Published online Feb 7, 2014. doi: 10.3748/wjg.v20.i5.1298
Revised: September 10, 2013
Accepted: November 3, 2013
Published online: February 7, 2014
AIM: To characterize the antinociceptive action of the novel melatonin receptor (MT) agonists, Neu-P11 and Neu-P12 in animal models of visceral pain.
METHODS: Visceral pain was induced by intracolonic (ic) application of mustard oil or capsaicin solution or by intraperitoneal (ip) administration of acetic acid. Neu-P11, Neu-P12, or melatonin were given ip or orally and their effects on pain-induced behavioral responses were evaluated. To identify the receptors involved, the non-selective MT1/MT2 receptor antagonist luzindole, the MT2 receptor antagonist 4-P-PDOT, or the μ-opioid receptor antagonist naloxone were injected ip or intracerebroventricularly (icv) prior to the induction of pain.
RESULTS: Orally and ip administered melatonin, Neu-P11, and Neu-P12 reduced pain responses in a dose-dependent manner. Neu-P12 was more effective and displayed longer duration of action compared to melatonin. The antinociceptive effects of Neu-P11 or Neu-P12 were antagonized by ip or icv. administered naloxone. Intracerebroventricularly, but not ip administration of luzindole or 4-P-PDOT blocked the antinociceptive actions of Neu-P11 or Neu-P12.
CONCLUSION: Neu-P12 produced the most potent and long-lasting antinociceptive effect. Further development of Neu-P12 for future treatment of abdominal pain seems promising.
Core tip: In search for new efficient therapies for the treatment of pain in the irritable bowel syndrome, the antinociceptive activity of two novel melatonin receptor agonists, Neu-P11 and Neu-P12, was characterized in a well-established mouse model of visceral pain. Neu-P12 produced a potent and long-lasting antinociceptive effect after intraperitoneal and oral administration. Further development of this novel compound for future treatment of abdominal pain seems promising.
- Citation: Chen C, Fichna J, Laudon M, Storr M. Antinociceptive effects of novel melatonin receptor agonists in mouse models of abdominal pain. World J Gastroenterol 2014; 20(5): 1298-1304
- URL: https://www.wjgnet.com/1007-9327/full/v20/i5/1298.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i5.1298
Melatonin is a hormone synthesized primarily in the pineal gland and in peripheral organs, including the gastrointestinal tract, bone marrow, and blood cells[1-3]. Following synthesis, melatonin is released into the blood stream and acts as an endocrine hormone controlling biological functions with circadian rhythms, like the sleep-wake cycle. It is a lipophilic compound diffusing rapidly through biological membranes and is amongst others involved in the regulation of intestinal reflexes, metabolism, and reproduction[4]. In the pineal gland melatonin is synthesized and secreted in a circadian manner, with secretion being highest during nighttime[5].
Morris and Lutsch[6] showed that darkness and elevated levels of melatonin decrease sensitivity to pain, suggesting that the hormone may play a significant role in the modulation of pain. In the following years, several studies characterized the antinociceptive effect of melatonin in multiple animal models[7]. Melatonin receptors (MT) mediate or modulate antinociceptive effects at spinal and supraspinal levels[8]. More recently, melatonin-induced antinociception has been reported using neuropathic pain models[9].
Recent clinical trials provided evidence for a beneficial role of melatonin in gastrointestinal functional pain[10]. Double-blinded placebo-controlled clinical trials showed that melatonin reduced extra-colonic symptoms and abdominal pain in patients with irritable bowel syndrome (IBS)[11-14].
Although melatonin has been studied for the treatment of many diseases such as cancer, cardiovascular diseases, depression, seasonal affective disorder, circadian rhythm sleep disorders and insomnia, its actions are limited by rapid degradation and short half-life. Recently, two novel melatonin receptor agonists with high affinity at MT receptors and prolonged duration of action, Neu-P11 and Neu-P12, were developed[15-17]. It was shown that Neu-P11 and Neu-P12 display long half-life and oral availability and are thus promising drug candidates, which require further characterization.
In the present study, we evaluated the possible antinociceptive effects of Neu-P11 and Neu-P12 in mouse models of visceral pain and compared their effects to those of melatonin. We also aimed at characterizing the mechanism of action of Neu-P11 and Neu-P12 via identifying the receptors involved.
Male Swiss albino CD1 mice, weighing 25-30 g were obtained from Charles River (Canada). Animals were housed at a constant temperature of 22 °C and kept at a constant photoperiod (12:12-h light-dark cycle) in sawdust-lined plastic cages with free access to standard laboratory chow and tap water. The animal use for these studies was approved by the University of Calgary Animal Care Committee and the experiments were performed in accordance with institutional animal ethics committee guidelines that are in agreement to the guidelines established by the Canadian Council on Animal Care.
Behavioral pain-related responses to intracolonic (ic) administration of mustard oil (MO) and capsaicin solution were determined in the morning as described previously[18-20]. Fifteen minutes after ip injection of Neu-P11, Neu-P12, or melatonin (25 and 50 mg/kg) or 20 min after oral gavage (25, 50 and 100 mg/kg), 50 μL of MO (1% vol/vol dissolved in 70% ethanol) or capsaicin (0.3% w/v in 10% ethanol, 10% Tween 80, 80% saline) were administered into the colon of anesthetized mice using a fine catheter (external diameter 0.61 mm, 4 cm long, Minipack, Portex, Hythe, United Kingdom). Vaseline was applied to the perianal area to avoid the stimulation of somatic areas by contact with MO or capsaicin. After the administration of MO or capsaicin, the animals were placed in individual plastic cages in a quiet environment. Five minutes later, spontaneous pain-related responses: licking of the abdomen, stretching the abdomen, squashing the lower abdomen against the floor, and abdominal retractions were counted for 20 min.
Typically, the number of behaviors in MO- and capsaicin-treated mice was 60-70 and 30-40, respectively. In these conditions even slight changes in the number of behaviors, caused by either anti- or pro-nociceptive action of studied compounds, can be noticed, but simultaneously only statistically significant differences in obtained values reflect pharmacologically relevant action.
The non-selective MT1/MT2 receptor antagonist luzindole (5 mg/kg), the selective MT2 receptor antagonist 4-P-PDOT (4 mg/kg) and the opioid receptor antagonist naloxone (1 mg/kg) were administered ip 15 min prior to Neu-P11, Neu-P12 and melatonin. To study the role of MT and opioid receptors in the central nervous system, luzindole (5 μg/animal), 4-P-PDOT (10 μg/animal) or naloxone (5 μg/animal) were injected icv. Five minutes prior to the ip administration of Neu-P11, Neu-P12 and melatonin. Vehicles only were used in control experiments.
The acetic acid test was performed as described recently[19-21]. Fifteen minutes after ip injection of Neu-P11, Neu-P12, or melatonin (1 and 5 mg/kg), mice received an ip injection of an acetic acid solution (0.5%, vol/vol in 0.9% NaCl). Animals were then placed individually in empty cages and after 5 min abdominal stretchings were counted for 15 min in 5 min intervals. A typical stretch was characterized by an elongation of the body and the development of tension in the abdominal muscles and hind paws. Vehicle was used in control experiments. Typically, the number of behaviors in acetic acid solution-treated mice was 30-40.
Melatonin, luzindole, 4-P-PDOT, naloxone and capsaicin were obtained from Tocris Bioscience (Tocris, Ellisville, Missouri, United States). Allyl isothiocyanate (mustard oil, MO) was purchased from Merck (Darmstadt, Germany). Neu-P11 and Neu-P12 were obtained from Neurim Pharmaceuticals Ltd., Israel. All drugs were dissolved in dimethyl sulfoxide and diluted in 0.9% saline to final concentrations.
PRISM 5.0 (GraphPad Software Inc., La Jolla, CA, United States) was used for statistical and curve-fitting analyses. One-way analysis of variance followed by Student-Newman-Keuls post hoc test was used for analysis of multiple treatment means. P values < 0.05 were considered significant. The data are expressed as mean ± SE.
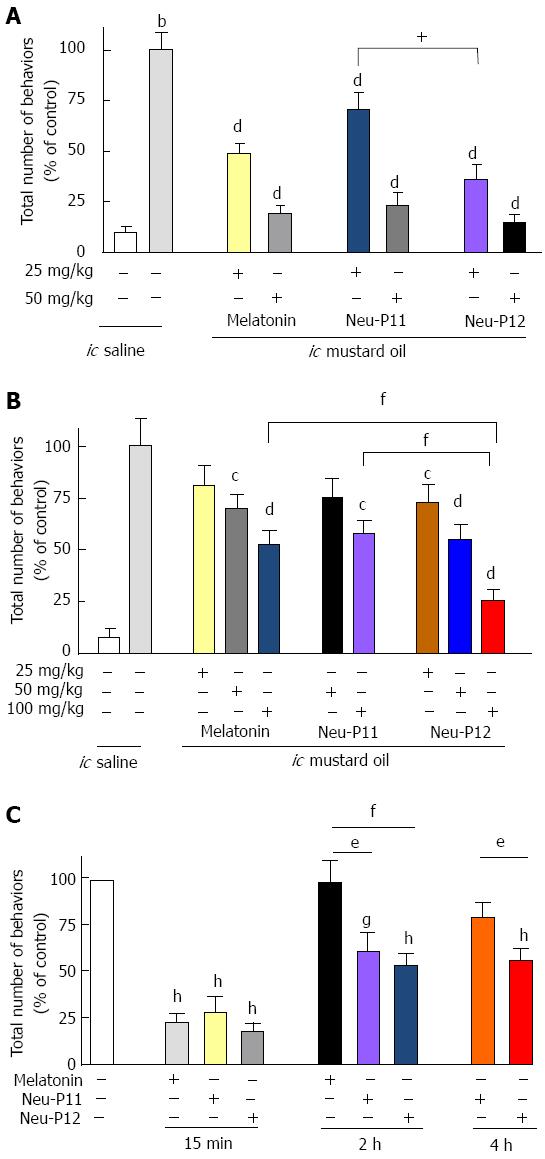
As shown in Figure 1, melatonin, Neu-P11 and Neu-P12 injected ip (Figure 1A) or given orally (Figure 1B) significantly reduced the number of pain-related behaviors in the OM visceral pain sensitivity test compared to control in a dose-depended fashion. Oral Neu-P12 was the most effective compound of all melatonin agonists in decreasing the pain behaviors.
The antinociceptive effect of Neu-P12 (ip 50 mg/kg) was observed up to 4 h after administration, the effect of Neu-P11 (ip 50 mg/kg) up to 2 h. Melatonin (ip 50 mg/kg) produced a short-lasting effect, which was observed at 15 min but disappeared at 2 h (Figure 1C).
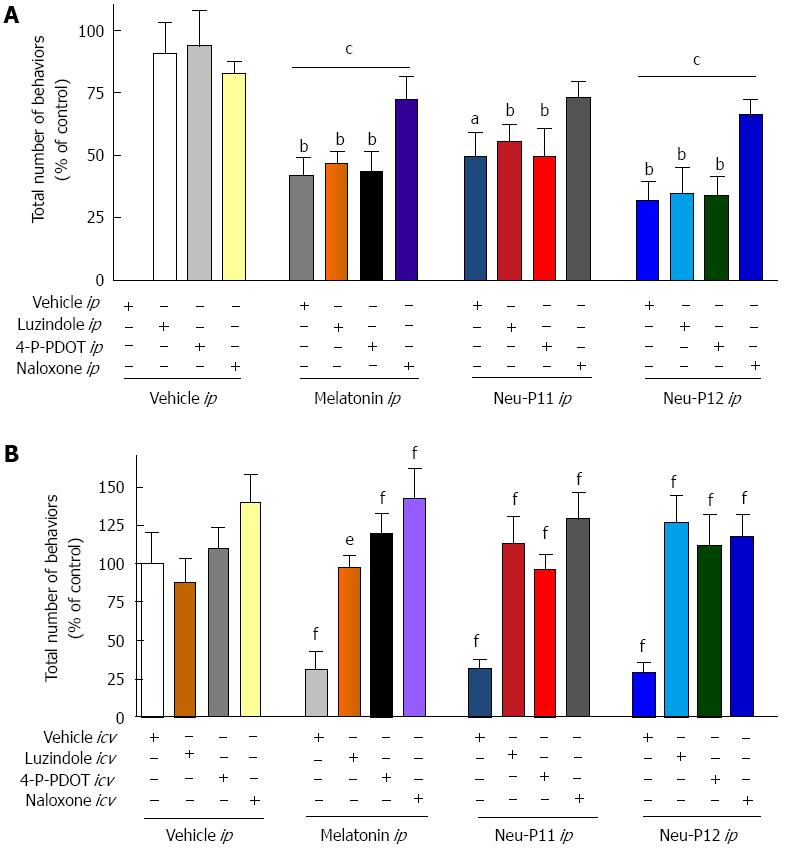
The effects of melatonin, Neu-P11 and Neu-P12 on the visceral pain-related behaviors in the MO sensitivity test were blocked by the ip administration of the opioid receptor antagonist naloxone, but not by ip application of the MT1/2 receptor antagonist luzindole or the MT2 antagonist 4-P-PDOT (Figure 2A). Naloxone and MT receptor antagonists administered directly to the central nervous system all effectively blocked behavioral responses in the visceral pain tests (Figure 2B).
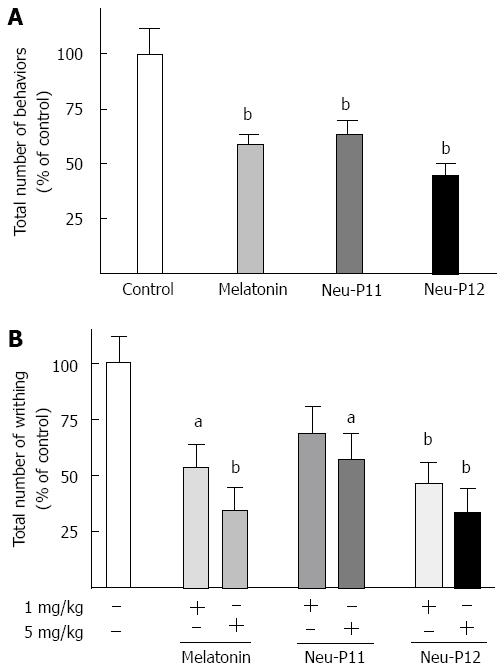
Melatonin, Neu-P11 and Neu-P12 administered ip (25 mg/kg) significantly decreased the number of pain induced behaviors following ic administration of capsaicin (Figure 3A). Of all compounds tested, Neu-P12 displayed the most potent antinociceptive action.
Melatonin, Neu-P11 and Neu-P12 (5 mg/kg, ip) all effectively reduced the total number of visceral pain behaviors induced by the ip injection of acetic acid (Figure 3B). Neu-P12 displayed the most potent antinociceptive effect.
In this study we showed that ip and oral administration of melatonin, Neu-P11 and Neu-P12 produced a potent antinociceptive effect in different animal models of visceral pain of abdominal origin. The analgesic action of all compounds was mediated by central and peripheral opioid and central, but not peripheral, MT receptors.
Melatonin significantly and dose-dependently decreased the total number of pain-related behaviors induced by OM, capsaicin and acetic acid solution in mice. This observation is in line with earlier reports suggesting a role of melatonin and MT receptors in pain signaling. It was shown by others that intrathecal co-administration of low doses of melatonin and morphine attenuated mechanical and thermal hyperalgesia in acute postoperative pain model in rats, suggesting that melatonin acts as a neuromodulator in the spinal cord[22].
In our studies, the two novel non-selective MT1/MT2 melatonergic agonists used, Neu-P11 and Neu-P12, produced a potent antinociceptive effect comparable to that of melatonin and this effect holds true in three different models of visceral pain. The in vivo experiments indicated that Neu-P12 is the most effective drug with the longest duration of action.
We showed that the antinociceptive effects of melatonin, Neu-P11 and Neu-P12 were blocked by the icv administration of a MT2 receptor antagonist 4-P-PDOT, as well as icv and ip injection of naloxone, suggesting a crucial role of central MT2 and opioid receptors, as well peripheral opioid receptors. The involvement of MT2 and opioid receptors in the antinociceptive effects of melatonin was earlier suggested by Ambriz-Tututi and Granados-Soto in models studying other forms of pain, who showed that intrathecal luzindole and 4-P-PDOT and intrathecal or subcutaneous naltrexone blocked the effect of melatonin on tactile allodynia in neuropathic rats[23]. Another study revealed that the antihyperalgesic effect of melatonin on nociceptin-induced hyperalgesia in mice was significantly antagonized by the icv. administration of luzindole or naloxone[24]. Similar results were recently reported by Mickle et al[25] suggesting that melatonin attenuates post-inflammatory hypersensitivity through a supra-spinal process linked to the central opioidergic system.
The involvement of opioid receptors in the antinociceptive effects of melatonin and melatonergic agonists observed in our study is an interesting, and complex phenomenon. Earlier receptor binding experiments confirmed the presence of mu- and delta-, but not kappa-opioid bind sites in bovine pinealocyte membranes[26]. (D-Ala2, N-MePhe4, Gly-ol)-enkephalin and (D-Pen2, D-Pen5)-enkephalin, which are mu- and delta-selective opioid receptor agonists, respectively, enhanced melatonin synthesis in pineal cultures by a naloxone-reversible manner[27,28] and acute administration of morphine induced melatonin release from rat pineal glands[29]. Despite these findings, there is no clear evidence for a melatonin-opioid system interaction or melatonergic ligand binding to opioid receptors. We might suggest a possible involvement of delta-opioid receptors in the antinociceptive activity of melatonin, as it was shown that its antihyperalgesic effect was partially inhibited by the selective delta opioid receptor antagonists, naltrexone and naltrindole, but not by a selective kappa opioid receptor antagonist 5’-guanidinonaltrindole[7]. Another possible explanation is that melatonin may interfere with the neural mechanisms involved in the development of tolerance to a delta-opioid agonist analgesia via its receptor[30]. Recently Wang reported that co-activating delta-opioid receptor and melatonin receptor could induce much longer analgesia by deltorphin-5-methoxytryptamine chimeric opioid peptides[31].
Some reports suggest that the interaction between melatonergic and opioidergic system may be explained at a molecular level. Melatonin may regulate changes in pain threshold by modulating fluctuations in opioid receptor expression and the release of beta-endorphin from pituitary gland[32-34]. It can be also suggested that melatonin, similarly to opioids, may modify K+ and Ca2+ ion channel function[35,36]. Furthermore, melatonin and opioid receptors are coupled to Gi proteins and may share the Gi-mediated intracellular pathways[27,37].
Rapid elimination of melatonin from the circulation is a major obstacle in its potential use in clinical treatment. The reported half-life of melatonin in blood is approximately 20-30 min[38-40]. However, administration of higher doses of melatonin induces a nocturnal decrease in body temperature and may desensitizes its receptors[41,42]. A possible solution to overcome limited melatonin bioavailability is by developing formulations with prolonged-release or the design of synthetic melatonergic agonists with prolonged action. A controlled-release melatonin formulation (Circadin®) has recently been approved in the European Union and other countries for the treatment of insomnia in patients aged 55 years and older[43].
Neu-P11, one of the novel melatonergic agonists used in our study was shown to be a melatonin agonist, serotonin 5-HT-1A and 5-HT-1D agonist and serotonin 5-HT2B antagonist[44]. Sleep promoting, anti-diabetic, antihypertensive, analgesic, anti-neurodegenerative, anxiolytic and antidepressant effects of Neu-P11 have been demonstrated in a series of relevant animal models[15,16]. The pharmacological actions of Neu-P12, which is a close derivative of Neu-P11, have not been fully studied yet. In the present study we report that both Neu-P12 and Neu-P11 display potent antinociceptive effects and have longer duration of action compared to melatonin and might thus become potential analgesics for future use in clinical treatment of visceral pain of abdominal origin. From our experience Neu-P12 has the longest duration of action and may thus be the most promising compound fur further development. It is finally important to mention that the effects are seen following ip or oral application as especially the oral activity is a good pre-requisite for future human use as oral application seems the most favorable delivery pathway.
Since melatonin receptors are also involved in the regulation of gastrointestinal motility, drugs targeting these receptors might be useful in the treatment of motility disorders where increased motility is a problem as in diarrhea or IBS. Thus, the effects of melatonin and its agonists on gastrointestinal motility worth additional research.
In summary melatonin, and its agonists Neu-P11, and Neu-P12 decreased visceral abdominal pain-related behaviors in mice. The antinociceptive action of the melatonergic agonists was mediated by central and peripheral opioid receptors, as well as central MT2 receptors. All analgesic effects were seen following oral application, an attribute that allows oral treatment if further developed for clinical use.
