Published online Dec 28, 2014. doi: 10.3748/wjg.v20.i48.18354
Revised: June 8, 2014
Accepted: July 11, 2014
Published online: December 28, 2014
AIM: To investigate T helper 17/regulatory T cell alterations in early severe hepatitis B and the effect of glucocorticoids.
METHODS: The study included 20 patients in the early stage of severe hepatitis B (SHB) and 11 healthy controls. All patients had elevated T helper 17 (Th17) levels, decreased regulatory T (Treg) cell levels, and significant Th17/Treg ratios.
RESULTS: After glucocorticoid treatment, 16 patients showed improvement with significant decreases in Th17 levels, increases in Treg, and rebalanced Th17/Treg ratios. The four patients who showed no improvement had increases in both Th17 and Treg levels and an even higher Th17/Treg ratio than before.
CONCLUSION: Glucocorticoid treatment can rectify Th17/Treg dysregulation in patients with SHB.
Core tip: Severe hepatitis B (SHB) is a fast progressing form of hepatitis infection with a low eradication rate. Patients with SHB have dysregulated ratios of T helper cells, observed as elevated levels of T helper 17 cells and reduced regulatory T cells. This study demonstrates that these levels can be rebalanced with glucocorticoid treatment. However, failure to rectify the imbalance likely will results in the progression of SHB and eventually cause death.
- Citation: Lu ZH, Huang XP, Sun W, Zhu YL, Cui JJ, Chen W, Huang LH, Kuai SG, Du HJ, Ju ZX, Gan JH. T helper cell dysregulation with hepatitis B and rebalance with glucocorticoids. World J Gastroenterol 2014; 20(48): 18354-18359
- URL: https://www.wjgnet.com/1007-9327/full/v20/i48/18354.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i48.18354
Hepatitis B is an infectious disease caused by the hepatitis B virus (HBV) that affects global health and becomes an important medical problem. Severe hepatitis B (SHB), also known as HBV-related liver failure, is the most detrimental type of Hepatitis B due to its fast progression and low eradication rate, with a mortality rate that can exceed 60%. Patients in the early stage of SHB are commonly seen in clinics. If SHB is not correctly treated, it can easily progress to a more severe stage with a higher death rate[1,2]. Glucocorticoids are a type of steroid hormone, which can be secreted by the zona fasciculata of the adrenal gland cortex. In SHB patients, glucocorticoids can suppress strong immune and inflammatory responses, decrease capillary blood vessel permeability, prevent the formation of bile bolt, rapidly diminish jaundice, promote appetite, improve mental state, shorten the disease course, relieve pain, decrease medical costs, and reduce the overall death rate. Therefore, glucocorticoids have become a focus in the current research concerning SHB, especially in the treatment of patients in the early stage.
T helper 17 (Th17) cells, a subtype of CD4+ T cells that have been identified in recent years, differ from traditional T helper cells such as Th1 and Th2 subtypes, as their secreted cytokines [mainly interleukin (IL)-17] can promote the mobilization, recruitment, and activation of neutrophils and mediate pro-inflammatory responses[3]. Th17 cells play an important role in the pathology of infectious diseases, autoimmune diseases, and cancers. Regulatory T (Treg) cells were confirmed as a subtype of T cells with in vivo immune-suppressive function in 1995. It has been reported that Th17 and Treg cells antagonize each other during differentiation. Under normal circumstances, a balance between the two subtypes maintains immune system stability. However, once the balance is broken, a series of immunopathological responses occurs[4,5].
Treatment for patients in the early stage of SHB should be standardized to prevent progression to the mid- and late stages of SHB[6,7]. There are currently very few reports concerning Th17/Treg alterations during SHB initiation, progression, and outcome. This study observed Th17/Treg in the sera of patients in the early stage of SHB before and after glucocorticoid treatment to provide more immunological evidence for the immune regulation and treatment of SHB. Therefore, treatment for patients in the early stage of SHB can be standardized to prevent progression to the mid- and late stages of SHB[6,7].
Twenty hospitalized patients in the early stage of SHB from The First Affiliated Hospital of Soochow University and Wuxi No. 5 People’s Hospital were included in this study. A total of 11 healthy people were recruited as controls. The patients met the following inclusion criteria: extreme fatigue with severe gastrointestinal symptoms such as anorexia, vomiting, and bloating; elevated jaundice level [total bilirubin (TBil): 51-171 μmol/L, and daily increases > 17 μmol/L]; bleeding tendency [plasma thromboplastin antecedent (PTA): 40%-50% or international normalized ratio (INR): 1.5-1.6]; and positive markers for HBV. Fasting peripheral venous blood (2 mL) was drawn from patients and healthy controls in the morning. The collected blood was put into test tubes with sodium heparin inverted five times to ensure thorough mixing for the detection of specific immune cells (before, during, and after treatment) by intracellular cytokine identification using in vitro stimulation and fixation/permeabilization followed by flow cytometry. The research plan was approved by the ethics committees of both hospitals. Written consent was obtained from all participants.
For these studies, a Beckman Coulter XL AT09010 (Beckman Coulter Inc., Pasadena, CA, United States) was used. The monoclonal anti-CD279(PD-1)/IL-17A antibody conjugated with phycoerythrin (PE), and Leukocyte Activation Cocktail with BD GolgiPlug™ were purchased from Becton Dickenson and Co. (Franklin Lakes, NJ, United States). Fluorescein isothiocyanate (FITC)-conjugated anti-CD4, PE-conjugated anti-CD127, ECD-conjugated anti-CD3, and PC5-conjugated anti-CD25 and anti-CD8 monoclonal antibodies and IntraPrep permeabilization reagents (including sheath fluid and wash buffer) were obtained from Beckman Coulter Inc.
The Th17 detection protocol is as follows: add 30 μL of stimulant (1:50 dilution of BD Pharmingen™ into 1640 Media with 10% heat-inactivated fetal bovine serum) to 50 μL of whole blood. Vortex and then incubate the sample for 4-6 h in the incubator at 37 °C with 5% CO2. Add 5 μL of anti-CD3-ECD and anti-CD8-PC5. Vortex and keep the sample away from light for 15 min. Add 100 μL of IntraPrep Permeabilization Reagent 1. Vortex and keep the sample away from light for 10 min. Add 1 mL of phosphate buffered saline (PBS). Vortex and centrifuge the mixture at 1500 rpm for 5 min. Discard the supernatant. Add 100 μL of IntraPrep Permeabilization Reagent 2. Vortex and incubate the sample for 15 min in an incubator at 37 °C with 5% CO2. Add 5 μL of anti-IL-17A-PE. Vortex and keep the sample away from light for 15 min. Add 2 mL of PBS. Vortex and then centrifuge at 1500 rpm for 5 min. Discard the supernatant and repeat twice. Re-suspend the sample in 500 μL of PBS and load the sample in a flow cytometry column. Set the gate using CD3-ECD and analyze 3000-5000 cells.
The Treg detection protocol is as follows: add 5 μL of anti-CD4-FITC, anti-CD25-PC5, and CD127-PE to 50 μL of whole blood. Vortex and keep the sample away from light for 15 min. Add 600 μL of homemade red cell lysis buffer A. Vortex until the lysate becomes clear and then add 265 μL of buffer B. Vortex and centrifuge at 1500 rpm for 5 min. Discard the supernatant. Add 2 mL of PBS. Vortex and centrifuge the mixture at 1500 rpm for 5 min. Discard the supernatant and repeat twice. Re-suspend the sample in 500 μL of PBS and load the sample in a flow cytometry column. Analyze 3000-5000 cells[8].
Patients were administered methylprednisolone (40-60 mg/d) in addition to the routine treatment involving liver protection, jaundice reversal, and anti-virus therapy. The dosage was reduced when gastrointestinal symptoms were alleviated or TBil was reduced by 25%. The dosage was initially reduced 5-10 mg/7 d until it reached 20 mg/d, and then reduced 5 mg/7 d until it reached 10 mg/d, and reduced 2.5 mg/7 d until it reached 2.5 mg/d. The final dosage was then maintained for one month. Fasting blood was collected in the morning before treatment was initiated, and again at 4, 8, and 15 d after treatment for flow cytometry analysis. Data from samples collected at 15 d are presented.
The primary endpoint was the survival rate at weeks 4, 12, 24, and 48. Secondary endpoints included improvement in clinical symptoms and signs such as fatigue, anorexia, bloating, oliguria, bleeding tendency, infection, and ascites and reflected by decreases in TBil, recovery of PTA (INR) values, and increases in serum albumin in blood biochemical tests.
Clinical standards for cure include: (1) disappearance of symptoms such as fatigue, anorexia, bloating, oliguria, bleeding tendency, and hepatic encephalopathy; (2) subsiding jaundice and the liver returning to its normal size; (3) recovery of liver function markers; and (4) recovery of PTA values. Clinical standards for improvement include: (1) significant improvement in symptoms such as fatigue, anorexia, bloating, and bleeding tendency; (2) significant improvement in jaundice and ascites; and (3) significantly improved liver function (TBil < 51 μmol/L). The clinical standard for uncured disease was deterioration leading to death despite active rescue. Of the 20 patients included here, 16 were cured or showed improvement and were labeled as improved after treatment, while the other four died due to disease deterioration and were labeled as uncured after treatment.
All data were processed by SPSS 17.0.1 (SPSS, Chicago, IL, United States). Comparisons were made with Students t-tests: (1) between controls and all patients before treatment; (2) in the improved group before and after treatment; (3) in the uncured group before and after treatment; (4) between the improved and uncured groups before treatment; (5) between the improved and uncured groups after treatment; (6) between controls and the improved groups after treatment; and (7) between controls and the uncured group after treatment. Values of P < 0.05 were considered to be statistically significant.
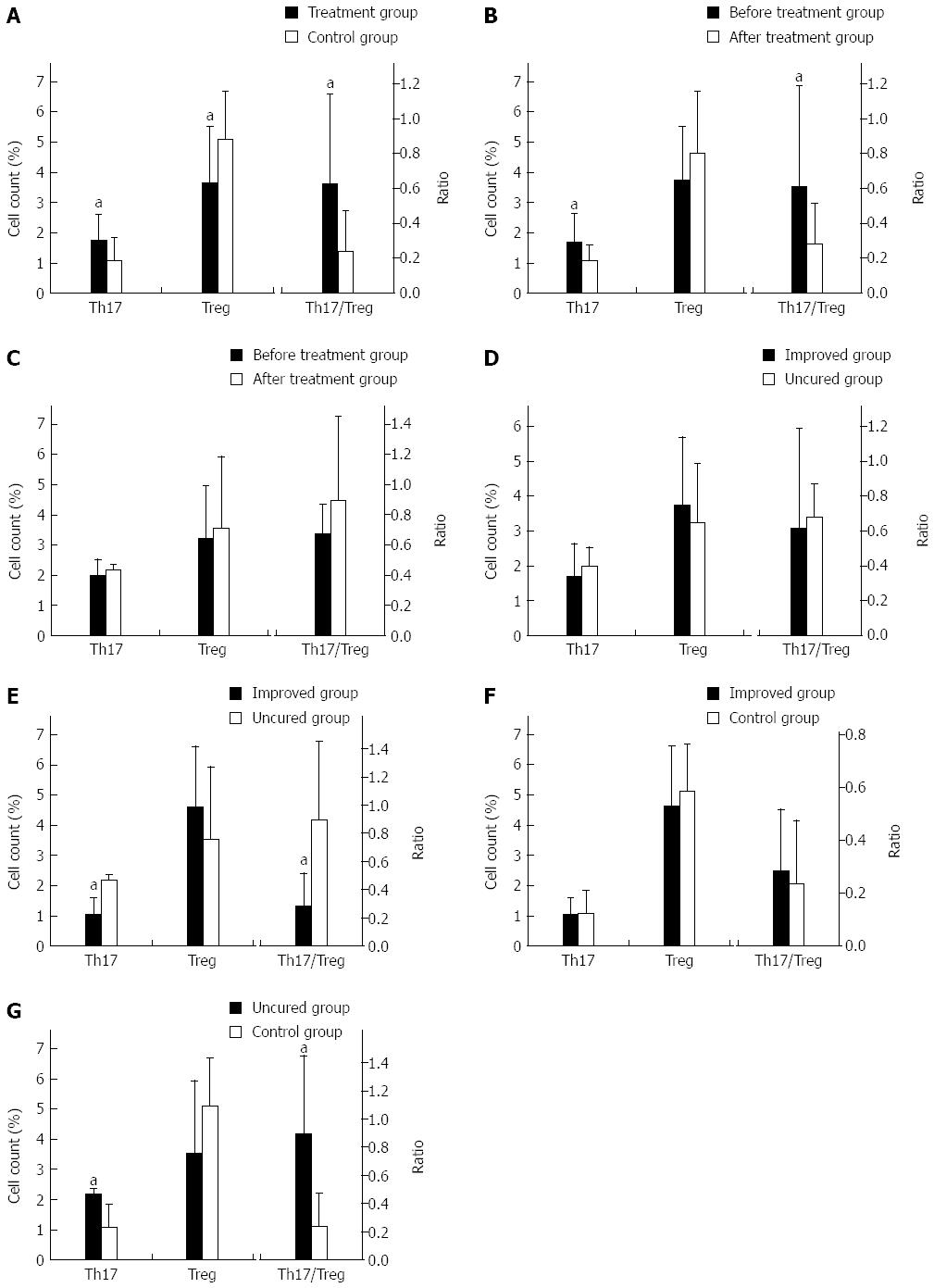
Comparison of all patients (n = 20) and controls (n = 11) before treatment showed that patients in the early stage of SHB had significantly higher levels of Th17 (P = 0.042), lower levels of Treg (P = 0.037), and elevated Th17/Treg ratios (P = 0.026) compared to controls (Figure 1A).
The patients in the improved group (n = 16) showed significant decreases in Th17 levels (P = 0.036) and Th17/Treg ratios (P = 0.037) after glucocorticoid treatment (Figure 1B). However, the increase in Treg levels was not statistically significant.
The patients in uncured group (n = 4) showed elevated Th17/Treg ratios after treatment (Figure 1C). Similarly, Th17 and Treg levels were slightly increased but not statistically significant.
Comparison between the improved (n = 16) and uncured (n = 4) groups before treatment showed that there were no significant differences in Th17 or Treg levels or Th17/Treg ratios (Figure 1D).
Compared with the uncured group (n = 4), patients in the improved group (n = 16) showed significant decreases in Th17 levels (P = 0.001) and Th17/Treg ratios (P = 0.003), but not Treg levels after treatment (Figure 1E).
There were no significant differences in Th17 or Treg levels, or their ratio, between controls (n = 11) and the improved group (n = 16) after treatment (Figure 1F).
Following glucocorticoid treatment, patients in the uncured group (n = 4) had significantly higher Th17 levels (P = 0.017) and Th17/Treg ratios (P = 0.006) compared to healthy controls (n = 11) (Figure 1G). However, Treg levels were not significantly different between the groups.
Patients with SHB suffer immensely. The fast progression, poorly understood pathological mechanisms, and lack of effective medications are fundamental reasons for its low cure rate and high death rate[9,10]. Ye et al[11] have identified four stages within the development and progression of SHB: early rising, rising, plateau, and recovery stages. During early rising stage, the body mainly suffers from immune injury. In the early phase of the rising stage, the body primarily bears the burden of immune and hypoxic-ischemic injury. During the mid- to late-phase of the rising stage, endotoxemia begins to contribute to the attack on the body. When it comes to the mid/late phase of the plateau and early phase of the recovery stage, the body is under an immunosuppression state and bears the pressure of endotoxemia[12,13]. Therefore, early treatment of SHB is critical. Because of the strong immune responses during the early rising stage and rising stage, glucocorticoids are currently the best treatment option.
Th17 and Treg cells are two recently discovered subtypes of CD4+ T cells that are distinct from Th1 and Th2 cells and have independent differentiation and regulatory mechanisms. Th17 cells mainly secret cytokines such as IL-17[14,15]. Treg cells are a subtype of T lymphocytes that express CD4, CD25, and Foxp3 and play a major role in immune suppression. Recent studies have revealed that dysregulation of the Th17/Treg ratio is closely associated with the development and treatment of various major human diseases such as autoimmune disease, infectious disease, allergies, malignant tumors, and transplant rejection. Tuncer et al[16] Park et al[17] and Barbi et al[18] were the first to hypothesize that IL-17 is a new marker for severe acute liver damage.
Recent research has revealed that Th17/Treg ratios are dysregulated in the peripheral blood and liver tissues of patients with chronic hepatitis B, cirrhosis, and liver cancers compared to healthy controls[19,20]. However, there are few reports concerning the alterations of Th17/Treg ratios during SHB initiation, progression, and outcome. Glucocorticoids can suppress Th17 cell differentiation and proliferation and reduce IL-17 production. Meanwhile, glucocorticoids can upregulate the expression of Foxp3, a key transcription factor in Treg cell development, at the mRNA and protein levels, and activate STAT5 pathways to increase the number of Treg cells[21,22]. Moreover, glucocorticoids are able to promote the repair of damaged Treg cells, allowing for recovery of their suppressive functions[23,24].
We recently discovered Th17/Treg dysregulation at the early stage of SHB regardless of disease subtype (acute, subacute, acute on chronic, or chronic). First, we found that before glucocorticoid treatment, Th17 elevations and Treg decreases were present in all patients in the early stage of SHB. The severe imbalance of Th17/Treg in these patients was higher than was found in the healthy population. After glucocorticoid treatment, 16 of 20 patients showed improvement, demonstrating significant Th17 decreases and slight Treg increases that were not significantly different from before treatment. The Th17/Treg dysregulation was corrected after treatment and the ratio did not differ from that of healthy people. The four uncured cases showed Th17 and Treg upregulation and an even higher Th17/Treg ratio, which was statistically significant compared to normal controls. Patients in the improved group showed significant Th17 and Th17/Treg decreases and Treg increases after treatment. Therefore, Th17/Treg dysregulation (or Th17 upregulation and Treg downregulation) is at least partially involved in the pathological mechanisms of SHB. If glucocorticoid treatment results in Th17 decreases and Treg increases, i.e., Th17/Treg rebalance, then it is very likely that the patient’s condition can be cured. If rebalance of Th17/Treg fails to be established after glucocorticoid treatment, it is more likely that SHB will progress and eventually lead to death. In this study, we chose 15 d after glucocorticoid treatment as the observation point because the glucocorticoids would have taken effect by that time and a longer observation period would probably delay treatment. Therefore, our study provides some insight in the selection of subjects who require liver transplantation. Patients in the early stage of SHB in whom the Th17/Treg balance is not re-established after glucocorticoid treatment can be considered good candidates for liver transplantation[25,26].
We greatly appreciate the contributions of all participants in this study.
Severe hepatitis B (SHB), also known as hepatitis B virus-related liver failure, is the most serious type of hepatitis B, as it features a fast progression and a low eradication rate. Patients in the early stage of SHB are commonly seen in clinics. If SHB is not correctly treated, it can easily progress to a more severe stage with a higher risk of death.
Glucocorticoids have become the focus of current research for SHB, particularly for the treatment of patients in the early stage. There are currently very few reports concerning T helper 17/regulatory T cell (Th17/Treg) alterations during SHB initiation, progression, and outcome.
Th17/Treg dysregulation was present in all patients in the early stage of SHB. If glucocorticoid treatment results in decreased Th17 levels, increased Treg levels, and, therefore, rebalanced Th17/Treg ratios, then it is very likely that the condition can be eradicated. If Th17/Treg rebalance does not occur after glucocorticoid treatment, SHB will likely progress and eventually cause death.
This study provides some insight in the selection of subjects who require liver transplantation. Patients in the early stage of SHB in whom the Th17/Treg balance is not re-established after glucocorticoid treatment can be considered good candidates for liver transplantation.
This is a good study in which authors analyze Th17/Treg dysregulation in the early stage of SHB and rebalance after glucocorticoid treatment. The results are interesting and suggest that Th17/Treg is a potential marker for SHB outcome, as well as a possible indicator for liver transplantation candidates.
P- Reviewer: Aghakhani A, Shimizu Y, Watanabe T S- Editor: Nan J L- Editor: AmEditor E- Editor: Liu XM
| 1. | Lee WM, Sorrell MF. Developing a World view toward acute liver failure. Hepatology. 1996;24:270-271. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 2. | Lee KC, Palacios Jimenez C, Alibhai H, Chang YM, Leckie PJ, Baker LA, Stanzani G, L Priestnall S, Mookerjee RP, Jalan R. A reproducible, clinically relevant, intensively managed, pig model of acute liver failure for testing of therapies aimed to prolong survival. Liver Int. 2013;33:544-551. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 3. | Moon YM, Lee J, Lee SY, Her YM, Ryu JG, Kim EK, Son HJ, Kwok SK, Ju JH, Yang CW. Gene associated with retinoid-interferon-induced mortality 19 attenuates murine autoimmune arthritis by regulation of th17 and treg cells. Arthritis Rheumatol. 2014;66:569-578. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 4. | Dong X, Gong Y, Zeng H, Hao Y, Wang X, Hou J, Wang J, Li J, Zhu Y, Liu H. Imbalance between circulating CD4+ regulatory T and conventional T lymphocytes in patients with HBV-related acute-on-chronic liver failure. Liver Int. 2013;33:1517-1526. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 5. | Singh A, Vajpayee M, Ali SA, Chauhan NK. Cellular interplay among Th17, Th1, and Treg cells in HIV-1 subtype “C” infection. J Med Virol. 2014;86:372-384. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 6. | O’Grady J. Bioartificial liver in acute liver failure: impostor or simply misunderstood? Hepatology. 2005;41:383-385. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | Hughes RD, Nagaki M, Keane H, Sheron N, Williams R. Artificial liver support in acute liver failure: a review of studies at King’s. Artif Organs. 1992;16:167-170. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Zhang GL, Xie DY, Lin BL, Xie C, Ye YN, Peng L, Zhang SQ, Zhang YF, Lai Q, Zhu JY. Imbalance of interleukin-17-producing CD4 T cells/regulatory T cells axis occurs in remission stage of patients with hepatitis B virus-related acute-on-chronic liver failure. J Gastroenterol Hepatol. 2013;28:513-521. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 9. | Joseph B. Liver failure. Liver failure. Surgical critical care and emergency surgery: clinical questions and answers. Chichester UK: John Wiley 2012; 165-171. [Cited in This Article: ] |
| 10. | Knisely AS. Patent ductus venosus and acute liver failure in the neonate: consider neonatal hemochromatosis with liver scarring. Liver Transpl. 2014;20:124. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Ye YN, Gao ZL. [Three shock hypotheses that may induce liver failure]. Zhonghua Ganzangbing Zazhi. 2009;17:638-640. [PubMed] [Cited in This Article: ] |
| 12. | Kelly D, Bremner R, Hartley J, Flynn D. The management of a child with acute liver failure. Hepatology and nutrition. Oxford UK: John Wiley 2013; 158-163. [Cited in This Article: ] |
| 13. | Rajanayagam J, Coman D, Cartwright D, Lewindon PJ. Pediatric acute liver failure: etiology, outcomes, and the role of serial pediatric end-stage liver disease scores. Pediatr Transplant. 2013;17:362-368. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 14. | Gu FM, Li QL, Gao Q, Jiang JH, Zhu K, Huang XY, Pan JF, Yan J, Hu JH, Wang Z. IL-17 induces AKT-dependent IL-6/JAK2/STAT3 activation and tumor progression in hepatocellular carcinoma. Mol Cancer. 2011;10:150. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 129] [Cited by in F6Publishing: 142] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 15. | Sehgal R, David P, Vyas A, Rawal P, Hissar S, Samudrala G, Patra S, Trivedi S, Sarin S, Trehanpati N. Reduction in CD4 T cells and altered monocytes/macrophages is associated with fatal acute liver failure hepatitis E virus infected pregnant patients. J Viral Hepat. 2013;20:39-40. [DOI] [Cited in This Article: ] |
| 16. | Tuncer C, Oo YH, Murphy N, Adams DH, Lalor PF. The regulation of T-cell recruitment to the human liver during acute liver failure. Liver Int. 2013;33:852-863. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 17. | Park JS, Lim MA, Cho ML, Ryu JG, Moon YM, Jhun JY, Byun JK, Kim EK, Hwang SY, Ju JH. p53 controls autoimmune arthritis via STAT-mediated regulation of the Th17 cell/Treg cell balance in mice. Arthritis Rheum. 2013;65:949-959. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 18. | Barbi J, Pardoll D, Pan F. Metabolic control of the Treg/Th17 axis. Immunol Rev. 2013;252:52-77. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 143] [Cited by in F6Publishing: 168] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 19. | Cho ML, Kang JW, Moon YM, Nam HJ, Jhun JY, Heo SB, Jin HT, Min SY, Ju JH, Park KS. STAT3 and NF-kappaB signal pathway is required for IL-23-mediated IL-17 production in spontaneous arthritis animal model IL-1 receptor antagonist-deficient mice. J Immunol. 2006;176:5652-5661. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 240] [Cited by in F6Publishing: 253] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 20. | Tammineni P, Anugula C, Mohammed F, Anjaneyulu M, Larner AC, Sepuri NB. The import of the transcription factor STAT3 into mitochondria depends on GRIM-19, a component of the electron transport chain. J Biol Chem. 2013;288:4723-4732. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 148] [Cited by in F6Publishing: 167] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 21. | Ikeda O, Mizushima A, Sekine Y, Yamamoto C, Muromoto R, Nanbo A, Oritani K, Yoshimura A, Matsuda T. Involvement of STAP-2 in Brk-mediated phosphorylation and activation of STAT5 in breast cancer cells. Cancer Sci. 2011;102:756-761. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 22. | Bansal MB. Acute liver failure. Hepatology. Oxford UK: John Wiley 2014; 280-293. [Cited in This Article: ] |
| 23. | Neuberger J. Management of the patient with fulminant hepatic failure awaiting liver transplantation. Liver Transplantation. Oxford UK: John Wiley 2013; 93-100. [Cited in This Article: ] |
| 24. | Benyoub K, Muller M, Bonnet A, Simon R, Gazon M, Duperret S, Aubrun F, Viale JP. Amounts of bile acids and bilirubin removed during single-pass albumin dialysis in patients with liver failure. Ther Apher Dial. 2011;15:504-506. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 25. | Barnabas A, O’Grady J. Assessment of the patient with acute liver failure. Liver transplantation: clinical assessment and management. Oxford UK: John Wiley 2013; 83-92. [Cited in This Article: ] |
| 26. | MacQuillan G. Predicting outcome in acute liver failure: are we there yet? Liver Transpl. 2007;13:1209-1211. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |