Copyright
©2014 Baishideng Publishing Group Inc.
World J Gastroenterol. Oct 28, 2014; 20(40): 14589-14597
Published online Oct 28, 2014. doi: 10.3748/wjg.v20.i40.14589
Published online Oct 28, 2014. doi: 10.3748/wjg.v20.i40.14589
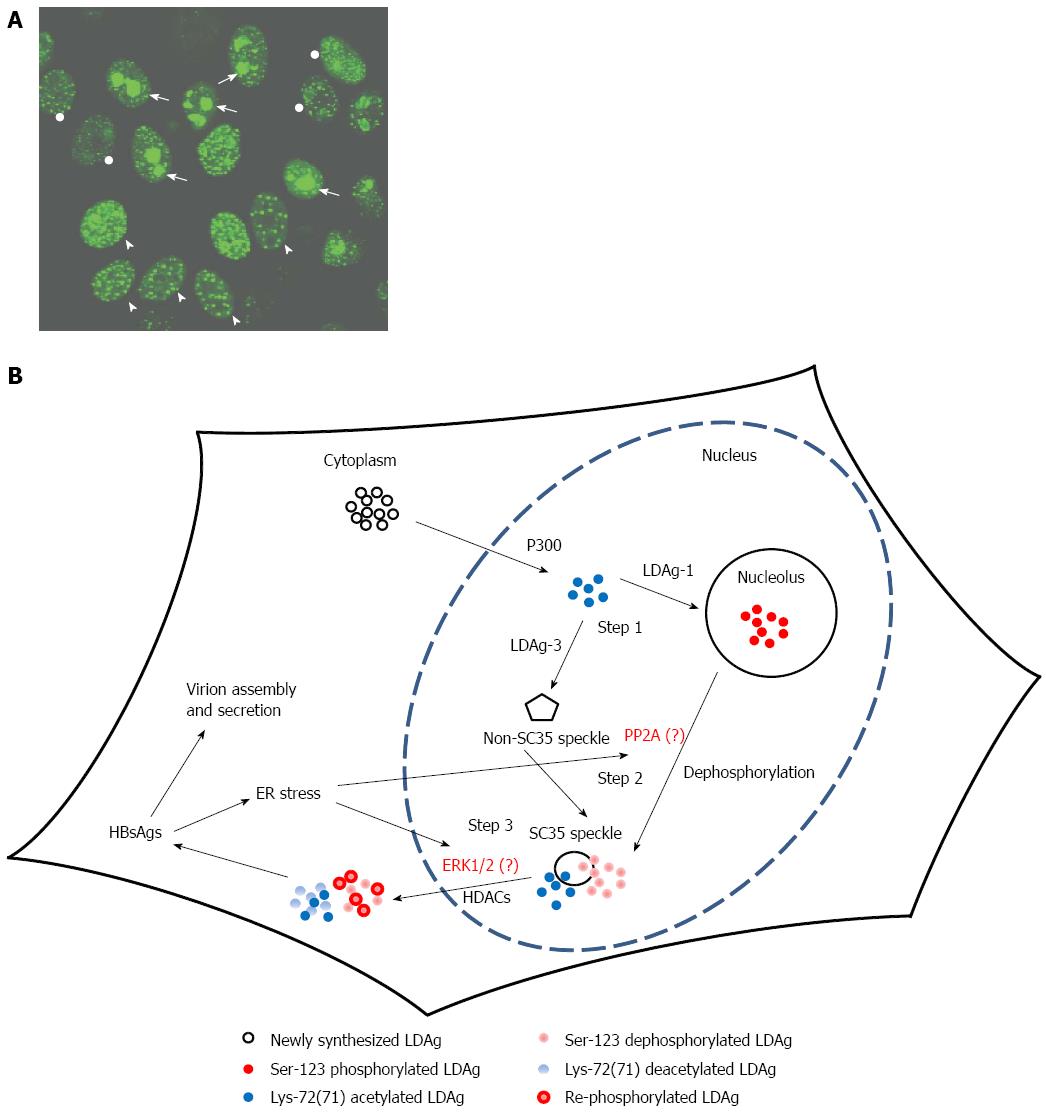
Figure 1 Large delta antigen resulting in different targeting patterns to various subcellular sites.
A: Green-fluorescent protein (GFP) fused with LDAg (GFP-LDAg) distribution shown by a fluorescence microscopic image. HeLa cells were transfected with GFP-LDAg expressing construct and the GFP signal was photographed under a fluorescence microscope. The distribution of GFP-LDAg is in different nuclear compartments indicated by the GFP signal. Three predominant locations of GFP-LDAg in GFP positive cells are nucleolus (Nu), nuclear speckles (SC35) and non-SC35 nuclear speckles (NS). The cells marked with arrow, arrowhead and circle indicate the most GFP signals in Nu, SC35 and NS, respectively; B: A schematic model of HBsAgs-induced LDAg nuclear exportation. The newly synthesized LDAg is acetylated by p300 (a type of acetyltransferase) followed by targeting to the nucleolus (genotype 1) or non-SC35 speckles (genotype 3). It is unclear whether there is a direct association between acetylation and/or serine-123 phosphorylation and LDAg localization to the nucleolus or nuclear speckles. Nevertheless, in both hepatitis D virus genotypes, LDAg moves to the SC35 speckles later (step 2) and it is speculated that phosphatase (PP2A) might contribute to the dephosphorylation of LDAgs. Afterwards, LDAg is deacetylated by histone deacetylases (HDACs) and exported out of the nucleus (step 3) and the ERK1/2 might participate in the re-phosphorylation of LDAgs. Finally, in the cytoplasm, the LDAg interacts with hepatitis B virus supplying surface antigens (HBsAgs) to assemble into empty viral particles or virions.
- Citation: Huang CR, Lo SJ. Hepatitis D virus infection, replication and cross-talk with the hepatitis B virus. World J Gastroenterol 2014; 20(40): 14589-14597
- URL: https://www.wjgnet.com/1007-9327/full/v20/i40/14589.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i40.14589