Neu-P12, which displayed the most significant analgesic effect and the longest duration of antinociceptive action and Neu-P11 have the potential to become future drugs for the treatment of pain in abdominal diseases including, but not limited to, IBS and ulcerative colitis.
Melatonin was reported to attenuate pain in animals and in patients with irritable bowel syndrome. However, due to low metabolic stability, it is rapidly degraded in physiological conditions.
The aim was to characterize the antinociceptive action of the novel melatonin receptor agonists, Neu-P11 and Neu-P12 in animal models of visceral pain and to compare their effects to that of melatonin.
Orally and intraperitoneally administered melatonin, Neu-P11, and Neu-P12 reduced pain responses in a dose-dependent manner. Neu-P12 was more effective and displayed longer duration of action compared to melatonin. The antinociceptive effects of Neu-P11 or Neu-P12 were antagonized naloxone. Centrally, but not intraperitoneally administration of melatonin receptor antagonists, luzindole or 4-P-PDOT blocked the antinociceptive actions of Neu-P11 or Neu-P12.
Neu-P12 produced a potent and long-lasting antinociceptive effect after intraperitoneal and oral administration. Further development of this novel compound for future treatment of abdominal pain seems promising.
Metabolic stability, resistance to degradation and attenuation of pharmacological action by various physiological factors, mainly enzymes. Irritable bowel syndrome, gastrointestinal disease characterized by motility disorders and abdominal pain.
The authors used the percentage of control (numbers of pain-related behaviors) instead of true numbers. An interesting study and the discussion is well-done.
P- Reviewers: Lee HC, Trecca A S- Editor: Gou SX L- Editor: A E- Editor: Wu HL
| 1. | Maestroni GJ. The immunotherapeutic potential of melatonin. Expert Opin Investig Drugs. 2001;10:467-476. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 165] [Cited by in F6Publishing: 176] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 2. | Maldonado MD, Mora-Santos M, Naji L, Carrascosa-Salmoral MP, Naranjo MC, Calvo JR. Evidence of melatonin synthesis and release by mast cells. Possible modulatory role on inflammation. Pharmacol Res. 2010;62:282-287. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 72] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 3. | Conti A, Conconi S, Hertens E, Skwarlo-Sonta K, Markowska M, Maestroni JM. Evidence for melatonin synthesis in mouse and human bone marrow cells. J Pineal Res. 2000;28:193-202. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 239] [Cited by in F6Publishing: 236] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 4. | Messner M, Huether G, Lorf T, Ramadori G, Schwörer H. Presence of melatonin in the human hepatobiliary-gastrointestinal tract. Life Sci. 2001;69:543-551. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 79] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 5. | Brzezinski A. Melatonin in humans. N Engl J Med. 1997;336:186-195. [PubMed] [Cited in This Article: ] |
| 6. | Morris RW, Lutsch EF. Daily susceptibility rhythm to morphine analgesia. J Pharm Sci. 1969;58:374-376. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 33] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | Arreola-Espino R, Urquiza-Marín H, Ambriz-Tututi M, Araiza-Saldaña CI, Caram-Salas NL, Rocha-González HI, Mixcoatl-Zecuatl T, Granados-Soto V. Melatonin reduces formalin-induced nociception and tactile allodynia in diabetic rats. Eur J Pharmacol. 2007;577:203-210. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 54] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 8. | Shin DJ, Jeong CW, Lee SH, Yoon MH. Receptors involved in the antinociception of intrathecal melatonin in formalin test of rats. Neurosci Lett. 2011;494:207-210. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Ambriz-Tututi M, Rocha-González HI, Cruz SL, Granados-Soto V. Melatonin: a hormone that modulates pain. Life Sci. 2009;84:489-498. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 105] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 10. | Mozaffari S, Rahimi R, Abdollahi M. Implications of melatonin therapy in irritable bowel syndrome: a systematic review. Curr Pharm Des. 2010;16:3646-3655. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 11. | Camilleri M, Andresen V. Current and novel therapeutic options for irritable bowel syndrome management. Dig Liver Dis. 2009;41:854-862. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 12. | Song GH, Leng PH, Gwee KA, Moochhala SM, Ho KY. Melatonin improves abdominal pain in irritable bowel syndrome patients who have sleep disturbances: a randomised, double blind, placebo controlled study. Gut. 2005;54:1402-1407. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 140] [Cited by in F6Publishing: 122] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 13. | Lu WZ, Gwee KA, Moochhalla S, Ho KY. Melatonin improves bowel symptoms in female patients with irritable bowel syndrome: a double-blind placebo-controlled study. Aliment Pharmacol Ther. 2005;22:927-934. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 93] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 14. | Saha L, Malhotra S, Rana S, Bhasin D, Pandhi P. A preliminary study of melatonin in irritable bowel syndrome. J Clin Gastroenterol. 2007;41:29-32. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 46] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 15. | She M, Deng X, Guo Z, Laudon M, Hu Z, Liao D, Hu X, Luo Y, Shen Q, Su Z. NEU-P11, a novel melatonin agonist, inhibits weight gain and improves insulin sensitivity in high-fat/high-sucrose-fed rats. Pharmacol Res. 2009;59:248-253. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 71] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 16. | Tian SW, Laudon M, Han L, Gao J, Huang FL, Yang YF, Deng HF. Antidepressant- and anxiolytic effects of the novel melatonin agonist Neu-P11 in rodent models. Acta Pharmacol Sin. 2010;31:775-783. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 17. | Paulis L, Simko F, Laudon M. Cardiovascular effects of melatonin receptor agonists. Expert Opin Investig Drugs. 2012;21:1661-1678. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 18. | Fichna J, Sibaev A, Sałaga M, Sobczak M, Storr M. The cannabinoid-1 receptor inverse agonist taranabant reduces abdominal pain and increases intestinal transit in mice. Neurogastroenterol Motil. 2013;25:e550-e559. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 19. | Fichna J, Lapointe T, Chapman K, Janecka A, Vergnolle N, Altier C, Storr MA. New neostigmine-based behavioral mouse model of abdominal pain. Pharmacol Rep. 2012;64:1146-1154. [PubMed] [Cited in This Article: ] |
| 20. | Fichna J, Dicay M, Lewellyn K, Janecka A, Zjawiony JK, MacNaughton WK, Storr MA. Salvinorin A has antiinflammatory and antinociceptive effects in experimental models of colitis in mice mediated by KOR and CB1 receptors. Inflamm Bowel Dis. 2012;18:1137-1145. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 21. | Gach K, do-Rego JC, Fichna J, Storr M, Delbro D, Toth G, Janecka A. Synthesis and biological evaluation of novel peripherally active morphiceptin analogs. Peptides. 2010;31:1617-1624. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 22. | Zahn PK, Lansmann T, Berger E, Speckmann EJ, Musshoff U. Gene expression and functional characterization of melatonin receptors in the spinal cord of the rat: implications for pain modulation. J Pineal Res. 2003;35:24-31. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 63] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 23. | Ambriz-Tututi M, Granados-Soto V. Oral and spinal melatonin reduces tactile allodynia in rats via activation of MT2 and opioid receptors. Pain. 2007;132:273-280. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 68] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 24. | Wang T, Li SR, Dai X, Peng YL, Chen Q, Wang R. Effects of melatonin on orphanin FQ/nociceptin-induced hyperalgesia in mice. Brain Res. 2006;1085:43-48. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 27] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 25. | Mickle A, Sood M, Zhang Z, Shahmohammadi G, Sengupta JN, Miranda A. Antinociceptive effects of melatonin in a rat model of post-inflammatory visceral hyperalgesia: a centrally mediated process. Pain. 2010;149:555-564. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 26. | Govitrapong P, Sawlom S, Chetsawang B, Sangchot P, Ebadi M. The bovine pineal gland contains delta and mu but not kappa or ORL1 opioid receptor subtypes. Proc West Pharmacol Soc. 2002;45:32-35. [PubMed] [Cited in This Article: ] |
| 27. | Chuchuen U, Ebadi M, Govitrapong P. The stimulatory effect of mu- and delta-opioid receptors on bovine pinealocyte melatonin synthesis. J Pineal Res. 2004;37:223-229. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 28. | Govitrapong P, Sawlom S, Ebadi M. The presence of delta and mu-, but not kappa or ORL(1) receptors in bovine pinealocytes. Brain Res. 2002;951:23-30. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 29. | Esposti D, Esposti G, Lissoni P, Parravicini L, Fraschini F. Action of morphine on melatonin release in the rat. J Pineal Res. 1988;5:35-39. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 43] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 30. | Dai X, Cui SG, Li SR, Chen Q, Wang R. Melatonin attenuates the development of antinociceptive tolerance to delta-, but not to mu-opioid receptor agonist in mice. Behav Brain Res. 2007;182:21-27. [PubMed] [Cited in This Article: ] |
| 31. | Wang J, Wang L, Li M, Jin Q, Dong S. Preliminary analgesic properties of deltorphin-5-methoxytryptamine chimeric opioid peptides. Peptides. 2011;32:1055-1059. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 32. | Lissoni P, Esposti D, Esposti G, Mauri R, Resentini M, Morabito F, Fumagalli P, Santagostino A, Delitala G, Fraschini F. A clinical study on the relationship between the pineal gland and the opioid system. J Neural Transm. 1986;65:63-73. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 77] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 33. | Barrett T, Kent S, Voudouris N. Does melatonin modulate beta-endorphin, corticosterone, and pain threshold? Life Sci. 2000;66:467-476. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 49] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 34. | Shavali S, Ho B, Govitrapong P, Sawlom S, Ajjimaporn A, Klongpanichapak S, Ebadi M. Melatonin exerts its analgesic actions not by binding to opioid receptor subtypes but by increasing the release of beta-endorphin an endogenous opioid. Brain Res Bull. 2005;64:471-479. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 79] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 35. | van den Top M, Buijs RM, Ruijter JM, Delagrange P, Spanswick D, Hermes ML. Melatonin generates an outward potassium current in rat suprachiasmatic nucleus neurones in vitro independent of their circadian rhythm. Neuroscience. 2001;107:99-108. [PubMed] [Cited in This Article: ] |
| 36. | Ayar A, Martin DJ, Ozcan M, Kelestimur H. Melatonin inhibits high voltage activated calcium currents in cultured rat dorsal root ganglion neurones. Neurosci Lett. 2001;313:73-77. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 41] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 37. | Ho MK, Yung LY, Chan JS, Chan JH, Wong CS, Wong YH. Galpha(14) links a variety of G(i)- and G(s)-coupled receptors to the stimulation of phospholipase C. Br J Pharmacol. 2001;132:1431-1440. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 50] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 38. | Pandi-Perumal SR, Srinivasan V, Poeggeler B, Hardeland R, Cardinali DP. Drug Insight: the use of melatonergic agonists for the treatment of insomnia-focus on ramelteon. Nat Clin Pract Neurol. 2007;3:221-228. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 49] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 39. | Srinivasan V, Pandi-Perumal SR, Trahkt I, Spence DW, Poeggeler B, Hardeland R, Cardinali DP. Melatonin and melatonergic drugs on sleep: possible mechanisms of action. Int J Neurosci. 2009;119:821-846. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 100] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 40. | Claustrat B, Brun J, Chazot G. The basic physiology and pathophysiology of melatonin. Sleep Med Rev. 2005;9:11-24. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 549] [Cited by in F6Publishing: 527] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 41. | Wurtman R. Ramelteon: a novel treatment for the treatment of insomnia. Expert Rev Neurother. 2006;6:957-964. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 23] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 42. | Gerdin MJ, Masana MI, Rivera-Bermúdez MA, Hudson RL, Earnest DJ, Gillette MU, Dubocovich ML. Melatonin desensitizes endogenous MT2 melatonin receptors in the rat suprachiasmatic nucleus: relevance for defining the periods of sensitivity of the mammalian circadian clock to melatonin. FASEB J. 2004;18:1646-1656. [PubMed] [Cited in This Article: ] |
| 43. | Wade AG, Ford I, Crawford G, McConnachie A, Nir T, Laudon M, Zisapel N. Nightly treatment of primary insomnia with prolonged release melatonin for 6 months: a randomized placebo controlled trial on age and endogenous melatonin as predictors of efficacy and safety. BMC Med. 2010;8:51. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 105] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 44. | Hardeland R. Investigational melatonin receptor agonists. Expert Opin Investig Drugs. 2010;19:747-764. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |