Published online Mar 14, 2015. doi: 10.3748/wjg.v21.i10.3013
Peer-review started: September 5, 2014
First decision: September 27, 2014
Revised: October 14, 2014
Accepted: December 5, 2014
Article in press: December 8, 2014
Published online: March 14, 2015
AIM: To evaluate the association between liver stiffness (LS) prior to the initiation of dual/triple therapy and viral response.
METHODS: LS was measured in all patients before treatment was administered. The therapeutic approach was based on hepatic, virological, and immunological evaluations and considered the fact that patients with severe fibrosis (F3) or compensated cirrhosis (F4) in Child-Pugh class A are the primary candidates for triple therapy. In total, 65 hepatitis C virus (HCV) patients were treated with Peg-interferon/ribavirin (Peg-IFN/RBV); 24 patients were classified as genotypes 1/4 (36.92%), and 41 patients were classified as genotypes 2/3 (63.08%) (dual therapy). In addition, 20 HCV treatment-experienced genotype 1 patients were treated with PegIFN-RBV and boceprevir (triple therapy). Wilcoxon rank-sum tests were used to compare the groups.
RESULTS: LS significantly differed between dual therapy and triple therapy (P = 0.002). The mean LS value before dual therapy treatment was 8.61 ± 5.79 kPa and was significantly different between patients achieving a sustained virologic response (SVR) 24 weeks after therapy and those who did not (7.23 ± 5.18 kPa vs 11.72 ± 5.99 kPa, respectively, P = 0.0003). The relative risk of non-response to therapy was 4.45 (95%CI: 2.32-8.55). The attributable risk of non-response to therapy was 49%. The mean LS value before triple therapy treatment was 13.29 ± 8.57 kPa and was significantly different between patients achieving and not achieving SVR24 (9.41 ± 5.05 vs 19.11 ± 9.74, respectively; P = 0.008). The relative risk of non-response to therapy was 5.57% (95%CI: 1.50-20.65). The attributable risk of non-response to therapy (70%) was increased compared with dual therapy patients. Pre-treatment stiffness > 12 kPa was significantly associated with non-SVR (P < 0.025) in both groups.
CONCLUSION: Pre-treatment liver stiffness may be useful for predicting the response to treatment in patients treated with either dual or triple anti-HCV therapy.
Core tip: Transient elastography is a non-invasive, easy, reproducible technique that is well tolerated for staging non-significant fibrosis or severe fibrosis/cirrhosis. The assessment of liver fibrosis using transient elastography may be useful to reduce the need for a liver biopsy. In this prospective study, liver stiffness values can also be used as a predictor of response to therapy. A liver stiffness value greater than 12 kPa is a negative predictor of response to both dual and triple therapy. Therefore, transient elastography could be used in clinical practice to improve the selection of patient treatment.
- Citation: Stasi C, Piluso A, Arena U, Salomoni E, Montalto P, Monti M, Boldrini B, Corti G, Marra F, Laffi G, Milani S, Zignego AL. Evaluation of the prognostic value of liver stiffness in patients with hepatitis C virus treated with triple or dual antiviral therapy: A prospective pilot study. World J Gastroenterol 2015; 21(10): 3013-3019
- URL: https://www.wjgnet.com/1007-9327/full/v21/i10/3013.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i10.3013
Liver fibrosis is a pathological process caused by an excessive accumulation of extracellular matrix that leads to liver cirrhosis progression. In fact, fibrosis generally progresses in untreated chronic viral liver diseases, thereby leading to excessive collagen deposition, which results in qualitative and quantitative changes of the extracellular matrix. The deposition of the fibrotic matrix is largely due to the recruitment of resident mesenchymal cells and possibly a small aliquot of circulatory cell types that are capable of differentiating into a myofibroblast phenotype[1,2]. Viral infection can cause a fibrogenic process that is initially concentrated around portal tracts and subsequently leads to the formation of portal-portal and portal-central septa. The fibrogenic process proceeds through various stages because the composition of the extracellular matrix changes with the advancement of fibrosis. The impact of the fibrosis stage on a patient’s response to anti-hepatitis C virus (HCV) treatment has been explored in several studies[3-5]. In fact, the decision of whether and when to start antiviral treatment for chronic HCV depends primarily on several variables, including the fibrotic stage[6]. Therefore, periodic liver biopsies are needed to assess the progression of fibrosis and select patients for antiviral treatment.
In HCV genotype 2 or 3 infected patients, liver biopsy is typically not performed given the high likelihood of a favourable therapeutic response to treatment with Peg-interferon (INF) and ribavirin (RBV) (Peg-INF/RBV)[6]. In several countries, including Italy, triple therapy with Peg-INF/RBV and either boceprevir or telaprevir currently serves as the standard care for HCV genotype 1 patients with previous treatment failure to dual therapy with Peg-INF/RBV. For patients who relapse on dual therapy, triple therapy should be initiated as soon as possible in the presence of advanced fibrosis (F3-F4, METAVIR classification)[7], whereas treatment for patients with moderate fibrosis can be considered on a case by case basis[8]. In naive patients with negative pre-treatment predictors of response, including genotype 1, CT-TT alleles of the IL28b polymorphism, and F3-F4 fibrosis stage, triple therapy should be introduced as first-line therapy.
In the last 10 years, various methods, such as serum markers of liver fibrosis, ultrasound[9], magnetic resonance imaging (MRI)[10], and transient[11] or MR-elastography[12] have been proposed as tests. These tests for staging and assessing the progression/regression of liver fibrosis are non-invasive, easily reproducible, and well tolerated by patients. Several studies[13-18] have evaluated the outcome of IFN/RBV treatment through the assessment of short- and long-term longitudinal assessment of liver stiffness (LS) by transient elastography in HCV infected patients.
New treatments for HCV infection involving triple therapy also require an accurate assessment of liver fibrosis for a correct therapeutic approach. Therefore, the objective of this study was to evaluate the value of LS prior to initiation of dual/triple therapy as a prognostic indicator of response to antiviral therapy in HCV patients.
Eighty-five patients with chronic HCV infection (35 women with a mean age of 56.31 ± 11.13 years and 50 men with a mean age 50.55 ± 10.12 years) referred to the hepatology outpatient ward of the Careggi University Hospital, Florence, Italy between January 2012 and December 2012 for assessment of liver disease stage and eligibility for antiviral treatment conforming to the recommendations of the Italian Association for the Study of the Liver[8] were considered for potential enrolment in the study. The enrolment inclusion criteria of this prospective study are as follows: presence of detectable serum HCV-RNA; eligibility for antiviral treatment; no antiviral or immunosuppressive therapies administered 6 mo prior to enrolment; abnormal liver enzyme levels; exclusion of Child-Pugh class B and C cirrhosis; absence of alcohol intake ≥ 40 g/d or use of hepatotoxic medications, hepatocellular carcinoma, acute viral hepatitis, co-infection with hepatitis B virus or human immunodeficiency virus, other hepatic disease (e.g., metabolic liver disease), pathologic conditions potentially affecting LS (i.e., cardiac failure), and physiological states contraindicated for this technique (e.g., pregnancy). We exclusively analysed patients who completed the course of therapy.
LS was measured in all patients before treatment. The therapeutic approach was based on hepatic, virological, and immunological evaluation and considered the fact that patients with advanced fibrosis (F3) or compensated cirrhosis (F4) in Child-Pugh class A are the primary candidates for triple therapy[8]. In total, 65 HCV patients were administered treatment with PegIFN/RBV (dual therapy), and 20 HCV genotype 1 treatment-experienced patients (relapser, partial responder or non-responder) were administered triple therapy (Peg-IFN/RBV/boceprevir) (triple therapy).
Patients treated with boceprevir who experienced an extended rapid virological response (eRVR), namely undetectable HCV RNA levels from week 4 through week 12, ended therapy at 28 wk. The other patients treated with boceprevir either without eRVR or with severe liver fibrosis in the presence of eRVR ended therapy at 48 wk. Patients with HCV genotypes 1/4 treated with double therapy stopped therapy at 48 wk; patients with genotypes 2/3 stopped all treatment at 24 wk[8].
The detection of HCV RNA in serum samples was determined by HCV-RNA Amplicor Monitor quantitative assay (Roche Diagnostics, Basel, Switzerland; limit of detection: 15 IU/mL), and HCV RNA was genotyped using INNO-LiPA HCV II (Immunogenetics, Gent, Belgium) conforming to the technical instruction of manufacturers.
The degree of liver function was evaluated through biohumoral liver function tests and the Child-Pugh score[19].
IL28B genotyping was performed on DNA extracted from whole blood samples using a specific custom TaqMan Single Nucleotide Polymorphism (SNP)-Genotyping Assay (SNP): rs12979860; Applied Biosystems, Foster City, CA, United States) based on allele-specific dual-labelled probes on a Rotor Gene 6000 (Corbett Research, Sidney, Australia)[20].
LS was measured in all patients before treatment after overnight fasting using FibroScan® (Echosens, Paris, France) conforming to the technical instruction of manufacturers. We considered as representative of LS the median values of 10 successful measurements, expressed in kiloPascal (kPa). We considered reliable the procedures with 10 successful acquisitions and a success rate greater than 60% and an interquartile range (IQR) ≤ 30% of the median value. LS values ≥ 12 kPa indicate advanced fibrosis[11,18].
Continuous variables were summarised by descriptive statistics and categorical variables were summarised using patient counts and percentages. All results are expressed as the mean ± SD. We used the Wilcoxon rank-sum test to compare two samples.
Relative risk (RR) is a measurement of the relationship between fibrosis and risk of non-response to treatment. A RR of 1.0 indicates an absence of association. When the RR is greater than 1.0, the risk of non-response to treatment is increased when the risk factor is present. Attributable risk (AR) is a measurement of the degree to which the risk of non-response to treatment is attributed to LS > 12 kPa. RR is calculated as follows: risk in patients with LS > 12 kPa/risk in patients with LS < 12 kPa. AR is calculated as follows: the incidence attributable to LS > 12 kPa/proportion of incidence attributable to LS > 12 kPa.
Logistic regression analysis was used to assess the factors associated with sustained virological response (SVR), which was defined as the absence of detectable HCV RNA in blood samples 24 wk after discontinuing the therapy. Multivariate logistic regression analysis was used to assess which baseline variables were predictive of SVR. In the final model, age, gender, stiffness, and viraemia (and viral genotype as well as IL28B genotype limited to dual therapy) were considered independent predictors of SVR.
All statistical tests were performed at the P≤ 0.05 level (two-sided). Statistical analysis and graphs were performed using Stata 11, (College Station, TX, United States) and SPSS v18 (SPSS Inc., Chicago, IL, United States).
The main features of the study population at baseline are summarised in Table 1.
| Baseline feature | Dual therapy | Triple therapy | Normal range | P value |
| Age (yr) | 51.82 ± 11.40 | 56.65 ± 8.24 | 0.16 | |
| BMI | 24.65 ± 3.45 | 26.17 ± 3.55 | 18-25 | 0.08 |
| Male sex | 40/25 | 10/10 | ||
| Albumin (g/dL) | 4.11 ± 0.34 | 4.08 ± 0.49 | 3.5-5.0 | 0.95 |
| Bilirubin (mg/dL) | 0.78 ± 0.41 | 1.05 ± 0.21 | 0.3-1 | 0.06 |
| Platelets (× 109/L) | 190.29 ± 60.04 | 168.16 ± 51.08 | 140-440 | 0.18 |
| ALT (U/L) | 116.75 ± 88.69 | 82.88 ± 47.86 | 5-40 | 0.28 |
| GGT (U/L) | 72.16 ± 80.53 | 93.38 ± 77.62 | 10-40 | 0.06 |
| C/T or T/T IL28B genotype | 19.50% | 100% | ||
| Liver stiffness (kPa) | 8.61 ± 5.79 | 13.29 ± 8.57 | 0.002 | |
| HCV genotypes 1/4, n (%) | 24 (36.92) | 20 (100) | ||
| HCV genotypes 2/3, n (%) | 41 (63.08) | |||
| HCV RNA level (IU × 1000) | 3459.135 ± 5541.24 | 4924.462 ± 6799.737 | 0.72 |
Among the 65 HCV patients treated with Peg-IFN/RBV dual therapy, 24 patients were infected with genotypes 1/4 (36.92%), whereas 41 patients were infected with genotypes 2/3 (63.08%) (dual therapy). Among the 20 HCV genotype 1 treatment-experienced patients treated with triple therapy, 1 patient was infected with genotype 1a, 1 patient was infected with genotype 1a/1b, and 18 patients were infected with genotype 1b. Only LS significantly differed in patients on dual vs triple therapy (P = 0.002) (Table 1). A diagnosis of liver cirrhosis (Child A) based on the histological and/or clinical and ultrasound data was made in 4 patients on dual therapy and in 8 patients on triple therapy.
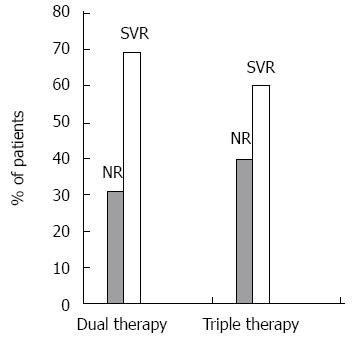
The rates of patients achieving a sustained virologic response 24 wk after therapy (SVR 24) are show in Figure 1.
In dual therapy patients, the mean LS value before treatment was 8.61 ± 5.79 kPa. Mean LS values significantly differed between patients achieving and not achieving SVR 24 (7.23 ± 5.18 kPa vs 11.72 ± 5.99 kPa, respectively, P = 0.0003). Liver stiffness > 12 kPa prior to the initiation of dual therapy was significantly associated with no SVR (P < 0.025). The relative risk of non-response to therapy was 4.45 (95%CI: 2.32-8.55). The attributable risk of non-response to therapy was 49%.
In triple therapy patients, the mean LS value before treatment was 13.29 ± 8.57 kPa. Pre-treatment LS values significantly differed between patients achieving SVR 24 and those who did not (9.41 ± 5.05 vs 19.11 ± 9.74, respectively, P = 0.008). Liver stiffness > 12 kPa prior to the initiation of triple therapy was significantly associated with no SVR (P < 0.025). The relative risk of non-response to therapy was 5.57% (95%CI: 1.50-20.65). The attributable risk of non-response to therapy was 70%. This result was increased compared with dual therapy patients, which is a consequence of eligibility for dual or triple therapy. In fact, boceprevir therapy is indicated for the treatment of difficult to treat patients, including patients with more advanced fibrosis. Univariate analysis was conducted by binary logistic regression analysis in both groups in which the response to treatment was the dependent variable; the results indicated that LS was a good predictor of response (dual therapy, P = 0.03; triple therapy, P = 0.0009) (Table 2). Multivariate regression analysis in dual therapy, where the independent variables included age, gender, stiffness, IL28B genotype, viraemia, and viral genotype, did not identify a predictor of treatment response. By contrast, in triple therapy, where the independent variables only included age, gender, stiffness, and viraemia (the IL28B polymorphism was always unfavourable regardless of the CT or TT alleles, and all patients were infected with genotype 1), LS was the only prognostic indicator of treatment response (P = 0.049) (Table 3).
| Dual therapy (P value) | Triple therapy (P value) | |
| Male sex | 0.33 | 0.39 |
| Age ≥ 55 yr | 0.40 | 0.47 |
| HCV genotype 1 and 4 | 0.46 | - |
| Liver stiffness > 12 kPa | 0.03 | 0.001 |
| C/T or T/T IL28B genotype | 0.12 | - |
| > 500000 IU HCV RNA titre | 0.30 | 0.06 |
| Dual therapy (P value) | Triple therapy (P value) | |
| Male sex | 0.465 | 0.913 |
| Age ≥ 55 yr | 0.465 | 0.954 |
| HCV genotypes 1 and 4 | 0.622 | - |
| Liver stiffness > 12 kPa | 0.733 | 0.049 |
| C/T or T/T IL28B genotype | 0.622 | - |
| > 500000 IU HCV RNA titre | 0.317 | 0.532 |
In this study, LS was demonstrated for the first time to predict a patient’s response to triple therapy. In a previous study[18], we observed that LS values greater than 12 kPa were a negative prognostic indicator of response to Peg-IFN/RBV therapy. In this different cohort of patients, the attributable risk of non-response to Peg-IFN/RBV therapy was similar but reduced compared with triple therapy. This difference was likely due to increased LS values in this latter group of patients.
The individual decision to start anti-HCV treatment depends largely on several factors, including liver histology, particularly in the difficult-to-treat HCV genotypes 1 and 4[6]. The extent of liver fibrosis may negatively affect the rate of response to antiviral therapy. Thus, cirrhotic patients, who may derive the most benefit from viral eradication, have the lowest SVR rates[3-5]. On the other hand, SVR may be associated with regression of liver fibrosis in patients with significant fibrosis. In this context, non-invasive tools for the assessment of liver fibrosis could be useful to eliminate the need of liver biopsy. The study by Roberts et al[3] demonstrated poor SVR rates among patients with advanced fibrosis compared with patients with moderate and absent or minimal fibrosis.
Transient elastography has been suggested as a possible machine for the diagnosis of absent/minimal fibrosis or severe fibrosis/cirrhosis in chronic HCV infected patients[21-25]. Moreover, the technique is characterised by high intra- and interobserver reproducibility[22,26]. However, recent food intake can affect the reliability of stiffness values in the diagnosis of fibrosis stage in patients with chronic HCV infection[27,28]. In addition, Arena et al[28] suggest that a fasting period of 120 min prior to obtaining liver stiffness measurements should be observed. A decline in the diagnostic need for liver biopsies in patients with HCV-related chronic liver disease was recently noted because the accuracy of non-invasive methods, especially in predicting non-significant or advanced liver fibrosis, suggests that LS can also be used as a predictor of response to therapy.
In fact, few data are available[18] regarding the interpretation of LS values as direct predictors of the treatment response. However, several studies[3-5] have demonstrated an association between the fibrotic stage in liver biopsies and response to treatment. In particular, Cheng et al[4] revealed a marked step-wise decline in SVR rates according to fibrotic stage. This observation is consistent with the present study that demonstrates a significant relationship between LS and the patient’s response to treatment. The LS values were particularly an independent predictor of response in those patients administered triple therapy.
Interestingly, the difference in the response rates between patients with LS < 12 vs > 12 was observed not only in patients treated with dual therapy but also in those treated with triple therapy with the direct acting antiviral (DAA) boceprevir. These results clearly confirm the potential utility of LS assessment in the evaluation of the response to anti-HCV treatment.
In Europe, the use of first-generation DAA boceprevir or telaprevir-based triple therapy has generally been restricted to patients with more advanced fibrosis stages given their high cost. The SPRINT-2[29] and RESPOND-2[30] trials demonstrated that the addition of boceprevir to Peg-INF/RBV results in significantly increased SVR rates in difficult-to-treat patients. Moreover, the duration of treatment was primarily based on fibrotic stage, with longer treatments providing the most benefit in patients with F3/4[31,32].
In conclusion, our data suggest that LS > 12 kPa should be considered a strong prognostic indicator of non-response to both dual and triple therapy. This index may potentially serve as a useful tool for accurate selection of patient treatment. Further studies are necessary to evaluate whether patients with LS < 12 kPa experience different clinical outcomes compared with patients with LS > 12.
New treatments for hepatitis C virus (HCV) infection require an accurate assessment of liver fibrosis for a correct therapeutic approach. The main objective of our study was to evaluate the value of liver stiffness as a prognostic indicator of response to antiviral therapy in HCV patients prior to initiation of dual/triple therapy.
First generation triple therapy is still a therapeutic option in many countries, where the polymerase inhibitors, inhibitors of the replication complex and protease inhibitors of the second generation are not yet available or still under way. This is mainly due to the living standards of the low and middle income countries. Interferon-free therapies are well tolerated and effective, but their high cost forces to let each patient follow cost-effective paths.
This prospective study points out that the liver stiffness values are significantly higher in patients who don’t respond to treatment suggesting that liver stiffness > 12 kPa should be considered a negative prognostic indicator.
The assessment of liver stiffness is a good option to evaluate liver fibrosis by means of a non invasive method. It can potentially be an useful tool for a more accurate selection of treatment.
The study is interesting and well done. In this prospective study of liver stiffness in 65 treatment-naive patients with HCV prior to commencing dual antiviral therapy, high liver stiffness values predicted a poor virological response. This group was compared with a group of patients who had been treated previously and were retreated with triple therapy. Liver stiffness was a strong predictor of response, especially in the latter group.
P- Reviewer: Badea R, Cosgrove D, Oramasionwu CU S- Editor: Ma YJ L- Editor: A E- Editor: Liu XM
| 1. | Pinzani M. Liver fibrosis. Springer Semin Immunopathol. 1999;21:475-490. [PubMed] [Cited in This Article: ] |
| 2. | Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209-218. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 211] [Cited by in F6Publishing: 202] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 3. | Roberts SK, Weltman MD, Crawford DH, McCaughan GW, Sievert W, Cheng WS, Rawlinson W, Desmond PV, Marks PS, Yoshihara M. Impact of high-dose peginterferon alfa-2A on virological response rates in patients with hepatitis C genotype 1: a randomized controlled trial. Hepatology. 2009;50:1045-1055. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 4. | Cheng WS, Roberts SK, McCaughan G, Sievert W, Weltman M, Crawford D, Rawlinson W, Marks PS, Thommes J, Rizkalla B. Low virological response and high relapse rates in hepatitis C genotype 1 patients with advanced fibrosis despite adequate therapeutic dosing. J Hepatol. 2010;53:616-623. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 5. | El Raziky M, Attia D, El Akel W, Shaker O, Khatab H, Abdo S, Elsharkawy A, Esmat G. Hepatic fibrosis and serum alpha-fetoprotein (AFP) as predictors of response to HCV treatment and factors associated with serum AFP normalisation after treatment. Arab J Gastroenterol. 2013;14:94-98. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 6. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: management of hepatitis C virus infection. J Hepatol. 2011;55:245-264. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 889] [Cited by in F6Publishing: 968] [Article Influence: 74.5] [Reference Citation Analysis (0)] |
| 7. | Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology. 1996;24:289-293. [PubMed] [Cited in This Article: ] |
| 8. | Associazione Italiana Per Lo Studio Del Fegato. Parere dell’Associazione Italiana per lo Studio del Fegato (AISF) sull’uso della triplice-terapia (Peg-IFN Ribavirina inibitore delle proteasi di prima generazione) per il trattamento dei pazienti con epatite cronica da HCV genotipo 1. Available from: http://www.webaisf.org/media/16360/position_paper_definitivo.pdf. [Cited in This Article: ] |
| 9. | Nishiura T, Watanabe H, Ito M, Matsuoka Y, Yano K, Daikoku M, Yatsuhashi H, Dohmen K, Ishibashi H. Ultrasound evaluation of the fibrosis stage in chronic liver disease by the simultaneous use of low and high frequency probes. Br J Radiol. 2005;78:189-197. [PubMed] [Cited in This Article: ] |
| 10. | Nojiri S, Kusakabe A, Fujiwara K, Shinkai N, Matsuura K, Iio E, Miyaki T, Joh T. Noninvasive evaluation of hepatic fibrosis in hepatitis C virus-infected patients using ethoxybenzyl-magnetic resonance imaging. J Gastroenterol Hepatol. 2013;28:1032-1039. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Stasi C, Arena U, Vizzutti F, Zignego AL, Monti M, Laffi G, Corti G, Pinzani M. Transient elastography for the assessment of liver fibrosis in patients with chronic viral hepatitis: the missing tool? Dig Liver Dis. 2009;41:863-866. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 12. | Ichikawa S, Motosugi U, Ichikawa T, Sano K, Morisaka H, Enomoto N, Matsuda M, Fujii H, Araki T. Magnetic resonance elastography for staging liver fibrosis in chronic hepatitis C. Magn Reson Med Sci. 2012;11:291-297. [PubMed] [Cited in This Article: ] |
| 13. | Ogawa E, Furusyo N, Toyoda K, Takeoka H, Maeda S, Hayashi J. The longitudinal quantitative assessment by transient elastography of chronic hepatitis C patients treated with pegylated interferon alpha-2b and ribavirin. Antiviral Res. 2009;83:127-134. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 112] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 14. | Arima Y, Kawabe N, Hashimoto S, Harata M, Nitta Y, Murao M, Nakano T, Shimazaki H, Kobayashi K, Ichino N. Reduction of liver stiffness by interferon treatment in the patients with chronic hepatitis C. Hepatol Res. 2010;40:383-392. [PubMed] [Cited in This Article: ] |
| 15. | Macías J, del Valle J, Rivero A, Mira JA, Camacho A, Merchante N, Pérez-Camacho I, Neukam K, Rivero-Juárez A, Mata R. Changes in liver stiffness in patients with chronic hepatitis C with and without HIV co-infection treated with pegylated interferon plus ribavirin. J Antimicrob Chemother. 2010;65:2204-2211. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 16. | Wang JH, Changchien CS, Hung CH, Tung WC, Kee KM, Chen CH, Hu TH, Lee CM, Lu SN. Liver stiffness decrease after effective antiviral therapy in patients with chronic hepatitis C: Longitudinal study using FibroScan. J Gastroenterol Hepatol. 2010;25:964-969. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 17. | Martinez SM, Foucher J, Combis JM, Métivier S, Brunetto M, Capron D, Bourlière M, Bronowicki JP, Dao T, Maynard-Muet M. Longitudinal liver stiffness assessment in patients with chronic hepatitis C undergoing antiviral therapy. PLoS One. 2012;7:e47715. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 18. | Stasi C, Arena U, Zignego AL, Corti G, Monti M, Triboli E, Pellegrini E, Renzo S, Leoncini L, Marra F. Longitudinal assessment of liver stiffness in patients undergoing antiviral treatment for hepatitis C. Dig Liver Dis. 2013;45:840-843. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 19. | Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646-649. [PubMed] [Cited in This Article: ] |
| 20. | Piluso A, Giannini C, Fognani E, Gragnani L, Caini P, Monti M, Petrarca A, Ranieri J, Urraro T, Triboli E. Value of IL28B genotyping in patients with HCV-related mixed cryoglobulinemia: results of a large, prospective study. J Viral Hepat. 2013;20:e107-e114. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Foucher J, Chanteloup E, Vergniol J, Castéra L, Le Bail B, Adhoute X, Bertet J, Couzigou P, de Lédinghen V. Diagnosis of cirrhosis by transient elastography (FibroScan): a prospective study. Gut. 2006;55:403-408. [PubMed] [Cited in This Article: ] |
| 22. | Fraquelli M, Rigamonti C, Casazza G, Conte D, Donato MF, Ronchi G, Colombo M. Reproducibility of transient elastography in the evaluation of liver fibrosis in patients with chronic liver disease. Gut. 2007;56:968-973. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 629] [Cited by in F6Publishing: 618] [Article Influence: 36.4] [Reference Citation Analysis (0)] |
| 23. | Coco B, Oliveri F, Maina AM, Ciccorossi P, Sacco R, Colombatto P, Bonino F, Brunetto MR. Transient elastography: a new surrogate marker of liver fibrosis influenced by major changes of transaminases. J Viral Hepat. 2007;14:360-369. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 484] [Cited by in F6Publishing: 441] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 24. | Arena U, Vizzutti F, Abraldes JG, Corti G, Stasi C, Moscarella S, Milani S, Lorefice E, Petrarca A, Romanelli RG. Reliability of transient elastography for the diagnosis of advanced fibrosis in chronic hepatitis C. Gut. 2008;57:1288-1293. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 206] [Cited by in F6Publishing: 217] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 25. | Sagir A, Erhardt A, Schmitt M, Häussinger D. Transient elastography is unreliable for detection of cirrhosis in patients with acute liver damage. Hepatology. 2008;47:592-595. [PubMed] [Cited in This Article: ] |
| 26. | Nobili V, Vizzutti F, Arena U, Abraldes JG, Marra F, Pietrobattista A, Fruhwirth R, Marcellini M, Pinzani M. Accuracy and reproducibility of transient elastography for the diagnosis of fibrosis in pediatric nonalcoholic steatohepatitis. Hepatology. 2008;48:442-448. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 297] [Cited by in F6Publishing: 283] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 27. | Mederacke I, Wursthorn K, Kirschner J, Rifai K, Manns MP, Wedemeyer H, Bahr MJ. Food intake increases liver stiffness in patients with chronic or resolved hepatitis C virus infection. Liver Int. 2009;29:1500-1506. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 182] [Cited by in F6Publishing: 191] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 28. | Arena U, Lupsor Platon M, Stasi C, Moscarella S, Assarat A, Bedogni G, Piazzolla V, Badea R, Laffi G, Marra F. Liver stiffness is influenced by a standardized meal in patients with chronic hepatitis C virus at different stages of fibrotic evolution. Hepatology. 2013;58:65-72. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 137] [Cited by in F6Publishing: 143] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 29. | Poordad F, McCone J, Bacon BR, Bruno S, Manns MP, Sulkowski MS, Jacobson IM, Reddy KR, Goodman ZD, Boparai N. Boceprevir for untreated chronic HCV genotype 1 infection. N Engl J Med. 2011;364:1195-1206. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1948] [Cited by in F6Publishing: 1951] [Article Influence: 150.1] [Reference Citation Analysis (0)] |
| 30. | Bacon BR, Gordon SC, Lawitz E, Marcellin P, Vierling JM, Zeuzem S, Poordad F, Goodman ZD, Sings HL, Boparai N. Boceprevir for previously treated chronic HCV genotype 1 infection. N Engl J Med. 2011;364:1207-1217. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1287] [Cited by in F6Publishing: 1288] [Article Influence: 99.1] [Reference Citation Analysis (0)] |
| 31. | Bruno S, Vierling JM, Esteban R, Nyberg LM, Tanno H, Goodman Z, Poordad F, Bacon B, Gottesdiener K, Pedicone LD. Efficacy and safety of boceprevir plus peginterferon-ribavirin in patients with HCV G1 infection and advanced fibrosis/cirrhosis. J Hepatol. 2013;58:479-487. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 32. | Tural C, Planas R. [Clinical use of telaprevir: stopping rules, predicting response, treatment length, and management of adverse effects]. Enferm Infecc Microbiol Clin. 2013;31 Suppl 3:19-25. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |