Published online Jan 7, 2015. doi: 10.3748/wjg.v21.i1.187
Peer-review started: May 26, 2014
First decision: June 18, 2014
Revised: July 10, 2014
Accepted: July 24, 2014
Article in press: July 25, 2014
Published online: January 7, 2015
AIM: To investigate dendritic cell-specific intercellular adhesion molecule-3-grabbing non-integrin (DC-SIGN) expression in intestinal epithelial cells (IECs) in inflammatory bowel disease (IBD).
METHODS: The expression of DC-SIGN in IECs was examined by immunohistochemistry of intestinal mucosal biopsies from 32 patients with IBD and 10 controls. Disease activity indices and histopathology scores were used to assess the tissue lesions and pathologic damage. Animal studies utilized BALB/c mice with dextran sodium sulfate (DSS)-induced colitis treated with anti-P-selectin lectin-EGF domain monoclonal antibody (PsL-EGFmAb). Controls, untreated and treated mice were sacrificed after 7 d, followed by isolation of colon tissue and IECs. Colonic expression of DC-SIGN, CD80, CD86 and MHC II was examined by immunohistochemistry or flow cytometry. The capacity of mouse enterocytes or dendritic cells to activate T cells was determined by co-culture with naïve CD4+ T cells. Culture supernatant and intracellular levels of interleukin (IL)-4 and interferon (IFN)-γ were measured by enzyme-linked immunosorbent assay and flow cytometry, respectively. The ability of IECs to promote T cell proliferation was detected by flow cytometry staining with carboxyfluorescein diacetate succinimidyl ester.
RESULTS: Compared with controls, DC-SIGN expression was significantly increased in IECs from patients with Crohn’s disease (P < 0.01) or ulcerative colitis (P < 0.05). DC-SIGN expression was strongly correlated with disease severity in IBD (r = 0.48; P < 0.05). Similarly, in the DSS-induced colitis mouse model, IECs showed upregulated expression of DC-SIGN, CD80, CD86 and MHC, and DC-SIGN expression was positively correlated with disease activity (r = 0.62: P < 0.01). IECs from mouse colitis stimulated naïve T cells to generate IL-4 (P < 0.05). Otherwise, dendritic cells promoted a T-helper-1-skewing phenotype by stimulating IFN-γ secretion. However, DC-SIGN expression and T cell differentiation were suppressed following treatment of mice with DSS-induced colitis with PsL-EGFmAb. The proliferation cycles of CD4+ T cells from mice with DSS-induced colitis appeared as five cycles, which was more than in the control and treated groups. These results suggest that IECs can promote T cell proliferation.
CONCLUSION: IECs regulate tissue-associated immune compartments under the control of DC-SIGN in IBD.
Core tip: Dendritic cell-specific intercellular adhesion molecule-3-grabbing non-integrin (DC-SIGN) functions as an adhesion and antigen-presenting molecule. We found that DC-SIGN was expressed by intestinal epithelial cells, which induced differentiation and proliferation of T cells under the control of DC-SIGN.
- Citation: Zeng JQ, Xu CD, Zhou T, Wu J, Lin K, Liu W, Wang XQ. Enterocyte dendritic cell-specific intercellular adhesion molecule-3-grabbing non-integrin expression in inflammatory bowel disease. World J Gastroenterol 2015; 21(1): 187-195
- URL: https://www.wjgnet.com/1007-9327/full/v21/i1/187.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i1.187
Inflammatory bowel disease (IBD), primarily comprised of Crohn’s disease and ulcerative colitis, is an idiopathic disease characterized by chronic, relapsing, nonspecific inflammatory reactions of the bowel[1,2]. The exact etiology of IBD is still unknown. Recent studies have provided substantial insight into how functional mucosal immunity is maintained and how the pathogenesis of IBD is initiated. IBD is generally attributed to inappropriate and continuing inflammatory stimulations[3-6].
Dendritic cells (DCs) play a key role in the initiation of inflammation, which is associated with the migration of DCs mediated by the adhesion molecule P-selectin. Adhesion and migration of DCs is inhibited by anti-P-selectin lectin-EGF domain monoclonal antibodies (PsL-EGFmAb), which target the carbohydrate recognition domain of P-selectin[7,8]. Our previous work demonstrated that PsL-EGFmAb had a blocking effect on DC-specific intercellular adhesion molecule-3-grabbing non-integrin (DC-SIGN) in a mouse model of nephritis and improved disease progression and outcome[7]. DC-SIGN, also designated as CD209, is a member of the C-type lectin superfamily, and has a carbohydrate recognition domain similar to P-selectin[9].
In this study, we investigated the expression of DC-SIGN in the intestinal tissues of patients with IBD and its significance in the disease activity. To further study the mechanisms of how DC-SIGN functions in colitis, we examined expression with PsL-EGFmAb treatment in an experimental model of dextran sodium sulfate (DSS)-induced colitis.
A total of 32 children with IBD were randomly recruited from the Department of Pediatrics, Ruijin Hospital between January 2006 and June 2010. All children were diagnosed with IBD by endoscopy and histopathologic examination according to the pediatric Crohn’s disease activity index (PCDAI) and pediatric ulcerative colitis activity index (PUCAI). The patients included 20 boys and 12 girls, with a mean age of 8.68 ± 5.21 years. We recruited two major disease groups: Crohn’s disease (n = 18) and ulcerative colitis (n = 14). Ten age- and sex-matched children with abdominal pain, diarrhea and no histologic enteritis were enrolled as controls. Human intestinal mucosal tissues from patients with Crohn’s disease, ulcerative colitis and the control group were collected by endoscopic biopsy.
The study was approved by the Ethical Committee of Shanghai Jiao Tong University School of Medicine, China.
The DSS-induced colitis mouse model of IBD was described by Okayasu et al[10]. Thirty female BALB/c mice (aged 6-8 wk, 16-20 g) were purchased from the Hayes Lake Experimental Animals Co. (Shanghai, China) and randomly assigned into three groups (n = 10 each): control, DSS-treated, and PsL-EGFmAb + DSS-treated. The DSS-treated group was orally administered a 5% DSS solution for 7 d. The PsL-EGFmAb + DSS-treated group were given daily injections with 2 mg/kg PsL-EGFmAb (ip) for 3 d during the 7 d of 5% DSS administration. Control animals were orally administered a sterile saline solution. Clinical Disease Activity Index for DSS-induced colitis was measured by weight loss, stool consistency, and bleeding[11]. All the mice were sacrificed at day 7, and intestinal mucosa and spleens were quickly removed for histologic and cellular function analyses.
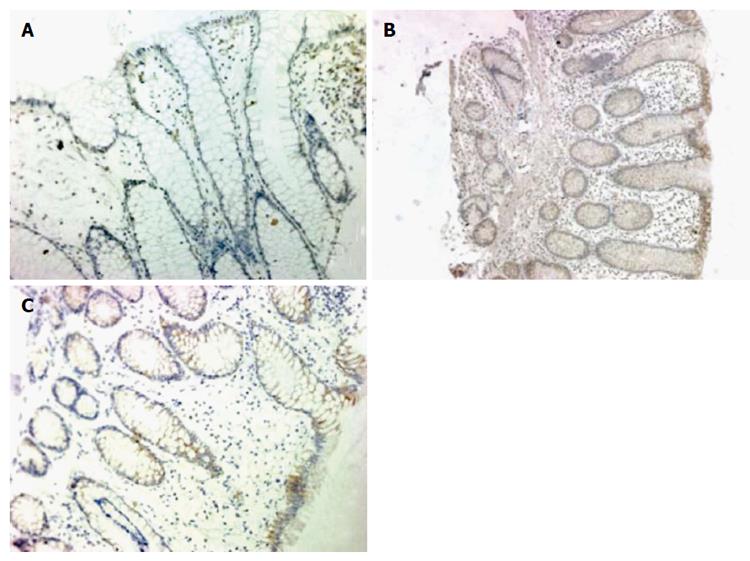
Paraffin sections of human and mouse intestinal mucosal tissues were treated with endogenous peroxidase and nonspecific protein blocking, and incubated with 1:100 primary antibody at 4 °C overnight and 1:400 secondary antibody for 1 h at room temperature. Antibodies used were as follows: mouse anti-human DC-SIGN mAb (R and D Systems, Minneapolis, MN, United States) and biotinylated anti-mouse IgG (Invitrogen of Thermo Fisher Scientific Inc., Waltham, MA, United States) for human tissues, and rat anti-mouse DC-SIGN mAb (eBioscience Inc., San Diego, CA, United States) with biotinylated anti-rat (Invitrogen) for mouse tissues. Finally, the sections were stained by diaminobenzidine for microscopic examination. The primary antibody was replaced with phosphate-buffered saline as a negative control and known positive sections were used as positive controls.
The positive cells showed distinct brown-orange coloration within the cell membrane or cytoplasm of epithelial cells. Immunohistochemistry scores were based on the percentage of positive cells (< 10% = 0; 10%-30% = 1; 31%-50% = 2; 51%-75% = 3; and > 75% = 4) multiplied by stain intensity (0 = negative, 1 = weak, 2 = moderate, 3 = strong) in five different high-power fields for each section. A score of 4+ was called “DC-SIGN positive”[12].
Paraffin-embedded sections (5 μm) prepared from the distal colons of experimental mice were stained with hematoxylin/eosin and examined under a Zeiss Axioplan 2 imaging microscope equipped with an AxioCam MRc5 camera (Carl Zeiss AG, Oberkochen, Germany). Histologic scoring was ranked according to the amount and depth of inflammation, and the amount of crypt damage[13]. Isolated mouse splenic cells (1 × 105 cells/mL) were incubated with fluorescein isothiocyanate-labeled CD4 mAb and stained with allophycocyanin-labeled interferon (IFN)-γ mAb and phycoerythrin-labeled interleukin (IL)-4 mAb to evaluate the systemic inflammatory response in mice[14].
Mouse intestinal epithelial cells (IECs) were sorted by flow cytometry using anti-mouse phycoerythrin-conjugated CD326 (epithelial cell adhesion molecule) and incubated with fluorescein isothiocyanate-labeled DC-SIGN, CD80, CD86 or MHC mAb at a density of 5 × 105 cells/mL. Phenotypic analysis was performed by flow cytometry using a FACS Calibur and FACSAria Cell Sorter (Becton, Dickenson and Co., Franklin Lakes, NJ, United States) and data were analyzed with FCS Express version 3.
Mouse splenic CD4+ T cells were isolated using mouse naïve CD4+ T cell isolation Kit (R and D Systems), and CD11c+ DCs were purified using magnetic activated cell sorting beads (Miltenyi Biotec, Bergisch Gladbach, Germany). The IECs (5 × 105/mL) or CD11c+ DCs (2 × 105/mL) were co-cultured with CD4+ T cells (1 × 106/mL) in 96-well plates in the presence of IL-2, anti-CD3 and anti-CD28 for 5 d. Cells were stimulated with phorbol 12-myristate 13-acetate (50 ng/mL), ionomycin (1 μg/mL) and brefeldin A (10 μg/mL) for 6 h, and harvested for intracellular IFN- γ or IL-4 staining with allophycocyanin-conjugated IFN-γ mAb and phycoerythrin -labeled IL-4 mAb, followed by flow cytometry analysis. In addition, IFN-γ or IL-4 levels in the co-culture supernatants were measured by enzyme-linked immunosorbent assay[15]. The ability of IECs to promote T cell proliferation was detected by flow cytometry after staining with carboxyfluorescein diacetate succinimidyl ester.
SPSS version 16.0 (SPSS Inc., Chicago, IL, United States) was used for the database analysis. Data are presented as mean ± standard deviation and were measured by nonparametric rank sum test and one-way analysis of variance. Numerical data were measured using Fisher’s exact test. The correlation between groups was analyzed using Spearman coefficients. A value of P < 0.05 was considered statistically significant.
Expression of DC-SIGN was rarely detected in the intestinal mucosa of healthy children, but was elevated in the intestinal mucosa of children with IBD, especially in the IECs and mesenchymal cells (Figure 1). DC-SIGN expression was significantly higher in children with Crohn’s disease (61%; P = 0.002) and ulcerative colitis (50%; P = 0.019) compared with controls (10%). However, there was no significant difference between expression in Crohn’s disease and ulcerative colitis.
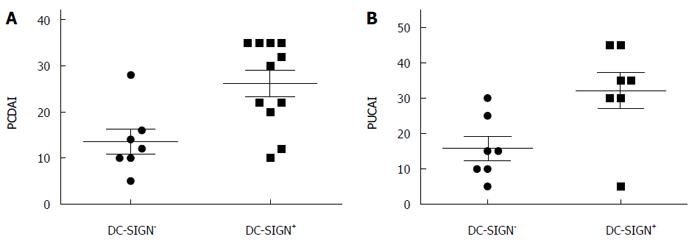
To determine if increased expression of DC-SIGN was correlated with IBD progression and severity, we used PCDAI and PUCAI to evaluate disease activity in children with IBD. The scores were significantly higher in the DC-SIGN-positive group than in the DC-SIGN-negative group (PCDAI: 25.91 ± 10.20 vs 13.93 ± 7.20, PUCAI: 32.14 ± 13.50 vs 15.71 ± 8.86; Ps < 0.01), and DC-SIGN expression was strongly correlated with disease severity in IBD (r = 0.48; P < 0.05) (Figure 2).
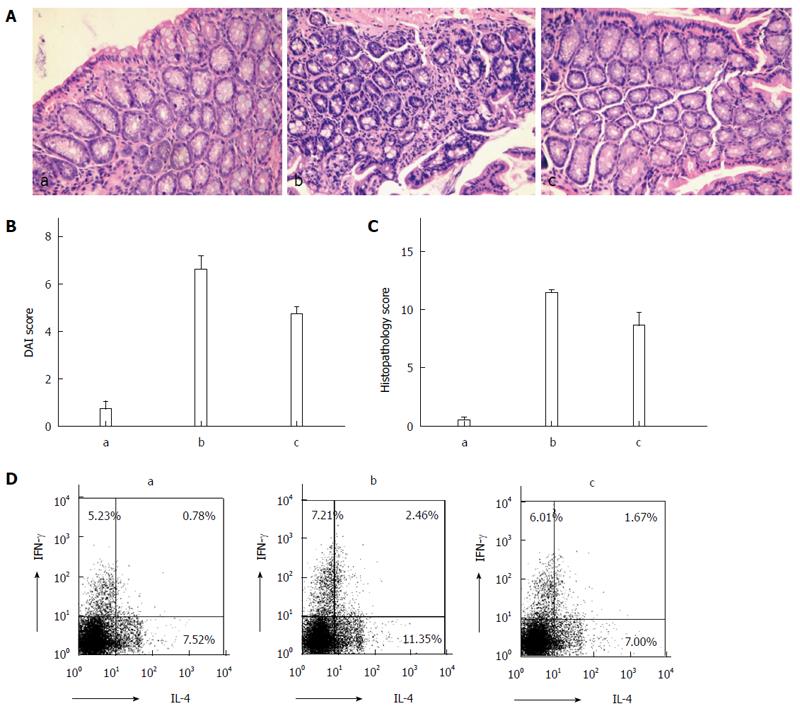
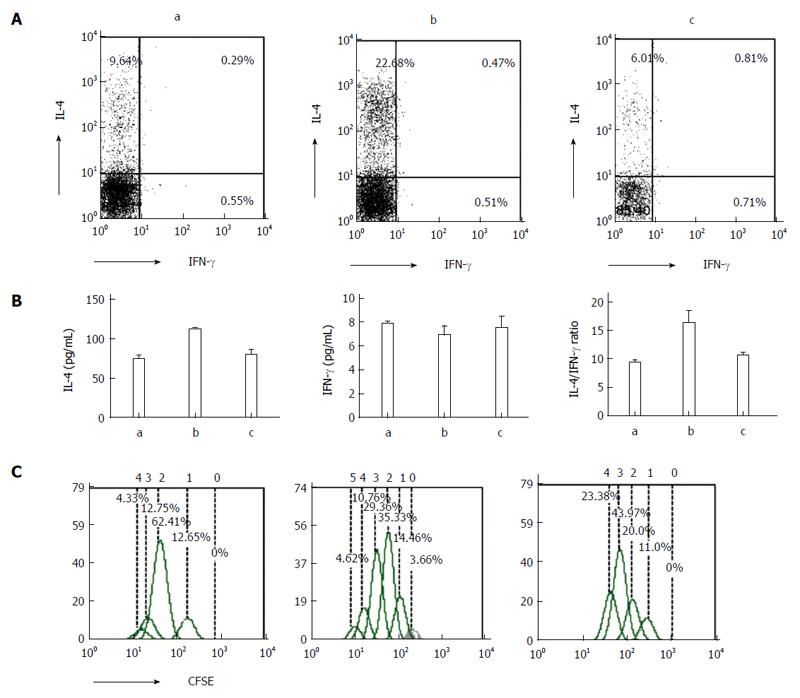
Hematoxylin and eosin staining revealed greater neutrophil infiltration in the intestinal tissue from the DSS and DSS + PSL-EGFmAb groups compared with the control group (Figure 3A). The disease activity index score was significantly elevated in DSS-treated mice compared with the controls (11.4 ± 0.70 vs 0.5 ± 0.53, P < 0.01), and was signficantly decreased by PsL-EGFmAb treatment (8.6 ± 3.60, P < 0.05) (Figure 3B). In addition, histologic examination of intestinal biopsies showed significantly higher scores in the DSS-treated group (6.6 ± 1.78, P < 0.01) compared with the controls (0.7 ± 1.06) but suppressed disease following treatment with PsL-EGFmAb (4.7 ± 1.06, P < 0.05) (Figure 3C). IL-4 and IFN-γ expression levels in mouse splenic CD4+ T cells were increased in the DSS-treated group compared with the control and DSS + PsL-EGFmAb groups (Figure 3D).
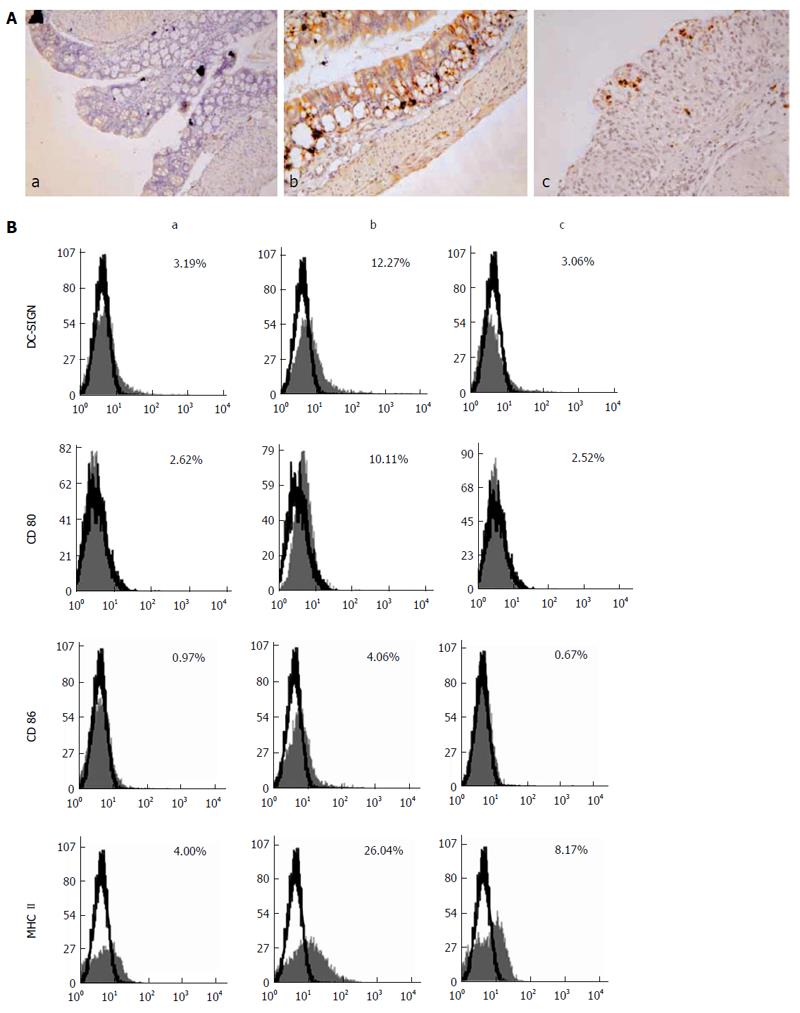
DC-SIGN expression was rarely detected in normal intestinal tissues, but was clearly observed in the intestinal tissues of the DSS-treated and DSS + PsL-EGFmAb groups (Figure 4A). Further analysis revealed that DC-SIGN expression was significantly correlated with disease activity scores (rs = 0.62; P < 0.01). Flow cytometric analysis revealed that, as well as co-stimulatory molecules, CD80, CD86 and MHC II were markedly elevated in IECs of DSS-treated mice and downregulated with PsL-EGFmAb treatment (Figure 4B).
IECs are not traditional antigen-presenting cells. However, we report here that after co-culturing naïve CD4+ T cells and IECs, T cells were activated and T helper (Th) cytokines (IFN-γ and IL-4) were detected by flow cytometry and enzyme-linked immunosorbent assay. The results show that compared with controls, IL-4 expression levels peaked in the DSS-treated group (P < 0.05), and increased in the PsL-EGFmAb + DSS-treated group (Figure 5A and B). No significant changes in IFN-γ were observed among the three groups. In addition, the IL-4/IFN-γ ratio of the co-culture supernatant was higher in the DSS-treated group, but downregulated with PsL-EGFmAb treatment (P < 0.05). The proliferation cycles of CD4+ T cells in the DSS-induced colitis group appeared as five cycles, which was more than in the other groups (Figure 5C).
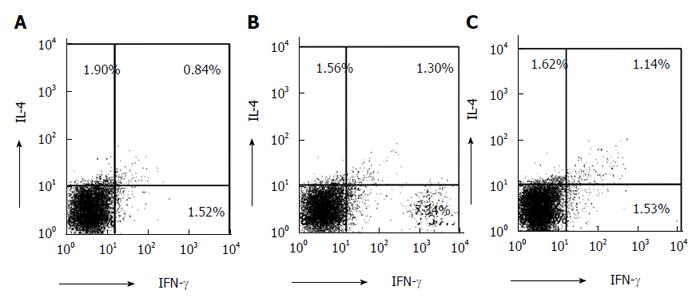
Co-culturing of naïve T cells from normal BALB/c mice and DCs from mouse spleens resulted in an increased proportion of IFN-γ in the DSS-treated group compared with the PsL-EGFmAb-treated and control groups (Figure 6).
IBD is a chronic intestinal disorder of unknown etiology and pathogenesis. However, it is generally believed that uncontrolled intestinal immune response facilitates onset and development of IBD[6,16,17]. Murine models of IBD have demonstrated that the imbalance in Th1/Th2 cells plays a pivotal role in determining the type of immune response generated in the gut and that distinct cytokine profiles characterize each CD4+ T cell subset[18,19].
The formation of a physical barrier by IECs plays an important role in innate immune defense[20-22]. Recently, it has been found that IECs are not only a passive barrier that limits the access of pathogens, but also participate in mucosal immune regulation through the pattern recognition receptors[23-25].
DCs are dysregulated in IBD, which leads to overproduction of chemokines and proinflammatory cytokines that stimulate the activation and differentiation of pathogenic Th cells[26-28]. DCs are antigen-presenting cells that are responsible for the regulation of abnormal T cell activation. Upon activation, a number of cell surface molecules and maturation markers are expressed, such as Toll-like receptors, Nod-like receptors, and C-type lectin receptors. Among them, DC-SIGN is a member of the C-type lectin superfamily, functioning as an adhesion receptor and a pattern recognition receptor[29-31]. It plays a critical role in regulating the migration of DCs and subsequent activation of T lymphocytes involved in the immunoregulation of infectious and inflammatory diseases[9,32].
Our study showed that IECs express DC-SIGN, which is significantly correlated with intestinal disease severity. In vitro, we further demonstrated that IECs stimulate CD4+ T cells to secrete IL-4, suggesting that they potently induce a Th2-predominant host immune response in experimental colitis. In contrast, DCs from animals with experimental colitis induced T cells towards a Th1-skewing phenotype by IFN-γ secretion. Based on the above results, we propose that the injured IECs might trans-differentiate, leading them to acquire immune properties. Trans-differentiation is a biologic process by which one differentiated cell type coverts into another[33,34]. In disease states, IECs exert antigen-presenting function by trans-differentiation and regulate mucosal immunity together with DCs in the local microenvironment, to determine the type of immune response. This phenomenon may be associated with the regulation of gut immune compartmentalization[35,36].
In summary, DC-SIGN modulates the trans-differentiation of IECs, interacts with DCs as well as the local intestinal immune compartment, and might play a vital role in facilitating damage to the gut mucosa in IBD. Further study is needed to investigate the underlying mechanisms for the immunomodulatory effects of IECs.
Dendritic cell-specific intercellular adhesion molecule-3-grabbing non-integrin (DC-SIGN) is a DC phenotypic molecule and plays an important role in mediating DC adhesion and migration, inflammation, and activation of primary T cells. This study aimed to confirm if intestinal epithelial cells (IECs) express DC-SIGN and explore if it plays a role in inflammatory bowel disease (IBD).
The authors found that DC-SIGN was expressed by IECs. This may have implications for cellular immunology.
This study confirmed that DC-SIGN expression increased significantly in IECs from patients with IBD and in the dextran sodium sulfate-induced colitis mouse model. IECs can promote T cell proliferation and differentiation to regulate tissue-associated immune compartments under the control of DC-SIGN.
The findings highlight DC-SIGN expression in IECs, which modulates the interaction of IECs with the local intestinal immune compartment in IBD. This provides new ideas for the underlying mechanism of IBD.
The carbohydrate recognition domain can selectively recognize sugar structures on the surface of pathogens. Anti-P-selectin lectin-EGF domain monoclonal antibody targets the targeting the carbohydrate recognition domain of P-selectin.
In this study, the authors investigated the expression of DC-SIGN in IECs and its significance in IBD, based on a mouse colitis model and human (pediatric) IBD samples. The idea is novel and interesting.
P- Reviewer: Freire-De-Lima CG, Li W S- Editor: Gou SX L- Editor: AmEditor E- Editor: Liu XM
| 1. | Hanauer SB. Inflammatory bowel disease: epidemiology, pathogenesis, and therapeutic opportunities. Inflamm Bowel Dis. 2006;12 Suppl 1:S3-S9. [PubMed] [Cited in This Article: ] |
| 2. | Hendrickson BA, Gokhale R, Cho JH. Clinical aspects and pathophysiology of inflammatory bowel disease. Clin Microbiol Rev. 2002;15:79-94. [PubMed] [Cited in This Article: ] |
| 3. | Roberts-Thomson IC, Fon J, Uylaki W, Cummins AG, Barry S. Cells, cytokines and inflammatory bowel disease: a clinical perspective. Expert Rev Gastroenterol Hepatol. 2011;5:703-716. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 83] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 4. | Khor B, Gardet A, Xavier RJ. Genetics and pathogenesis of inflammatory bowel disease. Nature. 2011;474:307-317. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1693] [Cited by in F6Publishing: 1716] [Article Influence: 132.0] [Reference Citation Analysis (1)] |
| 5. | Blumberg RS. Inflammation in the intestinal tract: pathogenesis and treatment. Dig Dis. 2009;27:455-464. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 63] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 6. | Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427-434. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2894] [Cited by in F6Publishing: 3122] [Article Influence: 183.6] [Reference Citation Analysis (9)] |
| 7. | Zhou T, Zhang Y, Sun G, Zou J, Li X, Cai M, Xiao Y, Zhang Y, Zhao Y, Chen N. PsL-EGFmAb inhibits the stimulatory functions of human dendritic cells via DC-SIGN. Front Biosci. 2008;13:7269-7276. [PubMed] [Cited in This Article: ] |
| 8. | Zhou T, Song W, Wang F, Ni PH, Chen N, Zhang DQ, Yu QW. [Cloning, expression of the lectin-EGF domain of P-selectin, and preparation of its monoclonal antibody]. Shengwu Huaxue Yu Shengwu Wuli Xuebao (Shanghai). 2003;35:172-176. [PubMed] [Cited in This Article: ] |
| 9. | Zhou T, Chen Y, Hao L, Zhang Y. DC-SIGN and immunoregulation. Cell Mol Immunol. 2006;3:279-283. [PubMed] [Cited in This Article: ] |
| 10. | Okayasu I, Hatakeyama S, Yamada M, Ohkusa T, Inagaki Y, Nakaya R. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology. 1990;98:694-702. [PubMed] [Cited in This Article: ] |
| 11. | Murano M, Maemura K, Hirata I, Toshina K, Nishikawa T, Hamamoto N, Sasaki S, Saitoh O, Katsu K. Therapeutic effect of intracolonically administered nuclear factor kappa B (p65) antisense oligonucleotide on mouse dextran sulphate sodium (DSS)-induced colitis. Clin Exp Immunol. 2000;120:51-58. [PubMed] [Cited in This Article: ] |
| 12. | Zhou T, Li X, Zou J, Cai M, Sun G, Zhang Y, Zhao Y, Zhang M, Zhang Y, Chen N. Effects of DC-SIGN expression on renal tubulointerstitial fibrosis in nephritis. Front Biosci (Landmark Ed). 2009;14:2935-2943. [PubMed] [Cited in This Article: ] |
| 13. | Dieleman LA, Peña AS, Meuwissen SG, van Rees EP. Role of animal models for the pathogenesis and treatment of inflammatory bowel disease. Scand J Gastroenterol Suppl. 1997;223:99-104. [PubMed] [Cited in This Article: ] |
| 14. | Dieleman LA, Palmen MJ, Akol H, Bloemena E, Peña AS, Meuwissen SG, Van Rees EP. Chronic experimental colitis induced by dextran sulphate sodium (DSS) is characterized by Th1 and Th2 cytokines. Clin Exp Immunol. 1998;114:385-391. [PubMed] [Cited in This Article: ] |
| 15. | Engvall E, Perlmann P. Enzyme-linked immunosorbent assay, Elisa. 3. Quantitation of specific antibodies by enzyme-labeled anti-immunoglobulin in antigen-coated tubes. J Immunol. 1972;109:129-135. [PubMed] [Cited in This Article: ] |
| 16. | Hisamatsu T, Kanai T, Mikami Y, Yoneno K, Matsuoka K, Hibi T. Immune aspects of the pathogenesis of inflammatory bowel disease. Pharmacol Ther. 2013;137:283-297. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 69] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 17. | Gersemann M, Wehkamp J, Stange EF. Innate immune dysfunction in inflammatory bowel disease. J Intern Med. 2012;271:421-428. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 112] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 18. | Asseman C, Read S, Powrie F. Colitogenic Th1 cells are present in the antigen-experienced T cell pool in normal mice: control by CD4+ regulatory T cells and IL-10. J Immunol. 2003;171:971-978. [PubMed] [Cited in This Article: ] |
| 19. | Kühn R, Löhler J, Rennick D, Rajewsky K, Müller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263-274. [PubMed] [Cited in This Article: ] |
| 20. | Mayer L, Eisenhardt D, Salomon P, Bauer W, Plous R, Piccinini L. Expression of class II molecules on intestinal epithelial cells in humans. Differences between normal and inflammatory bowel disease. Gastroenterology. 1991;100:3-12. [PubMed] [Cited in This Article: ] |
| 21. | Harrison OJ, Maloy KJ. Innate immune activation in intestinal homeostasis. J Innate Immun. 2011;3:585-593. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 22. | Shale M, Ghosh S. How intestinal epithelial cells tolerise dendritic cells and its relevance to inflammatory bowel disease. Gut. 2009;58:1291-1299. [PubMed] [Cited in This Article: ] |
| 23. | Geijtenbeek TB, Gringhuis SI. Signalling through C-type lectin receptors: shaping immune responses. Nat Rev Immunol. 2009;9:465-479. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 870] [Cited by in F6Publishing: 906] [Article Influence: 60.4] [Reference Citation Analysis (0)] |
| 24. | Rosenstiel P, Sina C, End C, Renner M, Lyer S, Till A, Hellmig S, Nikolaus S, Fölsch UR, Helmke B. Regulation of DMBT1 via NOD2 and TLR4 in intestinal epithelial cells modulates bacterial recognition and invasion. J Immunol. 2007;178:8203-8211. [PubMed] [Cited in This Article: ] |
| 25. | Cario E, Podolsky DK. Differential alteration in intestinal epithelial cell expression of toll-like receptor 3 (TLR3) and TLR4 in inflammatory bowel disease. Infect Immun. 2000;68:7010-7017. [PubMed] [Cited in This Article: ] |
| 26. | te Velde AA, van Kooyk Y, Braat H, Hommes DW, Dellemijn TA, Slors JF, van Deventer SJ, Vyth-Dreese FA. Increased expression of DC-SIGN+IL-12+IL-18+ and CD83+IL-12-IL-18- dendritic cell populations in the colonic mucosa of patients with Crohn’s disease. Eur J Immunol. 2003;33:143-151. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 109] [Cited by in F6Publishing: 113] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 27. | Rutella S, Locatelli F. Intestinal dendritic cells in the pathogenesis of inflammatory bowel disease. World J Gastroenterol. 2011;17:3761-3775. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 50] [Cited by in F6Publishing: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 28. | Niess JH. Role of mucosal dendritic cells in inflammatory bowel disease. World J Gastroenterol. 2008;14:5138-5148. [PubMed] [Cited in This Article: ] |
| 29. | van Kooyk Y, Geijtenbeek TB. DC-SIGN: escape mechanism for pathogens. Nat Rev Immunol. 2003;3:697-709. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 712] [Cited by in F6Publishing: 667] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 30. | Geijtenbeek TB, van Kooyk Y. Pathogens target DC-SIGN to influence their fate DC-SIGN functions as a pathogen receptor with broad specificity. APMIS. 2003;111:698-714. [PubMed] [Cited in This Article: ] |
| 31. | van Gisbergen KP, Aarnoudse CA, Meijer GA, Geijtenbeek TB, van Kooyk Y. Dendritic cells recognize tumor-specific glycosylation of carcinoembryonic antigen on colorectal cancer cells through dendritic cell-specific intercellular adhesion molecule-3-grabbing nonintegrin. Cancer Res. 2005;65:5935-5944. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 655] [Reference Citation Analysis (0)] |
| 32. | Cai M, Zhou T, Li X, Chen J, Mao C, Xi X, Chen N, Xu C. DC-SIGN modulates DC maturation and function in rat renal tubulointerstitial lesions. Front Biosci (Landmark Ed). 2012;17:1795-1803. [PubMed] [Cited in This Article: ] |
| 33. | Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420-1428. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6575] [Cited by in F6Publishing: 7377] [Article Influence: 491.8] [Reference Citation Analysis (0)] |
| 34. | Romero-Valdovinos M, Bobadilla-Sandoval N, Flisser A, Vadillo-Ortega F. The epithelial mesenchymal transition process may contribute to the pathogenesis of amniotic band syndrome. Med Hypotheses. 2014;83:306-311. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 6] [Reference Citation Analysis (0)] |
| 35. | Kunkel EJ, Campbell JJ, Haraldsen G, Pan J, Boisvert J, Roberts AI, Ebert EC, Vierra MA, Goodman SB, Genovese MC. Lymphocyte CC chemokine receptor 9 and epithelial thymus-expressed chemokine (TECK) expression distinguish the small intestinal immune compartment: Epithelial expression of tissue-specific chemokines as an organizing principle in regional immunity. J Exp Med. 2000;192:761-768. [PubMed] [Cited in This Article: ] |
| 36. | Kunkel EJ, Boisvert J, Murphy K, Vierra MA, Genovese MC, Wardlaw AJ, Greenberg HB, Hodge MR, Wu L, Butcher EC. Expression of the chemokine receptors CCR4, CCR5, and CXCR3 by human tissue-infiltrating lymphocytes. Am J Pathol. 2002;160:347-355. [PubMed] [Cited in This Article: ] |