
EC of the gastric mucosa revealed two different patterns, n-Pit and n-Pap, in the gastric corpus and antral regions, respectively. These differences are considered to arise due to differences in the glandular structures and arrangements between the two regions[27]. In the gastric corpus, the collecting venules and the true capillaries form networks that surround the gastric glands, feeding into the collecting venules which descend vertically. In the antrum, the collecting venules tend to run obliquely and the capillaries are expanded. Furthermore, surface mucous cells form papillary structures and expand deeply into the lamina propria. We can also identify cellular level information about the goblet cells using endocytoscopy. Additionally, due to the higher magnification that is possible, more structural change can be seen than when using ME-NBI, although the microvessels are less clear than in ME-NBI.
Our results demonstrate that the presence of the n-Pit morphology in the gastric corpus, observed using EC, corresponds well with histopathologically proven normal mucosa, with a high sensitivity and specificity. Moreover, the excellent inter-observer agreement shows that the observation of the n-Pit mucosal pattern in the corpus is a clear-cut, practical indicator of normal mucosa, even for non-specialists. The sensitivity and specificity of n-Pap for identifying normal antral region mucosa were 92.0% and 86.7%, respectively. Although the sensitivity of n-Pap was much higher than that of wDRP, as reported by Yagi et al, n-Pap was not as reliable for identifying normal mucosa as n-Pit in our series. However, for identifying closed-type gastritis[16,28,29], n-Pap in the antral region appears to predict the presence of histological gastritis.
The specificities and PPVs of n-Pit and n-Pap for identifying H. pylori-negative mucosa were considered to be sufficient to be useful for excluding the presence of H. pylori infection. The sensitivities and NPVs of n-Pit and n-Pap associated with normal mucosa were not as reliable, probably due to cases of natural H. pylori eradication or false negative H. pylori test results.
In conclusion, EC determinations of n-Pit and n-Pap are useful predictors of normal mucosa and the absence of H. pylori infection. The results also correspond well with the presence of normal fundic and antral glands. This was a pilot study and further study is needed.
Endocytoscopy (EC) was developed as an ultra-magnifying technology for in vivo pathological diagnoses.
EC enables detailed examinations, with images comparable to those obtained using microscopy.
EC can differentiate gastric mucosal patterns of minimal change and appears to reliably exclude Helicobacter pylori (H. pylori) infection.
EC of the normal gastric mucosa revealed two different patterns, normal pit-dominant type (n-Pit) and normal papilla-dominant type (n-Pap), in the gastric corpus and antral regions, respectively.
This is a study about endocytoscopic examination of gastric mucosa according to the presence of H. pylori infection. The results showed that EC determinations of n-Pit and n-Pap are useful predictors of normal mucosa and the absence of H. pylori infection.
P- Reviewer: Chen JX, Iizuka T, Kim GH S- Editor: Ma YJ L- Editor: Roemmele A E- Editor: Ma S
| 1. | Inoue H, Kudo SE, Shiokawa A. Novel endoscopic imaging techniques toward in vivo observation of living cancer cells in the gastrointestinal tract. Clin Gastroenterol Hepatol. 2005;3:S61-S63. [PubMed] |
| 2. | Inoue H, Honda T, Yoshida T, Nishikage T, Nagahama T, Nagai K, Kawano T, Yoshino K, Tani M, Takeshita K. Ultra-high Magnification Endoscopy of the Normal Esophageal Mucosa. Dig Endosc. 1996;8:134-138. |
| 3. | Inoue H, Sasajima K, Kaga M, Sugaya S, Sato Y, Wada Y, Inui M, Satodate H, Kudo SE, Kimura S. Endoscopic in vivo evaluation of tissue atypia in the esophagus using a newly designed integrated endocytoscope: a pilot trial. Endoscopy. 2006;38:891-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 106] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 4. | Kumagai Y, Kawada K, Yamazaki S, Iida M, Momma K, Odajima H, Kawachi H, Nemoto T, Kawano T, Takubo K. Endocytoscopic observation for esophageal squamous cell carcinoma: can biopsy histology be omitted? Dis Esophagus. 2009;22:505-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 5. | Inoue H, Tianle KM, Ikeda H, Hosoya T, Onimaru M, Yoshida A, Minami H, Kudo SE. Peroral endoscopic myotomy for esophageal achalasia: technique, indication, and outcomes. Thorac Surg Clin. 2011;21:519-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 127] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 6. | Inoue H, Ikeda H, Hosoya T, Yoshida A, Onimaru M, Minami H, Kudo SE. [Per-oral endoscopic myotomy (POEM) for esophageal achalasia]. Nihon Shokakibyo Gakkai Zasshi. 2012;109:728-731. [PubMed] |
| 7. | Ichimasa K, Kudo SE, Mori Y, Wakamura K, Ikehara N, Kutsukawa M, Takeda K, Misawa M, Kudo T, Miyachi H. Double staining with crystal violet and methylene blue is appropriate for colonic endocytoscopy: an in vivo prospective pilot study. Dig Endosc. 2014;26:403-408. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 8. | Kumagai Y, Kawada K, Yamazaki S, Iida M, Ochiai T, Momma K, Odajima H, Kawachi H, Nemoto T, Kawano T. Endocytoscopic observation of esophageal squamous cell carcinoma. Dig Endosc. 2010;22:10-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Fujishiro M, Takubo K, Sato Y, Kaise M, Niwa Y, Kato M, Muto M. Potential and present limitation of endocytoscopy in the diagnosis of esophageal squamous-cell carcinoma: a multicenter ex vivo pilot study. Gastrointest Endosc. 2007;66:551-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 10. | Shimizu Y, Takahashi M, Yoshida T, Ono S, Mabe K, Kato M, Asaka M, Hatanaka K, Sakamoto N. Endoscopic in vivo cellular imaging of superficial squamous cell carcinoma of the head and neck by using an integrated endocytoscopy system (with video). Gastrointest Endosc. 2013;78:351-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 11. | Hanaoka N, Uedo N, Ishihara R, Higashino K, Takeuchi Y, Inoue T, Chatani R, Hanafusa M, Tsujii Y, Kanzaki H. Clinical features and outcomes of delayed perforation after endoscopic submucosal dissection for early gastric cancer. Endoscopy. 2010;42:1112-1115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 91] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 12. | Kitahara F, Kobayashi K, Sato T, Kojima Y, Araki T, Fujino MA. Accuracy of screening for gastric cancer using serum pepsinogen concentrations. Gut. 1999;44:693-697. [PubMed] |
| 13. | Watabe H, Mitsushima T, Yamaji Y, Okamoto M, Wada R, Kokubo T, Doi H, Yoshida H, Kawabe T, Omata M. Predicting the development of gastric cancer from combining Helicobacter pylori antibodies and serum pepsinogen status: a prospective endoscopic cohort study. Gut. 2005;54:764-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 310] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 14. | Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996;20:1161-1181. [PubMed] |
| 15. | Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159-174. [PubMed] |
| 16. | Kimura K, Takemoto T. An endoscopic recognition of the atrophic border and its significance in chronic gastritis. Endoscopy. 1969;1:87-97. |
| 17. | Asaka M, Kato M, Takahashi S, Fukuda Y, Sugiyama T, Ota H, Uemura N, Murakami K, Satoh K, Sugano K; Japanese Society for Helicobacter Research. Guidelines for the management of Helicobacter pylori infection in Japan: 2009 revised edition. Helicobacter. 2010;15:1-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 294] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 18. | Laine L, Cohen H, Sloane R, Marin-Sorensen M, Weinstein WM. Interobserver agreement and predictive value of endoscopic findings for H. pylori and gastritis in normal volunteers. Gastrointest Endosc. 1995;42:420-423. [PubMed] |
| 19. | Bah A, Saraga E, Armstrong D, Vouillamoz D, Dorta G, Duroux P, Weber B, Froehlich F, Blum AL, Schnegg JF. Endoscopic features of Helicobacter pylori-related gastritis. Endoscopy. 1995;27:593-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 55] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 20. | Machado RS, Viriato A, Kawakami E, Patrício FR. The regular arrangement of collecting venules pattern evaluated by standard endoscope and the absence of antrum nodularity are highly indicative of Helicobacter pylori uninfected gastric mucosa. Dig Liver Dis. 2008;40:68-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 21. | Yagi K, Nakamura A, Sekine A. Comparison between magnifying endoscopy and histological, culture and urease test findings from the gastric mucosa of the corpus. Endoscopy. 2002;34:376-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 82] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 22. | Yagi K, Aruga Y, Nakamura A, Sekine A, Umezu H. The study of dynamic chemical magnifying endoscopy in gastric neoplasia. Gastrointest Endosc. 2005;62:963-969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 50] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 23. | Inoue H, Yokoyama A, Kudo SE. [Ultrahigh magnifying endoscopy: development of CM double staining for endocytoscopy and its safety]. Nihon Rinsho. 2010;68:1247-1252. [PubMed] |
| 24. | Nakamura M, Shibata T, Tahara T, Yoshioka D, Okubo M, Mizoguchi Y, Kuroda M, Arisawa T, Hirata I. The usefulness of magnifying endoscopy with narrow-band imaging to distinguish carcinoma in flat elevated lesions in the stomach diagnosed as adenoma by using biopsy samples. Gastrointest Endosc. 2010;71:1070-1075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 25. | Hayee B, Inoue H, Sato H, Santi EG, Yoshida A, Onimaru M, Ikeda H, Kudo SE. Magnification narrow-band imaging for the diagnosis of early gastric cancer: a review of the Japanese literature for the Western endoscopist. Gastrointest Endosc. 2013;78:452-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 26. | Bansal A, Ulusarac O, Mathur S, Sharma P. Correlation between narrow band imaging and nonneoplastic gastric pathology: a pilot feasibility trial. Gastrointest Endosc. 2008;67:210-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 70] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 27. | Tsuchihashi Y, Tani T, Maruyama K, Yorioka S, Okada K, Sudo H, Ashihara T, Fujita S, Kawai K. Structural alterations of mucosal microvascular system in human chronic gastritis. Microcirculation in circulatory disorders: Springer 1988; 161-169. |
| 28. | Asaka M, Kato M, Kudo M, Katagiri M, Nishikawa K, Yoshida J, Takeda H, Miki K. Relationship between Helicobacter pylori infection, atrophic gastritis and gastric carcinoma in a Japanese population. Eur J Gastroenterol Hepatol. 1995;7 Suppl 1:S7-10. [PubMed] |
| 29. | Hiyama T, Haruma K, Kitadai Y, Masuda H, Miyamoto M, Ito M, Kamada T, Tanaka S, Uemura N, Yoshihara M. Clinicopathological features of gastric mucosa-associated lymphoid tissue lymphoma: a comparison with diffuse large B-cell lymphoma without a mucosa-associated lymphoid tissue lymphoma component. J Gastroenterol Hepatol. 2001;16:734-739. [PubMed] |