Published online Oct 28, 2014. doi: 10.3748/wjg.v20.i40.14778
Revised: May 1, 2014
Accepted: June 12, 2014
Published online: October 28, 2014
Organ fibrosis and architectural remodeling can severely disrupt tissue function, often with fatal consequences. Fibrosis is the end result of chronic inflammatory reactions induced by a variety of stimuli, and the key cellular mediator of fibrosis comprises the myofibroblasts which, when activated, serve as the primary collagen-producing cells. Complex links exist between fibrosis, regeneration and carcinogenesis, and the concept that all organs contain common tissue fibrosis pathways that could be potential therapeutic targets is an attractive one. Because of the major impact of fibrosis on human health there is an unmet need for safe and effective therapies that directly target fibrosis. Halofuginone inhibits tissue fibrosis and regeneration, and thereby affects the development of tumors in various tissues along the gastrointestinal tract. The high efficacy of halofuginone in reducing the fibrosis that affects tumor growth and tissue regeneration is probably due to its dual role in inhibiting the signaling pathway of transforming growth factor β, on the one hand, and inhibiting the development of Th17 cells, on the other hand. At present halofuginone is being evaluated in a clinical trial for other fibrotic indication, and any clinical success in that trial would allow the use of halofuginone, also for all other fibrotic indications, including those of the gastrointestinal tract.
Core tip: Fibrosis is a pathological process associated with excessive deposition of extracellular matrix that leads to destruction of organ architecture and function. Fibrosis contributes enormously to deaths worldwide, thus therapies are of a great need. The concept of common fibrosis pathways that could be therapeutic targets in all organs is an attractive one. Halofuginone is a novel anti-fibrotic therapy that inhibits tissue fibrosis, regeneration, and development of tumors in tissues along the gastrointestinal tract. At present halofuginone is being evaluated in a clinical trial for another fibrotic indication, and any clinical success there would allow its use for all other fibrotic indications.
- Citation: Pines M. Halofuginone for fibrosis, regeneration and cancer in the gastrointestinal tract. World J Gastroenterol 2014; 20(40): 14778-14786
- URL: https://www.wjgnet.com/1007-9327/full/v20/i40/14778.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i40.14778
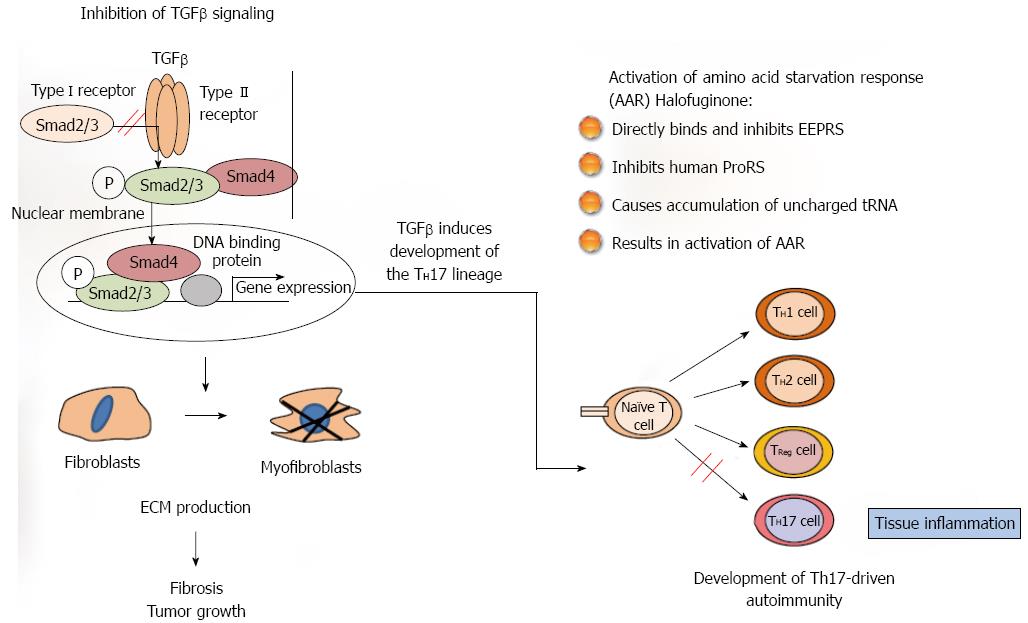
The synthesis and turnover of the extracellular matrix (ECM) is essential for normal tissue organization and function, both during development and adaptive homeostasis and also in the tissue remodeling that occurs after injury. As a result of the dysregulation of ECM synthesis caused by acute or chronic injury a pathological scarring process-fibrosis-occurs; it leads to destruction of organ architecture and impairment of organ function and, if highly progressive, the process of fibrosis eventually leads to organ malfunction and death. Fibrosis plays a role in the progression of many chronic diseases, tissue regeneration and tumor growth and although it follows similar pathways in divers organs and exhibits similar histomorphology in all of them, each organ has unique fibrotic features[1]. The myofibroblasts, which are responsible for the major increase in matrix synthesis in fibrosis, are unique mesenchymal cells with properties inherent in both muscle and non-muscle cells, and their cytoskeletal protein expression and contractile properties distinguish them from other cell types[2]. The myofibroblasts may derive from resident fibroblasts, from the circulating fibroblast population, from a vascular bed, or from hemopoietic progenitor or stromal cells derived from the bone marrow and from epithelial-mesenchymal transitions (EMTs). It is important to note that the myofibroblasts are heterogeneous populations of cells[3], probably having differing origins, expressing differing sets of genes, and possibly having differing functions. Fibrosis, in most cases, is associated with inflammation, and the inflammatory cells are the main source of the tumor growth factor (TGF) β that is one of the leading candidates for eliciting overproduction of ECM proteins in various fibrotic conditions and in the tumor stroma. TGFβ meets the criteria for being a major participant in fibroblast activation: it is present in the affected tissues, it induces profibrotic cellular and transcriptional responses, and it induces EMT during cancer progression[4]. Following binding of TGFβ to its receptor, signaling to the nucleus occurs predominantly by phosphorylation of cytoplasmatic mediators of the Smad family.
A high proportion of all deaths in the developed world are attributed to chronic fibroproliferative diseases, therefore, the demand for safe and effective antifibrotic drugs is on the rise. However, in spite of its vast impact on human health, there are currently no approved treatments that directly target fibrosis.
For centuries in China the roots and leaves of Dichroa febrifuga Lour. were used against malarial fever, and out of 600 plants tested for their antimalarial effects, D. febrifuga was found to yield one of the most effective extracts. An active alkaloid named febrifugine, with the molecular formula C16H21O3N3, was isolated, but its high antimalarial activity was accompanied by a gastrointestinal toxicity, therefore it was used as a lead compound in the synthesis of active molecules with lower toxicity. It has been demonstrated that most febrifugine analogs bearing a modified or unmodified 4-quinazolinone moiety are active, but analogs produced through modification of the side chain attached to the N3 position of the 4-quinazolinone ring have proved to be ineffective. Furthermore, a synthetically prepared racemic febrifugine was reported to be only about half as effective as natural febrifugine. These results suggested that the 4-quinazolinone moiety, the nitrogen atom of the piperidine ring, and the hydroxyl group are necessary for the antimalarial activity, and that the absolute configuration of these functional groups plays an important role[5]. Halofuginone hydrobromide,7-bromo-6-chloro-3-[3-(3-hydroxy-2-piperidinyl)-2-oxopropyl]-4(3H)-quinazolinone, one of the febrifugine analogs, is an FDA-approved feed additive for prevention of coccidiosis in poultry (For reviews see[6-8]). At first, halofuginone was identified as an inhibitor of collagen synthesis, and the observation that it inhibited collagen α1(I) gene expression paved the way for the use of halofuginone as an anti-fibrotic drug. At present, halofuginone is being evaluated in a Phase 1b FDA-approved clinical trial for Duchenne muscular dystrophy, in which fibrosis is the main complication.
The most studied mode of action, and probably the canonical one, is inhibition of Smad3 phosphorylation downstream of the TGFβ signaling pathway[9]. In most animal models of fibrosis, regardless of tissue type, halofuginone had a minimal effect on the ECM protein contents in the non-fibrotic control animals, whereas it exhibited a profound inhibitory effect in the fibrotic organs. These results suggest that there was differing regulation, on the one hand, of the housekeeping expression - usually low level - of ECM genes and, on the other hand, of the TGFβ-driven over-expression induced by the fibrogenic stimulus, which is usually an aggressive and rapid process. Halofuginone was found to inhibit the collagen synthesis that was activated by TGFβ in human skin fibroblasts and by activated fibroblasts derived from scleroderma and from cGvHD patients[10]. It also inhibited both Smad3 phosphorylation and collagen synthesis in TGFβ-activated tight-skin (Tsk+)-mouse fibroblasts[11], in the mdx mouse model of muscular dystrophy[12], but not in control cells[10,13]. In various fibrotic models no effect of halofuginone was observed on the expression of the TGFβ receptors gene or on TGFβ levels[12-14] -a finding that supports the hypothesis that the halofuginone target is downstream in the TGFβ pathway. In chemically induced liver fibrosis halofuginone affected TGFβ-regulated genes through inhibition of Smad3 phosphorylation of activated hepatic stellate cells (HSCs)[15]. Halofuginone inhibited TGFβ-dependent Smad3 phosphorylation and elevated the expression of the inhibitory Smad7 in a variety of cell types, such as fibroblasts, hepatic and pancreatic stellate cells (PSCs), tumor cells and myoblasts[14-17]. The inhibition of Smad3 phosphorylation was due, at least in part, to halofuginone-dependent activation of Akt MAPK/ERK and p38 MAPK phosphorylation[16] (Figure 1).
An additional mechanism suggested for the halofuginone-dependent inhibition of fibrosis involves selective prevention of the development of Th17 cells, by activation of the amino acid starvation response[18,19]. Halofuginone treatment reduced the severity and incidence of autoimmune encephalomyelitis associated with Th17 cells that are characterized by production of interleukin-17 (IL-17), which promotes fibrosis by both exacerbating the upstream inflammatory response and regulating the downstream activation of fibroblasts. The mechanism by which halofuginone and other febrifugine analogs inhibit the amino acid starvation response is by binding to glutamyl-prolyl-tRNA synthetase and inhibiting prolyl-tRNA synthetase activity[20], by simultaneously occupying two different substrate binding sites on prolyl-transfer RNA synthetase[21]. Only the 2R,3S isomer of halofuginone, which matches the absolute configuration of febrifugine, exhibits biological activity. Thus, halofuginone could potentially be used to address any autoimmune or inflammatory disease associated with Th17 cells. It should be noted that TGFβ is required for facilitation of differentiation of the inflammatory Th17 cell subset[22,23], which suggests the existence of a link between the TGFβ signaling pathway and the amino acid starvation response. The interactions among fibroblasts, macrophages, and CD4 T cells likely play general and critical roles in initiating, perpetuating, and resolving fibrosis. Together, these findings identify the IL-1β-IL-17A-TGF-β1 cytokine axis as an important pathway in inflammation-driven fibrosis.
Various organs of the gastrointestinal tract are affected by fibrosis, in suffering many and varied insults, and all of these organs could benefit immensely from an antifibrotic therapy (Table 1).
| Fibrosis | Resolution of fibrosis | Strictures formation | Regeneration | Tumor development | |
| Esophagus | ↓ | ↓ | |||
| Small intestine | ↓ | ↓ | |||
| Liver | ↓ | ↑ | ↑ | ↓ | |
| Pancreas | ↓ | ↓ |
Esophageal strictures are a major clinical problem, with a high degree of morbidity[24]. They are caused by post-traumatic fibrosis after surgical interventions, ingestion of corrosive materials, endoscopic mucosectomy (EEM), and radiotherapy or combined chemo-radio-therapy for head and neck cancer[25,26]. Halofuginone treatment caused an increase in esophageal patency, reduction in esophageal wall thickness, and increased survival in rats with caustic-dependent esophageal stricture[27,28], reduced esophageal fibrosis and collagen synthesis after head and neck radiation[29], and decreased stricture formation in pigs after endoscopic mucosectomy[30]. Similar results were observed in urethral[31], abdominal[32,33], uterine horn stricture[34], and subglottic stenosis in a canine model[35].
Liver and pancreas fibrosis, triggered by chronic tissue damage with a wide variety of etiologies, constitute a major cause of morbidity and mortality worldwide. The fibrosis is characterized by an accumulation of ECM and it results in formation of a fibrotic scar that eventually alters the cellular and functional balance of the liver[36]. Moreover, the excessive ECM provides a pivotal microenvironment for tumor development, both in the liver[37,38] and in the pancreas[39]. The interactions of various cell populations, some residing within the liver and the pancreas and some recruited from the bone marrow, are required for the tissue fibrinogenesis. A key role in fibrogenesis is played by myofibroblasts, which produce ECM proteins and coordinate the “wound healing” response[36,40,41]. HSC and pancreatic stellate cells (PSC) are classically considered to be a major source of myofibroblasts[40,42,43], but other cell types have been hypothesized to contribute to the expansion of the myofibroblast population observed during injury; they include portal myofibroblasts and cells recruited from the bone marrow[40]. It is believed that the involvement of various populations of fibrogenic cells is dependent on the etiology of the damage and the resulting development of distinct spatial patterns of fibrosis[44]. The activation of the HSC involves the initiatory or pre-inflammatory stage, which results from early changes in gene expression that are caused by paracrine stimuli from damaged resident liver and pancreas cells, and the perpetuation stage, which is caused by maintenance of these stimuli by autocrine and paracrine stimuli. In both the liver and the pancreas TGFβ and platelet-derived growth factor (PDGF) have been considered to be the key fibrogenic and proliferative stimuli to SC[45,46] - a hypothesis that raises the possibility of a common mechanism and clear targets[47].
Three rat models, dimethylnitrosamine (DMN), thioacetamide (TAA), and concanavalin A (ConA)-induced liver fibrosis/cirrhosis, and a mouse model of cerulein-dependent pancreas fibrosis were used to evaluate the efficacy of halofuginone in preventing hepatic and pancreaticfibrosis[14,48-51]. Halofuginone, independently of the route of administration, prevented the increase in collagen α1(I) gene expression and collagen content in all models, and prevented the decrease in liver weight, reduced plasma alkaline phosphatase activity and reduced the mortality rate, all of which are characteristic of tissue fibrosis/cirrhosis. Halofuginone inhibited Smad3 phosphorylation downstream of the TGFβ signaling, resulting in inhibition of an array of TGFβ-dependent and SC-specific genes[15], and reduction of the number of activated HSCs and PSCs[14,49]. Halofuginone also affected the cross-talk between the hepatocytes and the SCs by up-regulating the synthesis and secretion of insulin-like growth factor binding protein-1 (IGFBP-1), which inhibits SC migration[50].
Moreover, halofuginone caused resolution of established liver fibrosis[49], similarly to its actions that were observed in skin of Tsk mice[11], in muscles of mice with muscular dystrophy[52], and in a chronic graft-versus-host cGvHD patient[53,54]. This was probably due to up-regulation of the collagen degradation pathway by inhibition of the tissue inhibitors of matrix metalloproteinase 2 (TIMP2), and activation of matrix metalloproteinases activities[55].
Hepatic regeneration leads to restoration of remnant hepatic parenchyma after partial hepatectomy. Liver regeneration is an essential component of the reparative process that follows liver injury and surgical resection, and in its absence, morbidity and mortality rates are often increased[56,57]. Post-hepatectomy liver insufficiency is one of the most serious problems associated with liver surgery, especially in the cirrhotic liver, which has less function reserve than the normal one[58]. In the cirrhotic liver the rate and extent of regeneration are retarded and diminished after partial hepatectomy, and the resulting dysfunction and insufficient regeneration often make the patients vulnerable to postoperative liver failure, which frequently leads to multiple organ failure. As hepatocellular carcinoma (HCC) is often seen in cirrhotic livers, the degree of liver cirrhosis limits the safe extent of hepatic resection[59,60]. The extensive resection needed to prevent recurrence of malignant tumors is questionable in light of the resulting impaired regenerative capacity, and morbidity and mortality rates after hepatectomy are enhanced in these patients[61]. Moreover, liver transplantation in patients with cirrhosis results in a very low survival benefit, and may not represent the optimal use of scarce, donated liver organs[62]. Thus, the need to improve liver regeneration in HCC-associated liver fibrosis is of great importance. Various attempts to improve regeneration in cirrhotic livers were made by using granulocyte-macrophage colony-stimulating factor (GM-CSF)[63], hepatocyte growth factor[64], cardiotrophin-1 (CT-1)[65], and human growth hormone[66]. Blockade of angiotensin-1 receptors by lostran increased hepatic blood flow and reduced HSC activation and liver fibrosis, but interfered with hepatocyte proliferation after partial hepatectomy in cirrhotic livers[67]. On the other hand, addition of low concentrations of halofuginone to the diet prior to and following partial hepatectomy in rats did not inhibit normal liver regeneration, as indicated by the number of proliferating cells and restored liver mass. When given to rats with established fibrosis, halofuginone caused significant reductions in αSMA, TIMP-2, collagen type I gene expression and collagen deposition. Such animals exhibited improved capacity for regeneration, suggesting the possible beneficial use of halofuginone before and during fibrotic/cirrhotic liver regeneration[68]. Heparan sulfate proteoglycans (HSPGs) are major components of the ECM, and cleavage of HSPGs influenced normal and pathological processes[69]. Heparanase, an endoglycosidase capable of cleavage of heparan sulfate is not expressed in the mature healthy liver, but is expressed following partial hepatectomy during the early stages of fibrosis[70]. Heparanase enhanced liver regeneration, probably by inducing proliferation of hepatocytes[71]. Elevated expression of heparanase mRNA in hepatic SCs, together with improved capacity to regenerate following partial hepatectomy was observed after halofuginone treatment[72].
Regulation of the expression of the early genes of regeneration, such as tyrosine phosphatase (PRL-1) and IGFBP-1, is part of the mode of action of halofuginone in improving cirrhotic liver regeneration[50,73]. PRL-1 gene expression was rapidly induced in a liver remnant that was undergoing regeneration after partial hepatectomy and was mediated by zinc-finger transcription factor early growth response-1 (Egr-1)[74,75]. The involvement of PRL-1 in cell proliferation is due to stimulation of progression of hepatocytes from G1 into S phase in a cyclin-kinase-dependent manner[76]. Furthermore, the cellular localization of PRL-1 is also associated with its role in cell-cycle regulation[77]. In culture, halofuginone increased PRL-1 expression in primary rat hepatocytes and in hepatocellular carcinoma (HCC) cell lines[73]. The halofuginone-dependent increase in PRL-1 gene expression was correlated with an increase in the Egr-1 expression and inversely correlated with the inhibition of cell proliferation. Halofuginone also affected the PRL-1 sub-cellular localization that was cell-cycle-dependent. In addition, halofuginone augmented PRL-1 and IGFBP-1 expression in the remnant liver after partial hepatectomy.
In many types of tumor a clear association is observed between tissue fibrosis and increased risk of tumor development. The fibrosis disrupts the normal cell-cell and cell-ECM interactions and thus leads to further loss of control over cell growth. Furthermore, there is increasing awareness that signals provided by the stroma can induce genetic alterations that trigger tumor formation and can stimulate tumor growth. For example, the leading risk factor for HCC is cirrhosis of the liver, and its associated inflammation, regeneration and fibrosis[37,78,79]. Alcohol and aflatoxins are well-recognized non-viral exogenous agents, associated with the pathogenesis of HCC and fibrosis. Accordingly, the risk of HCC increased in parallel with progression of hepatic fibrosis[80], and approximately 80% of HCCs were developed in macro-nodular cirrhosis. Even after successful resection, the remnant cirrhotic liver frequently develops new HCC lesions, seriously impairing long-term survival[81]. Also, patients with pancreatic fibrosis as a consequence of chronic pancreatitis have a substantially increased risk of developing pancreatic cancer[82,83]. Such increased cancer risk was observed in patients with pancreatitis that started early in life, which suggests that the duration of exposure to the fibrotic stimulus is a major factor[84]. Taken together, these results suggest that stroma cells and the ECM components provide the pivotal microenvironment for tumor development and, therefore, represent potential new therapy targets.
Halofuginone reduced tumor growth and mortality in xenograph mice implanted with human hepatoma cells[85]. In chemically induced liver-fibrotic rats that developed HCC and spontaneously metastasized to the lungs, halofuginone reduced the number of liver tumors and the percentage of the lung surface infiltrated by metastasis[86]. More tumors developed in mouse fibrotic pancreas than in normal pancreas, and prevention of tissue fibrosis by halofuginone greatly reduced tumor development[87,88]. Halofuginone-dependent reduction in growth occurred also in other tumors, such as prostate cancer, von Hippel-Lindau pheochromocytoma, Wilm’s tumor and glioma, in all cases in association with ECM reduction[89-92]. The important role of halofuginone in myofibroblast activation and tumor formation was demonstrated by implanting tumor cells together with myofibroblasts: tumor numbers increased significantly when the myofibroblasts were derived from fibrotic pancreas, but were almost nowhere to be found if the donor mice had been treated with halofuginone. Halofuginone, by inhibiting Smad3 phosphorylation, specifically affected stroma cells that expressed both contractile and collagen biosynthesis genes - characteristics of activated myofibroblasts. As with other tumors[93], in pancreatic xenografts, combination of treatments with halofuginone, which inhibits stroma cell infiltration, causes apoptosis of myofibroblasts and inhibits Smad3 phosphorylation, together with chemotherapy, which increases tumor-cell apoptosis without affecting Smad3 phosphorylation, was more efficacious than either treatment alone[88]. Taken together these findings emphasize that ECM production by activated PSCs/myofibroblasts is essential for tumor establishment and growth. Thus, inhibition of activation of these cells is a viable means of reducing pancreatic tumor development.
Thanks to the histomorphological similarities that are common to fibrosis in all organs, the concept of common tissue fibrosis pathways that could be potential therapeutic targets in all organs is an attractive one. Therapeutics that simultaneously target important inflammatory mediators and profibrotic cytokines will probably emerge as the most successful way to treat this highly complex and difficult-to-manage pathology[94]. Thus, if halofuginone were to be found successful in a clinical trial addressed to a specific fibrotic indication, it might be considered as an antifibrotic therapy for all other indications as well. The therapeutic potential of halofuginone, originally intended for use as an anti-malarial drug, but which was eventually used as a veterinary drug against coccidiosis, was revealed by serendipity. The high efficacy of halofuginone in reducing fibrosis, which affects tumor growth and tissue regeneration in various tissues, springs from its dual role in inhibiting the TGFβ signaling, on the one hand, and inhibiting the development of Th17 cells, on the other hand. Halofuginone did not result from any drug discovery program, and its structure was not designed specifically for inhibition of TGFβ signaling. Thus, a specifically designed combinatory chemistry approach, using halofuginone structure in an oriented drug discovery scheme, together with development of biological assays, might lead to a much needed new arsenal of drugs that would be more specific with respect to various steps in recruitment, activation and function of myofibroblasts, that would be better tolerated by patients, and that would exhibit enhanced efficacy.
P- Reviewer: Wong GLH S- Editor: Qi Y L- Editor: A E- Editor: Wang CH
| 1. | Zeisberg M, Kalluri R. Cellular mechanisms of tissue fibrosis. 1. Common and organ-specific mechanisms associated with tissue fibrosis. Am J Physiol Cell Physiol. 2013;304:C216-C225. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 302] [Cited by in F6Publishing: 326] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 2. | Kramann R, DiRocco DP, Humphreys BD. Understanding the origin, activation and regulation of matrix-producing myofibroblasts for treatment of fibrotic disease. J Pathol. 2013;231:273-289. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 157] [Cited by in F6Publishing: 162] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 3. | Magness ST, Bataller R, Yang L, Brenner DA. A dual reporter gene transgenic mouse demonstrates heterogeneity in hepatic fibrogenic cell populations. Hepatology. 2004;40:1151-1159. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 197] [Cited by in F6Publishing: 191] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 4. | Verrecchia F, Mauviel A. Transforming growth factor-beta signaling through the Smad pathway: role in extracellular matrix gene expression and regulation. J Invest Dermatol. 2002;118:211-215. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 5. | Takaya Y, Tasaka H, Chiba T, Uwai K, Tanitsu M, Kim HS, Wataya Y, Miura M, Takeshita M, Oshima Y. New type of febrifugine analogues, bearing a quinolizidine moiety, show potent antimalarial activity against Plasmodium malaria parasite. J Med Chem. 1999;42:3163-3166. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 120] [Cited by in F6Publishing: 122] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 6. | McLaughlin NP, Evans P, Pines M. The chemistry and biology of febrifugine and halofuginone. Bioorg Med Chem. 2014;22:1993-2004. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 7. | Pines M, Nagler A. Halofuginone: a novel antifibrotic therapy. Gen Pharmacol. 1998;30:445-450. [PubMed] [Cited in This Article: ] |
| 8. | Pines M, Vlodavsky I, Nagler A. Halofuginone: From veterinary use to human therapy. Drug Develop Res. 2000;50:371-378. [Cited in This Article: ] |
| 9. | Pines M. Targeting TGFβ signaling to inhibit fibroblast activation as a therapy for fibrosis and cancer: effect of halofuginone. Expert Opin Drug Discov. 2008;3:11-20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 10. | Halevy O, Nagler A, Levi-Schaffer F, Genina O, Pines M. Inhibition of collagen type I synthesis by skin fibroblasts of graft versus host disease and scleroderma patients: effect of halofuginone. Biochem Pharmacol. 1996;52:1057-1063. [PubMed] [Cited in This Article: ] |
| 11. | Pines M, Domb A, Ohana M, Inbar J, Genina O, Alexiev R, Nagler A. Reduction in dermal fibrosis in the tight-skin (Tsk) mouse after local application of halofuginone. Biochem Pharmacol. 2001;62:1221-1227. [PubMed] [Cited in This Article: ] |
| 12. | Turgeman T, Hagai Y, Huebner K, Jassal DS, Anderson JE, Genin O, Nagler A, Halevy O, Pines M. Prevention of muscle fibrosis and improvement in muscle performance in the mdx mouse by halofuginone. Neuromuscul Disord. 2008;18:857-868. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 105] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 13. | McGaha TL, Phelps RG, Spiera H, Bona C. Halofuginone, an inhibitor of type-I collagen synthesis and skin sclerosis, blocks transforming-growth-factor-beta-mediated Smad3 activation in fibroblasts. J Invest Dermatol. 2002;118:461-470. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 14. | Zion O, Genin O, Kawada N, Yoshizato K, Roffe S, Nagler A, Iovanna JL, Halevy O, Pines M. Inhibition of transforming growth factor beta signaling by halofuginone as a modality for pancreas fibrosis prevention. Pancreas. 2009;38:427-435. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 53] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 15. | Gnainsky Y, Kushnirsky Z, Bilu G, Hagai Y, Genina O, Volpin H, Bruck R, Spira G, Nagler A, Kawada N. Gene expression during chemically induced liver fibrosis: effect of halofuginone on TGF-beta signaling. Cell Tissue Res. 2007;328:153-166. [PubMed] [Cited in This Article: ] |
| 16. | Roffe S, Hagai Y, Pines M, Halevy O. Halofuginone inhibits Smad3 phosphorylation via the PI3K/Akt and MAPK/ERK pathways in muscle cells: effect on myotube fusion. Exp Cell Res. 2010;316:1061-1069. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 17. | Zeplin PH. Halofuginone down-regulates Smad3 expression and inhibits the TGFbeta-induced expression of fibrotic markers in human corneal fibroblasts. Ann Plast Surg. 2014;72:489. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 18. | Sundrud MS, Koralov SB, Feuerer M, Calado DP, Kozhaya AE, Rhule-Smith A, Lefebvre RE, Unutmaz D, Mazitschek R, Waldner H. Halofuginone inhibits TH17 cell differentiation by activating the amino acid starvation response. Science. 2009;324:1334-1338. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 303] [Cited by in F6Publishing: 300] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 19. | Pietrella D, Rachini A, Pines M, Pandey N, Mosci P, Bistoni F, d’Enfert C, Vecchiarelli A. Th17 cells and IL-17 in protective immunity to vaginal candidiasis. PLoS One. 2011;6:e22770. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 86] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 20. | Keller TL, Zocco D, Sundrud MS, Hendrick M, Edenius M, Yum J, Kim YJ, Lee HK, Cortese JF, Wirth DF. Halofuginone and other febrifugine derivatives inhibit prolyl-tRNA synthetase. Nat Chem Biol. 2012;8:311-317. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 242] [Cited by in F6Publishing: 273] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 21. | Zhou H, Sun L, Yang XL, Schimmel P. ATP-directed capture of bioactive herbal-based medicine on human tRNA synthetase. Nature. 2013;494:121-124. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 113] [Cited by in F6Publishing: 113] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 22. | Mangan PR, Harrington LE, O’Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR, Weaver CT. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231-234. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2372] [Cited by in F6Publishing: 2430] [Article Influence: 135.0] [Reference Citation Analysis (0)] |
| 23. | Das J, Ren G, Zhang L, Roberts AI, Zhao X, Bothwell AL, Van Kaer L, Shi Y, Das G. Transforming growth factor beta is dispensable for the molecular orchestration of Th17 cell differentiation. J Exp Med. 2009;206:2407-2416. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 176] [Cited by in F6Publishing: 176] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 24. | Nunes AC, Romãozinho JM, Pontes JM, Rodrigues V, Ferreira M, Gomes D, Freitas D. Risk factors for stricture development after caustic ingestion. Hepatogastroenterology. 2002;49:1563-1566. [PubMed] [Cited in This Article: ] |
| 25. | Contini S, Scarpignato C. Caustic injury of the upper gastrointestinal tract: a comprehensive review. World J Gastroenterol. 2013;19:3918-3930. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 231] [Cited by in F6Publishing: 183] [Article Influence: 16.6] [Reference Citation Analysis (6)] |
| 26. | Prisman E, Miles BA, Genden EM. Prevention and management of treatment-induced pharyngo-oesophageal stricture. Lancet Oncol. 2013;14:e380-e386. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 27. | Ozçelik MF, Pekmezci S, Saribeyoğlu K, Unal E, Gümüştaş K, Doğusoy G. The effect of halofuginone, a specific inhibitor of collagen type 1 synthesis, in the prevention of esophageal strictures related to caustic injury. Am J Surg. 2004;187:257-260. [PubMed] [Cited in This Article: ] |
| 28. | Arbell D, Udassin R, Koplewitz BZ, Ohana M, Genina O, Pines M, Nagler A. Prevention of esophageal strictures in a caustic burn model using halofuginone, an inhibitor of collagen type I synthesis. Laryngoscope. 2005;115:1632-1635. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 29. | Dabak H, Karlidag T, Akpolat N, Keles E, Alpay HC, Serin M, Kaygusuz I, Yalcin S, Isik O. The effects of methylprednisolone and halofuginone on preventing esophageal and hypopharyngeal fibrosis in delivered radiotherapy. Eur Arch Otorhinolaryngol. 2010;267:1429-1435. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 30. | Wu Y, Schomisch SJ, Cipriano C, Chak A, Lash RH, Ponsky JL, Marks JM. Preliminary results of antiscarring therapy in the prevention of postendoscopic esophageal mucosectomy strictures. Surg Endosc. 2014;28:447-455. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 31. | Nagler A, Gofrit O, Ohana M, Pode D, Genina O, Pines M. The effect of halofuginone, an inhibitor of collagen type i synthesis, on urethral stricture formation: in vivo and in vitro study in a rat model. J Urol. 2000;164:1776-1780. [PubMed] [Cited in This Article: ] |
| 32. | Nagler A, Rivkind AI, Raphael J, Levi-Schaffer F, Genina O, Lavelin I, Pines M. Halofuginone--an inhibitor of collagen type I synthesis--prevents postoperative formation of abdominal adhesions. Ann Surg. 1998;227:575-582. [PubMed] [Cited in This Article: ] |
| 33. | Washburn S, Jennell JL, Hodges SJ. Halofuginone- and chitosan-coated amnion membranes demonstrate improved abdominal adhesion prevention. ScientificWorldJournal. 2010;10:2362-2366. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 34. | Nagler A, Genina O, Lavelin I, Ohana M, Pines M. Halofuginone, an inhibitor of collagen type I synthesis, prevents postoperative adhesion formation in the rat uterine horn model. Am J Obstet Gynecol. 1999;180:558-563. [PubMed] [Cited in This Article: ] |
| 35. | Eliashar R, Ochana M, Maly B, Pines M, Sichel JY, Nagler A. Halofuginone prevents subglottic stenosis in a canine model. Ann Otol Rhinol Laryngol. 2006;115:382-386. [PubMed] [Cited in This Article: ] |
| 36. | Hernandez-Gea V, Friedman SL. Pathogenesis of liver fibrosis. Annu Rev Pathol. 2011;6:425-456. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1096] [Cited by in F6Publishing: 1229] [Article Influence: 94.5] [Reference Citation Analysis (0)] |
| 37. | Hernandez-Gea V, Toffanin S, Friedman SL, Llovet JM. Role of the microenvironment in the pathogenesis and treatment of hepatocellular carcinoma. Gastroenterology. 2013;144:512-527. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 486] [Cited by in F6Publishing: 552] [Article Influence: 50.2] [Reference Citation Analysis (0)] |
| 38. | Kocabayoglu P, Friedman SL. Cellular basis of hepatic fibrosis and its role in inflammation and cancer. Front Biosci (Schol Ed). 2013;5:217-230. [PubMed] [Cited in This Article: ] |
| 39. | Apte MV, Pirola RC, Wilson JS. Pancreatic stellate cells: a starring role in normal and diseased pancreas. Front Physiol. 2012;3:344. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 184] [Cited by in F6Publishing: 229] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 40. | Friedman SL. Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol Rev. 2008;88:125-172. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2084] [Cited by in F6Publishing: 2041] [Article Influence: 127.6] [Reference Citation Analysis (0)] |
| 41. | Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008;134:1655-1669. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1928] [Cited by in F6Publishing: 2053] [Article Influence: 128.3] [Reference Citation Analysis (0)] |
| 42. | Cohen-Naftaly M, Friedman SL. Current status of novel antifibrotic therapies in patients with chronic liver disease. Therap Adv Gastroenterol. 2011;4:391-417. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 148] [Cited by in F6Publishing: 146] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 43. | Apte MV, Wilson JS, Lugea A, Pandol SJ. A starring role for stellate cells in the pancreatic cancer microenvironment. Gastroenterology. 2013;144:1210-1219. [PubMed] [Cited in This Article: ] |
| 44. | Guyot C, Lepreux S, Combe C, Doudnikoff E, Bioulac-Sage P, Balabaud C, Desmoulière A. Hepatic fibrosis and cirrhosis: the (myo)fibroblastic cell subpopulations involved. Int J Biochem Cell Biol. 2006;38:135-151. [PubMed] [Cited in This Article: ] |
| 45. | Jiao J, Friedman SL, Aloman C. Hepatic fibrosis. Curr Opin Gastroenterol. 2009;25:223-229. [PubMed] [Cited in This Article: ] |
| 46. | Talukdar R, Tandon RK. Pancreatic stellate cells: new target in the treatment of chronic pancreatitis. J Gastroenterol Hepatol. 2008;23:34-41. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 47. | Schuppan D, Koda M, Bauer M, Hahn EG. Fibrosis of liver, pancreas and intestine: common mechanisms and clear targets? Acta Gastroenterol Belg. 2008;63:366-370. [PubMed] [Cited in This Article: ] |
| 48. | Pines M, Knopov V, Genina O, Lavelin I, Nagler A. Halofuginone, a specific inhibitor of collagen type I synthesis, prevents dimethylnitrosamine-induced liver cirrhosis. J Hepatol. 1997;27:391-398. [PubMed] [Cited in This Article: ] |
| 49. | Bruck R, Genina O, Aeed H, Alexiev R, Nagler A, Avni Y, Pines M. Halofuginone to prevent and treat thioacetamide-induced liver fibrosis in rats. Hepatology. 2001;33:379-386. [PubMed] [Cited in This Article: ] |
| 50. | Gnainsky Y, Spira G, Paizi M, Bruck R, Nagler A, Abu-Amara SN, Geiger B, Genina O, Monsonego-Ornan E, Pines M. Halofuginone, an inhibitor of collagen synthesis by rat stellate cells, stimulates insulin-like growth factor binding protein-1 synthesis by hepatocytes. J Hepatol. 2004;40:269-277. [PubMed] [Cited in This Article: ] |
| 51. | Liang J, Zhang B, Shen RW, Liu JB, Gao MH, Li Y, Li YY, Zhang W. Preventive effect of halofuginone on concanavalin A-induced liver fibrosis. PLoS One. 2013;8:e82232. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 52. | Halevy O, Genin O, Barzilai-Tutsch H, Pima Y, Levi O, Moshe I, Pines M. Inhibition of muscle fibrosis and improvement of muscle histopathology in dysferlin knock-out mice treated with halofuginone. Histol Histopathol. 2013;28:211-226. [PubMed] [Cited in This Article: ] |
| 53. | Nagler A, Pines M. Topical treatment of cutaneous chronic graft versus host disease with halofuginone: a novel inhibitor of collagen type I synthesis. Transplantation. 1999;68:1806-1809. [PubMed] [Cited in This Article: ] |
| 54. | Pines M, Snyder D, Yarkoni S, Nagler A. Halofuginone to treat fibrosis in chronic graft-versus-host disease and scleroderma. Biol Blood Marrow Transplant. 2003;9:417-425. [PubMed] [Cited in This Article: ] |
| 55. | Popov Y, Patsenker E, Bauer M, Niedobitek E, Schulze-Krebs A, Schuppan D. Halofuginone induces matrix metalloproteinases in rat hepatic stellate cells via activation of p38 and NFkappaB. J Biol Chem. 2006;281:15090-15098. [PubMed] [Cited in This Article: ] |
| 56. | Riehle KJ, Dan YY, Campbell JS, Fausto N. New concepts in liver regeneration. J Gastroenterol Hepatol. 2011;26 Suppl 1:203-212. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 155] [Cited by in F6Publishing: 153] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 57. | Duncan AW, Soto-Gutierrez A. Liver repopulation and regeneration: new approaches to old questions. Curr Opin Organ Transplant. 2013;18:197-202. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 58. | Moser MA, Kneteman NM, Minuk GY. Research toward safer resection of the cirrhotic liver. HPB Surg. 2000;11:285-297. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 59. | Hsu KY, Chau GY, Lui WY, Tsay SH, King KL, Wu CW. Predicting morbidity and mortality after hepatic resection in patients with hepatocellular carcinoma: the role of Model for End-Stage Liver Disease score. World J Surg. 2009;33:2412-2419. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 59] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 60. | Ruzzenente A, Valdegamberi A, Campagnaro T, Conci S, Pachera S, Iacono C, Guglielmi A. Hepatocellular carcinoma in cirrhotic patients with portal hypertension: is liver resection always contraindicated? World J Gastroenterol. 2011;17:5083-5088. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 33] [Cited by in F6Publishing: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 61. | Nagasue N, Yukaya H, Ogawa Y, Kohno H, Nakamura T. Human liver regeneration after major hepatic resection. A study of normal liver and livers with chronic hepatitis and cirrhosis. Ann Surg. 1987;206:30-39. [PubMed] [Cited in This Article: ] |
| 62. | Berry K, Ioannou GN. Are patients with Child’s A cirrhosis and hepatocellular carcinoma appropriate candidates for liver transplantation? Am J Transplant. 2012;12:706-717. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 63. | Eroğlu A, Demirci S, Akbulut H, Sever N, Demirer S, Unal AE. Effect of granulocyte-macrophage colony-stimulating factor on hepatic regeneration after 70% hepatectomy in normal and cirrhotic rats. HPB (Oxford). 2002;4:67-73. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 64. | Kaido T, Yoshikawa A, Seto S, Yamaoka S, Furuyama H, Arii S, Takahashi Y, Imamura M. Pretreatment with soluble thrombomodulin prevents intrasinusoidal coagulation and liver dysfunction following extensive hepatectomy in cirrhotic rats. Thromb Haemost. 1999;82:1302-1306. [PubMed] [Cited in This Article: ] |
| 65. | Yang ZF, Lau CK, Ngai P, Lam SP, Ho DW, Poon RT, Fan ST. Cardiotrophin-1 enhances regeneration of cirrhotic liver remnant after hepatectomy through promotion of angiogenesis and cell proliferation. Liver Int. 2008;28:622-631. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 66. | Luo SM, Liang LJ, Lai JM. Effects of recombinant human growth hormone on remnant liver after hepatectomy in hepatocellular carcinoma with cirrhosis. World J Gastroenterol. 2004;10:1292-1296. [PubMed] [Cited in This Article: ] |
| 67. | Bahde R, Kebschull L, Stöppeler S, Zibert A, Siaj R, Hölzen JP, Minin E, Schmidt HH, Spiegel HU, Palmes D. Role of angiotensin-1 receptor blockade in cirrhotic liver resection. Liver Int. 2011;31:642-655. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 68. | Spira G, Mawasi N, Paizi M, Anbinder N, Genina O, Alexiev R, Pines M. Halofuginone, a collagen type I inhibitor improves liver regeneration in cirrhotic rats. J Hepatol. 2002;37:331-339. [PubMed] [Cited in This Article: ] |
| 69. | Iozzo RV, San Antonio JD. Heparan sulfate proteoglycans: heavy hitters in the angiogenesis arena. J Clin Invest. 2001;108:349-355. [PubMed] [Cited in This Article: ] |
| 70. | Goldshmidt O, Yeikilis R, Mawasi N, Paizi M, Gan N, Ilan N, Pappo O, Vlodavsky I, Spira G. Heparanase expression during normal liver development and following partial hepatectomy. J Pathol. 2004;203:594-602. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 36] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 71. | Carmel J, Arish A, Shoshany G, Baruch Y. Heparanase accelerates the proliferation of both hepatocytes and endothelial cells early after partial hepatectomy. Exp Mol Pathol. 2012;92:202-209. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 72. | Ohayon O, Mawasi N, Pevzner A, Tryvitz A, Gildor T, Pines M, Rojkind M, Paizi M, Spira G. Halofuginone upregulates the expression of heparanase in thioacetamide-induced liver fibrosis in rats. Lab Invest. 2008;88:627-633. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 73. | Gnainsky Y, Spira G, Paizi M, Bruck R, Nagler A, Genina O, Taub R, Halevy O, Pines M. Involvement of the tyrosine phosphatase early gene of liver regeneration (PRL-1) in cell cycle and in liver regeneration and fibrosis effect of halofuginone. Cell Tissue Res. 2006;324:385-394. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 74. | Peng Y, Du K, Ramirez S, Diamond RH, Taub R. Mitogenic up-regulation of the PRL-1 protein-tyrosine phosphatase gene by Egr-1. Egr-1 activation is an early event in liver regeneration. J Biol Chem. 1999;274:4513-4520. [PubMed] [Cited in This Article: ] |
| 75. | Mueller L, Broering DC, Meyer J, Vashist Y, Goettsche J, Wilms C, Rogiers X. The induction of the immediate-early-genes Egr-1, PAI-1 and PRL-1 during liver regeneration in surgical models is related to increased portal flow. J Hepatol. 2002;37:606-612. [PubMed] [Cited in This Article: ] |
| 76. | Werner SR, Lee PA, DeCamp MW, Crowell DN, Randall SK, Crowell PL. Enhanced cell cycle progression and down regulation of p21(Cip1/Waf1) by PRL tyrosine phosphatases. Cancer Lett. 2003;202:201-211. [PubMed] [Cited in This Article: ] |
| 77. | Wang J, Kirby CE, Herbst R. The tyrosine phosphatase PRL-1 localizes to the endoplasmic reticulum and the mitotic spindle and is required for normal mitosis. J Biol Chem. 2002;277:46659-46668. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 92] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 78. | Nissen NN, Martin P. Hepatocellular carcinoma: the high-risk patient. J Clin Gastroenterol. 2002;35:S79-S85. [PubMed] [Cited in This Article: ] |
| 79. | Wu SD, Ma YS, Fang Y, Liu LL, Fu D, Shen XZ. Role of the microenvironment in hepatocellular carcinoma development and progression. Cancer Treat Rev. 2012;38:218-225. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 77] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 80. | Okita K, Sakaida I, Hino K. Current strategies for chemoprevention of hepatocellular carcinoma. Oncology. 2002;62 Suppl 1:24-28. [PubMed] [Cited in This Article: ] |
| 81. | Okuda K. Hepatocellular carcinoma. J Hepatol. 2000;32:225-237. [PubMed] [Cited in This Article: ] |
| 82. | Menke A, Adler G. TGFbeta-induced fibrogenesis of the pancreas. Int J Gastrointest Cancer. 2002;31:41-46. [PubMed] [Cited in This Article: ] |
| 83. | Go VL, Gukovskaya A, Pandol SJ. Alcohol and pancreatic cancer. Alcohol. 2005;35:205-211. [PubMed] [Cited in This Article: ] |
| 84. | Maisonneuve P, Lowenfels AB. Chronic pancreatitis and pancreatic cancer. Dig Dis. 2002;20:32-37. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 66] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 85. | Nagler A, Ohana M, Shibolet O, Shapira MY, Alper R, Vlodavsky I, Pines M, Ilan Y. Suppression of hepatocellular carcinoma growth in mice by the alkaloid coccidiostat halofuginone. Eur J Cancer. 2004;40:1397-1403. [PubMed] [Cited in This Article: ] |
| 86. | Taras D, Blanc JF, Rullier A, Dugot-Senant N, Laurendeau I, Bièche I, Pines M, Rosenbaum J. Halofuginone suppresses the lung metastasis of chemically induced hepatocellular carcinoma in rats through MMP inhibition. Neoplasia. 2006;8:312-318. [PubMed] [Cited in This Article: ] |
| 87. | Spector I, Honig H, Kawada N, Nagler A, Genin O, Pines M. Inhibition of pancreatic stellate cell activation by halofuginone prevents pancreatic xenograft tumor development. Pancreas. 2010;39:1008-1015. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 88. | Spector I, Zilberstein Y, Lavy A, Nagler A, Genin O, Pines M. Involvement of host stroma cells and tissue fibrosis in pancreatic tumor development in transgenic mice. PLoS One. 2012;7:e41833. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 89. | Abramovitch R, Dafni H, Neeman M, Nagler A, Pines M. Inhibition of neovascularization and tumor growth, and facilitation of wound repair, by halofuginone, an inhibitor of collagen type I synthesis. Neoplasia. 1999;1:321-329. [PubMed] [Cited in This Article: ] |
| 90. | Gavish Z, Pinthus JH, Barak V, Ramon J, Nagler A, Eshhar Z, Pines M. Growth inhibition of prostate cancer xenografts by halofuginone. Prostate. 2002;51:73-83. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 53] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 91. | Gross DJ, Reibstein I, Weiss L, Slavin S, Dafni H, Neeman M, Pines M, Nagler A. Treatment with halofuginone results in marked growth inhibition of a von Hippel-Lindau pheochromocytoma in vivo. Clin Cancer Res. 2003;9:3788-3793. [PubMed] [Cited in This Article: ] |
| 92. | Pinthus JH, Sheffer Y, Nagler A, Fridman E, Mor Y, Genina O, Pines M. Inhibition of Wilms tumor xenograft progression by halofuginone is accompanied by activation of WT-1 gene expression. J Urol. 2005;174:1527-1531. [PubMed] [Cited in This Article: ] |
| 93. | Sheffer Y, Leon O, Pinthus JH, Nagler A, Mor Y, Genin O, Iluz M, Kawada N, Yoshizato K, Pines M. Inhibition of fibroblast to myofibroblast transition by halofuginone contributes to the chemotherapy-mediated antitumoral effect. Mol Cancer Ther. 2007;6:570-577. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 43] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 94. | Barron L, Wynn TA. Fibrosis is regulated by Th2 and Th17 responses and by dynamic interactions between fibroblasts and macrophages. Am J Physiol Gastrointest Liver Physiol. 2011;300:G723-G728. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 184] [Cited by in F6Publishing: 196] [Article Influence: 15.1] [Reference Citation Analysis (0)] |