Published online Jan 28, 2014. doi: 10.3748/wjg.v20.i4.1119
Revised: July 31, 2013
Accepted: August 16, 2013
Published online: January 28, 2014
Processing time: 278 Days and 19.6 Hours
A 63-year-old woman was referred to our hospital for further examination because of an incidental finding of early gastric cancer. Endoscopic submucosal dissection (ESD) was successfully performed for complete resection of the tumor. On the first post-ESD day, the patient suddenly complained of abdominal pain after an episode of vomiting. Abdominal computed tomography (CT) showed delayed perforation after ESD. The patient was conservatively treated with an intravenous proton pump inhibitor and antibiotics. On the fifth post-ESD day, CT revealed a gastric wall abscess in the gastric body. Gastroscopy revealed a gastric fistula at the edge of the post-ESD ulcer, and pus was found flowing into the stomach. An intradrainage stent and an extradrainage nasocystic catheter were successfully inserted into the abscess for endoscopic transgastric drainage. After the procedure, the clinical symptoms and laboratory test results improved quickly. Two months later, a follow-up CT scan showed no collection of pus. Consequently, the intradrainage stent was removed. Although the gastric wall abscess recurred 2 wk after stent removal, it recovered soon after endoscopic transgastric drainage. Finally, after stent removal and oral antibiotic treatment for 1 mo, no recurrence of the gastric wall abscess was found.
Core tip: In this report, we describe for the first time a case in which a gastric wall abscess caused by delayed perforation after endoscopic submucosal dissection was conservatively treated with endoscopic drainage via the gastric lumen and antibiotics.
- Citation: Dohi O, Dohi M, Inoue K, Gen Y, Jo M, Tokita K. Endoscopic transgastric drainage of a gastric wall abscess after endoscopic submucosal dissection. World J Gastroenterol 2014; 20(4): 1119-1122
- URL: https://www.wjgnet.com/1007-9327/full/v20/i4/1119.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i4.1119
Endoscopic submucosal dissection (ESD), a technique developed in Japan, is associated with a high success rate for en-bloc resection in early gastric cancer (EGC). This procedure is associated with a low risk of lymph node metastasis, but a high risk of such complications as perforation. The risk of perforation during ESD is approximately 4%[1]. In contrast, delayed perforation is a rare complication, that occurs 1 to 2 d after the procedure and often requires emergency surgery[2,3]. One study reported that such perforations occurred in 6 (0.5%) out of 1159 consecutive patients with 1329 EGCs who underwent ESD[2]. Another report indicated two cases (0.43%) of delayed perforation occurring after the completion of ESD for 468 gastric noninvasive gastric neoplasias including EGCs and gastric adenomas[3]. In a few cases, however, delayed perforations were treated with conservative therapy instead of surgery[4-6]. A gastric wall abscess is an uncommon suppurative infection of the stomach. The prognosis of gastric wall abscess has recently improved because of the use of endoscopic drainage techniques and antibiotics[7-10]. Here, we report a case in which a patient developed a gastric wall abscess after delayed perforation caused by ESD for EGC. Surgery was avoided in this case, and the patient was conservatively treated with endoscopic transgastric drainage and antibiotics.
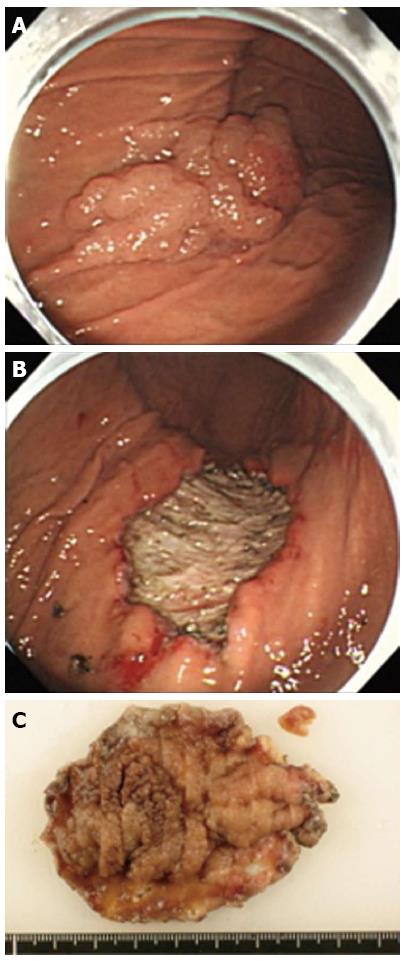
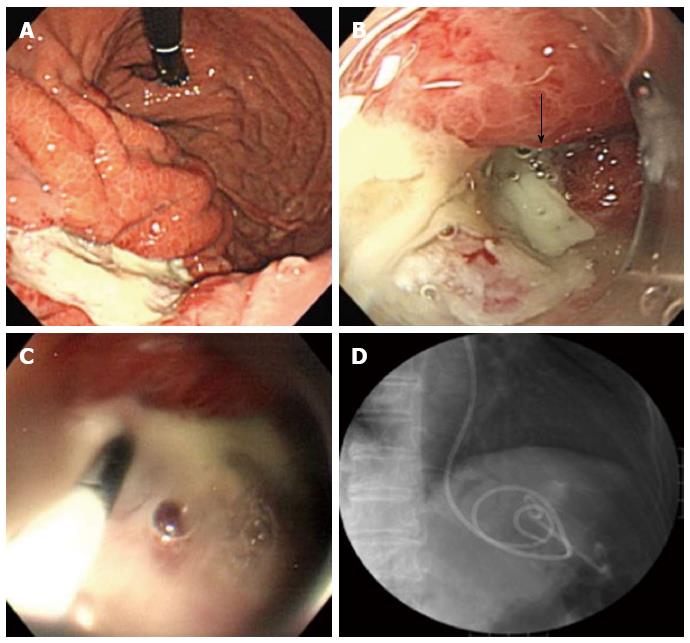
A 63-year-old woman with suspected EGC on the gastric body based on gastroscopy, was referred to our hospital for further examination. The patient’s medical history and laboratory test results were unremarkable. A 65-mm-wide I+IIa lesion was localized in the greater curvature of the lower part of the gastric body (Figure 1A). Examination of biopsy specimens confirmed a well-differentiated adenocarcinoma. No abnormalities were detected upon abdominal computed tomography (CT). The lesion was safely and completely removed en-bloc with ESD (Figure 1B), using an endoscope (GIF-Q260J, Olympus, Tokyo, Japan), an electrosurgical generator (VIO 300D, Erbe Co., Tübingen, Germany), a needle-knife (KD-10Q-1, Olympus, Tokyo, Japan), and an insulated-tip (IT) knife 2 (KD-611 L, Olympus, Tokyo, Japan). The size of the mucosa that was resected en-bloc was 75 mm × 60 mm (length × width), including 65 mm × 40 mm of the cancer lesion (Figure 1C). On the first post-ESD day, the patient suddenly complained of abdominal pain after an episode of vomiting. Initial enhanced abdominal CT showed a portion of the thickened gastric wall and a small amount of free air and ascites around the stomach (Figure 2A). The patient was diagnosed with delayed perforation after ESD. Because the CT revealed a small amount of free air and ascites, we expected the delayed perforation to be very small, and we did not perform emergency endoscopy. The patient was treated with a regimen that comprised fasting, an intravenous proton pump inhibitor, and the antibiotic cefotiam (CTM 3g/d). On the fifth post-ESD day, despite the continued absence of abdominal pain, the patient was febrile. Abdominal T2-weighted magnetic resonance imaging (MRI) showed a moderately high-intensity tumor with niveau formation, 5 cm in size, located in the posterior wall of the fundus of the stomach (Figure 2B). The images demonstrated a gastric wall abscess in the gastric body. Gastroscopic reexamination revealed a smooth, elevated lesion shaped like a submucosal tumor at the greater curvature of the upper body on the oral side of the post-ESD ulcer (Figure 3A). A gastric fistula was found at the edge of the post-ESD ulcer, and pus was found to flow into the stomach (Figure 3B). An endoscopic retrograde cholangiopancreatography (ERCP) catheter (MTW Endoskopie, Wesel, Germany) was inserted through the fistula into the abscess. Then, two 0.035-inch guidewires (VisiGlide; Olympus, Tokyo, Japan) were inserted over the catheter into the abscess. Finally, a 7.0-Fr double pigtail stent (Zimmon Biliary Stent, Cook Japan, Tokyo, Japan) for the internal fistula and a 7.5-Fr nasocystic catheter (Flexima ENBD Catheter, Boston Scientific Japan, Tokyo, Japan) for the external fistula were successfully inserted into the abscess using the double-wire technique for endoscopic transgastric drainage (Figure 3C). Five days later, the abscess contents had cleared, the nasocystic catheter was removed, and the stent was left in to allow continued drainage. A week after the procedure, the patient was released from the hospital. At discharge, she was not prescribed any antibiotics and was scheduled to be followed up at our hospital. When a follow-up CT scan conducted 2 mo later showed the absence of pus collection, the intradrainage stent was removed. Two weeks after the intradrainage stent removal, the patient developed fever and epigastralgia. CT revealed a recurrence of the gastric wall abscess in the gastric body. Gastroscopy revealed a gastric fistula in the center of the post-ESD ulcer scar. As before, an ERCP catheter (MTW Endoskopie, Wesel, Germany) was inserted through the fistula into the abscess. Endoscopic transgastric drainage was repeated by inserting the intradrainage stent and the extradrainage catheter into the abscess. A few days later, the abscess contents had cleared, and the nasocystic catheter was removed. Two months later, a follow-up CT scan showed no collection of pus. The intradrainage stent was then removed, and an oral antibiotic (amoxicillin 750 mg/d) was administered for 1 mo. A follow-up CT scan conducted 1 mo later revealed no abnormal findings. At the 24-mo follow-up examination, the patient was asymptomatic, and the abdominal CT scan showed no abnormalities.
The mechanism underlying delayed perforation is thought to involve electrical cautery during submucosal dissection or repeated coagulation that causes ischemic changes in the gastric wall and results in necrosis[3]. The shape of the delayed perforation was round, and the color of the surrounding muscle layer was whitish, suggesting necrosis of the muscle layer related to the delayed perforation[6]. In our case, necrosis of the muscle layer by electrical cautery combined with an increase in the abdominal pressure caused by vomiting may have led to the perforation because its location was coincident with the lesion coagulated with electrical cautery; moreover, the patient had suddenly experienced peritoneal irritation after vomiting. Emergency surgery was not required in our case because the peritonitis was localized and improved with conservative antibiotics treatment.
Until recently, surgical drainage using antibiotics was the recommended treatment for a gastric wall abscess. However, surgical therapy has been replaced by technically advanced radiologic and endoscopic interventions. Recent studies have reported that the prognosis of gastric wall abscess has improved because of the introduction of endoscopic drainage with or without antibiotics[7-11]. To our knowledge, this is the first report of a case in which a gastric wall abscess caused by delayed perforation after ESD was treated with endoscopic drainage via the gastric lumen and antibiotics. Because there was a fistula between the abscess and the post-ESD ulcer, endoscopic transgastric drainage was easily performed. A pigtail catheter was left in the stomach for 2 mo to allow additional drainage of the purulent fluid. Although the ESD ulcer was almost healed, the gastric wall abscess recurred because of a residual fistula after stent removal. Therefore, it was necessary to continue antibiotic treatment until the fistula closed. Although delayed perforations after ESD can be conservatively treated, endoscopists must keep in mind that a gastric wall abscess may develop and that it is necessary to perform the procedure carefully.
P- Reviewers: Grassi R, Shi RH, Yoshi N S- Editor: Wen LL L- Editor: A E- Editor: Zhang DN
| 1. | Gotoda T, Yamamoto H, Soetikno RM. Endoscopic submucosal dissection of early gastric cancer. J Gastroenterol. 2006;41:929-942. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 485] [Cited by in F6Publishing: 489] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 2. | Hanaoka N, Uedo N, Ishihara R, Higashino K, Takeuchi Y, Inoue T, Chatani R, Hanafusa M, Tsujii Y, Kanzaki H. Clinical features and outcomes of delayed perforation after endoscopic submucosal dissection for early gastric cancer. Endoscopy. 2010;42:1112-1115. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 80] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 3. | Kato M, Nishida T, Tsutsui S, Komori M, Michida T, Yamamoto K, Kawai N, Kitamura S, Zushi S, Nishihara A. Endoscopic submucosal dissection as a treatment for gastric noninvasive neoplasia: a multicenter study by Osaka University ESD Study Group. J Gastroenterol. 2011;46:325-331. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 88] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 4. | Onozato Y, Iizuka H, Sagawa . A case report of delayed perforation due to endoscopic submucosal dissection (ESD) for early gastric cancer. Progr Dig Endosc. 2006;68:114-115. [Cited in This Article: ] |
| 5. | Hirasawa T, Yamamoto Y, Okada K. A case of the delayed perforation due to endoscopic submucosal dissection for the gastric cancer of the residual stomach. Progr Dig Endosc. 2009;74:52-53. [Cited in This Article: ] |
| 6. | Ikezawa K, Michida T, Iwahashi K, Maeda K, Naito M, Ito T, Katayama K. Delayed perforation occurring after endoscopic submucosal dissection for early gastric cancer. Gastric Cancer. 2012;15:111-114. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 7. | Choong NW, Levy MJ, Rajan E, Kolars JC. Intramural gastric abscess: case history and review. Gastrointest Endosc. 2003;58:627-629. [PubMed] [Cited in This Article: ] |
| 8. | Will U, Masri R, Bosseckert H, Knopke A, Schönlebe J, Justus J. Gastric wall abscess, a rare endosonographic differential diagnosis of intramural tumors: successful endoscopic treatment. Endoscopy. 1998;30:432-435. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 18] [Article Influence: 0.7] [Reference Citation Analysis (1)] |
| 9. | Eley A, Richards HG. A case of localized gastric abscess. Br J Surg. 1959;47:97-98. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Marcos WC, Petrini BG, Xavier RL, Starling RM, Couto JC, Ribeiro GJ. Gastric wall abscess--an uncommon condition treated by an alternative form. Clinics (Sao Paulo). 2010;65:819-821. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Yang CW, Yen HH. Endoscopic sonography in the diagnosis and treatment of a gastric wall abscess: a case report and review of the literature. J Clin Ultrasound. 2012;40:105-108. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |