Published online Oct 21, 2014. doi: 10.3748/wjg.v20.i39.14455
Revised: March 18, 2014
Accepted: June 14, 2014
Published online: October 21, 2014
Processing time: 280 Days and 22.5 Hours
AIM: To investigate the mechanism leading to perioperative mortality in patients undergoing major liver resection and presenting with metabolic disorders.
METHODS: The link between Metabolic Syndrome and non-alcoholic fatty liver disease is currently demonstrated. Various metabolic disorders and the Metabolic Syndrome (the association of ≥ 3 metabolic disorders) have been recently described as a risk factor of perioperative mortality in major liver resection. Patients who passed away during perioperative course of major liver resection and presenting with the association of ≥ 2 metabolic disorders without any other known cause of liver disorders were reviewed.
RESULTS: From January 2001 to May 2010 in a tertiary centre, ten patients presenting with ≥ 2 metabolic disorders without any other known cause of liver disorders died during perioperative course of major liver resection. The same four-consecutive-steps sequence of events occurred, including jaundice. The analysis of this series suggested a rapidly deteriorating congestive liver resulting in an increased portal hypertension leading to hepatorenal syndrome and lately to multiorgan failure (mimicking septic collapse) as the mechanism leading to exitus. The acute portal hypertension is mainly related to the surgical procedure. The chronic portal hypertension is indeterminate. Patients with ≥ 2 metabolic disorders should be considered as potentially presenting with portal hypertension possibly evolving towards hepatorenal syndrome; thus, they should be considered as having a high perioperative risk and should be carefully evaluated before undergoing major liver resection.
CONCLUSION: As fibrosis was not present or marginal in liver specimens, the real cause of portal hypertension in patients with multiple metabolic disorders should be investigated with further studies.
Core tip: Patients who passed away during perioperative course of major liver resection and presenting with the association of ≥ 2 metabolic disorders without any other known cause of liver disorders were reviewed. The same four-consecutive-steps sequence of events occurred, including jaundice/ascites, renal failure, hemodynamic collapse with inotrope use and death. The analysis suggested a rapidly deteriorating congestive liver resulting in an increased portal hypertension leading to hepatorenal syndrome and lately to multiorgan failure as the mechanism leading to exitus. As fibrosis was marginal in liver specimens, cause of portal hypertension in patients with multiple metabolic disorders should be investigated with further studies.
- Citation: Zarzavadjian Le Bian A, Costi R, Sbai-Idrissi MS, Smadja C. Liver resection and metabolic disorders: An undescribed mechanism leading to postoperative mortality. World J Gastroenterol 2014; 20(39): 14455-14462
- URL: https://www.wjgnet.com/1007-9327/full/v20/i39/14455.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i39.14455
Metabolic syndrome (MS)[1] consists in the association of three (or more) criteria among increased waist circumference, increased fasting glucose, arterial hypertension, increased triglycerides and reduced high-density lipoprotein (HDL) cholesterol. With a rising incidence in Western countries[2] and a demonstrated link to non-alcoholic fatty liver disease (NAFLD)[3-5] and hepatocellular carcinoma[3,6], MS has become a major concern in the field of Hepatology.
Aside from that, and in spite of a trend towards aggressive strategies, advances in hepatic surgical care have led to a significant improvement in perioperative outcome after liver resection. This mortality has gradually decreased from 8.6%-14.7%[7,8] to 3.1%-8.7%[9-14] in the last three decades. Recently, we reported an unusually high perioperative mortality after right hepatectomy in patients with two or more metabolic disorders[15]. Interestingly, mortality was related to a recurrent sequence leading to multiorgan failure (MOF). The pathogenetic mechanism of this MOF remains unclear.
As we noted this recurrent sequence of events finally leading to death, we hypothesized that a keen analysis of surgical procedures, peri- and postoperative outcomes, laboratory examinations and medical managements may possibly allow for a better clinical understanding of the response of patients presenting with the association of ≥ 2 metabolic disorders (or metabolic disorders association, MDA) and undergoing major hepatic resection. Here we present a retrospective analysis of consecutive patients presenting with two or more metabolic disorders, undergoing major liver resection and who postoperatively passed away.
From January 2001 to May 2010, a retrospective analysis was performed in the Service de Chirurgie Hépatobiliaire of Antoine Béclère Hospital, an academic tertiary centre. All consecutive patients presenting with at least two metabolic disorders, undergoing major liver resection and who deceased during the perioperative course, were included in the study. Metabolic disorders included[1]: increased waist circumference, increased fasting glucose (fasting plasma glucose > 5.5 mmol/L), arterial hypertension (> 130/85 mmHg), increased triglycerides (≥ 1.7 mmol/L) and reduced HDL cholesterol (< 1 mmol/L in males or < 1.3 mmol/L in females).
The presence of two or more metabolic disorders is defined as MDA, whereas the presence of three or more metabolic disorders is defined as MS, in accordance with previous publications[6,15]. Major liver resection is defined as the resection of three or more Couinaud’s liver segments[16]. Renal insufficiency is defined as glomerular filtration rate (GFR) < 50 mL/min (Cockroft formula[17]) or oliguria without obstructive process; oliguria is defined as an urine ouput < 500 mL/d (Measure of Creatinine using Creatinine Reagents OSR6178 and OSR 6678 from Beckman Coulter®). Postoperative hepatocellular insufficiency is defined as prothrombin ratio (PR) < 50% and serum total bilirubin (STB) > 50 μmol/L, according to the fifty-fifty score[18] (measure of prothrombin time using neoplastine CI from Beckman Coulter®); jaundice is defined as STB > 50 μmol/L (measure of total bilirubin using total bilirubin reagents OSR6112, OSR 6212 and OSR 6512 from Beckman Coulter® and measure of direct bilirubin using direct bilirubin reagents OSR6111, OSR 6211 and OSR 6511). Arterial hypotension is defined as systolic blood pressure < 90 mmHg or diastolic < 60 mmHg.
All patients presenting with any of the following conditions were excluded from the analysis: positive serology for hepatitis B or C virus, autoimmune liver diseases (serum autoantibodies), alcohol abuse (alcoholic consumption > 40 g/d), or genetic hemochromatosis (positive genetic testing or hepatic iron index > 1.9).
Parenchymal transection was performed using “the clamp-crushing technique” or electrothermal bipolar vessel sealing system; when required, vessels and bile ducts were ligated and divided intra-parenchymally or managed by mechanical stapling.
Collected data included: Preoperative data, including age, gender, body mass index (BMI), American Society of Anesthesiology score, metabolic disorders, coronary disease, renal insufficiency, tobacco/alcohol consumption, and preoperative treatment (chemotherapy/embolization); Intraoperative management, including type of procedure, number and type of resected hepatic veins, blood loss, pedicular clamping and its duration, hepatic vascular exclusion, procedure duration, associated procedures; Perioperative outcome, including any postoperative events and morbidity, need of surgical/radiologicaly-guided drainage, intensive care unit (ICU) stay, postoperative day of death, cause of death; Histopathology of tumor(s) and adjacent parenchyma, specimen weight.
Steatosis was considered absent when less than 5% of hepatocytes were involved, otherwise it was defined as mild (5% to 33%), moderate (34% to 66%) or severe (> 66%). Morphologically, steatosis was described as macrovacuolar, microvacuolare or mixed. Fibrosis was staged according to Kleiner et al[19]: stage 0 (no fibrosis), stage 1 (zone 3 or perisinusoidal and portal fibrosis), stage 2 (perisinusoidal and portal fibrosis without bridging), stage 3 (bridging fibrosis) or stage 4 (cirrhosis) (stage 4). Lobular inflammation was reported.
The patients have been anonymized in order to meet Ethical and Legal obligations. The study was cleared by IRB.
From January 2001 to May 2010, 27 patients out of 769 undergoing liver resection deceased perioperatively (mortality rate: 3.5%). Out of 27 deceased patients, 24 patients underwent major liver resection. Out of 24 patients who underwent a major liver resection and perioperatively passed away, ten patients had no underlying cause of liver disease except only MDA/MS. These ten patients are the studied sample.
Patients characteristics are presented in Table 1, metabolic disorders are reported in Table 2. Prior surgical procedure, no patient had known cirrhosis/renal insufficiency; coronaropathy (without contraindication to hepatectomy) was reported in three patients. No patient declared an alcohol consumption exceeding 20 g/d; only one patient smoked (60 cigarettes/d).
| Patients characteristics | Value |
| Age (range) (yr) | 67.7 (52.1-79) |
| ASA score: Median (range) | 2 (2-3) |
| Body mass index: Median (range) (kg/m²) | 26.1 (20.7-38.6) |
| Metabolic disorders: | |
| 2 disorders | 2 |
| 3 disorders | 4 |
| 4 disorders | 1 |
| 5 disorders | 3 |
| Hepatic tumor histopathology: | |
| Hepatocellular carcinoma | 4 |
| Colorectal cancer metastasis | 3 |
| Intrahepatic cholangiocarcinoma | 2 |
| Muco-secreting adenocarcinoma metastasis | 1 |
| Patient | Gender | Increased waist circum-ference | Arterial hyper-tension | Increased fasting glucose | Decreased HDL choleste-rol/increased trigly-cerides | Procedure: Segments and wedge resections3 | First day in ICU | First day of jaundice or ascites | First day of renal failure or oliguria/first day of hemofil--tration | First day of inotropes use | Day of death |
| Patient 11 | Male | Yes | Yes | Yes | No / No | V, VI, VII, VIII | 1 | 2 | 1/6 | 1 | 7 |
| Patient 2 | Male | Yes | Yes | Yes | Yes/Yes | V, VI, VII, VIII | 22 | 4 | 2/6 | 1 | 10 |
| Patient 3 | Male | Yes | Yes | Yes | Yes/Yes | V, VI, VII, VIII | 12 | 6 | 4/14 | 14 | 15 |
| Patient 4 | Male | Yes | Yes | Yes | Yes/Yes | Partial IV, V, VI, VII, VIII | 3 | 3 | 3/9 | 8 | 18 |
| Patient 5 | Male | No | Yes | Yes | Yes/No | V, VI, VII, VIII | 12 | 7 | 4/6 | 4 | 25 |
| Patient 6 | Male | No | Yes | No | Yes/No | V, VI, VII, VIII | 7 | 7 | 8/no | 18 | 29 |
| Patient 7 | Male | Yes | No | No | Yes/Yes | Partial IV, V, VI, VII, VIII | 12 | 2 | 10/no | 1 | 35 |
| Patient 8 | Male | Yes | Yes | No | No/No | II, III, VII | 12 | 3 | 5/8 | 1 | 38 |
| 3 Wedge resections: IV, V, VIII | |||||||||||
| Patient 9 | Male | Yes | Yes | No | Yes/Yes | V, VI, VII, VIII | 8 | 6 | 8/22 | 8 | 43 |
| Patient 10 | Lady | No | Yes | Yes | Yes/No | Partial IV, V, VI, VII, VIII, | 12 | 4 | 4/9 | 10 | 52 |
| Wedge of III | |||||||||||
| Total or median (range) | 9 M/1 F | 7 | 9 | 6 | 8/5 | N/A | 1 | 4 | 4 (1-10)/8.5 (6-22) | 6 | 27 |
| (1-8) | (2-7) | (1-18) | (7-52) |
Considering preoperative usual blood tests, median PR was 87% (range: 79%-100%), median creatininemia was 92.5 μmol/L (range: 56-117 μmol/L), median GFR was 71.85 mL/min (range: 45.1-126.8 mL/min, with only one patient < 50 mL/min) and median STB was 13 μmol/L (range: 5-51 μmol/L, with only one patient > 20 μmol/L -a stented hilar cholangiocarcinoma).
Surgical decisions were made during multidisciplinary team meetings. Two patients received chemotherapy 4 to 6 wk prior the liver resection: Leucovorin, fluorouracil and irinotecan in one case, and bevacizumab, leucovorin, fluorouracil and oxaliplatin and cetuximab in the other.
In all patients, preoperative volumetric liver evaluation showed a remnant liver > 30% of total volume (in four patients, after a right portal branch embolisation performed three to five weeks preoperatively).
Surgical procedures are summarized in Table 2. The surgical procedure was the first hepatic resection in all but one patient (who previously underwent a left lobe wedge resection[16]). A right portal branch thrombus was removed in one patient. A middle hepatic vein resection was performed in six patients. Intermittent pedicle clamping technique (15-min-ischemia, 5-min-reperfusion) was used in six patients, with a median clamping time of 22 min (range 19-80 min). An hepaticojejunostomy (Roux-en-Y anastomosis) was completed in one patient, without specific complication. A drain was left in place in one patient. Median blood loss was 1000 mL (range: 300-3000 mL). An inferior vena cava injury was reported during a laparoscopic right hepatectomy, requiring a conversion to laparotomy.
Median specimen weight was 950 g (range: 620-1380 g). Median liver steatosis percentage was 10% (range 0%-60%). Steatosis was evaluated as absent, mild and moderate, in one, six and one patient, respectively (in two patients, steatosis was not evaluated). Steatosis was defined as macrovacuolar, “mixed” and microvacuolare, in seven patients, two patients and no patient, respectively.
Considering fibrosis, stage 0, 1, 2 and 4 were found in six, one, one and two patients, respectively. Unspecific inflammatory infiltration, without Mallory bodies, was reported in six patients.
Postoperative course is summarized in Table 2. All patients presented with four common steps.
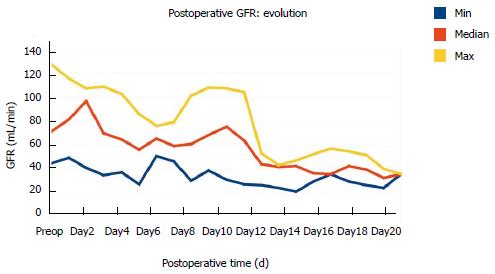
Acute renal failure: Acute renal failure, associated with oligoanuria and non-responsiveness to volume expansion: Patients’ GFR evolution before hemodialysis is reported in Figure 1 (hemodialysis was used in eight patients).
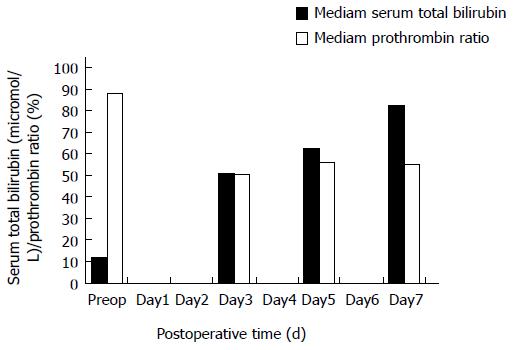
Jaundice/ascitis: Jaundice/ascitis without any sign of hepatic insufficiency, such as hepatic encephalopathy (inverted sleep-wake pattern, confusion, flapping tremors) and with PR stably > 50%. STB and PR evolution is reported in Figure 2. All patients had several abdominal imaging (including CT-scan and US-scan): no investigation demonstrated any biliary dilatation/obstruction, nor portal/hepatic vein thrombosis.
Arterial hypotension: Arterial hypotension, non-responsive to volume expansion and (later) inotropes administration (noradrenalin). In all patients, arterial hypotension was attributed to sepsis of intra-abdominal origin in spite of repeated bacteriological examinations on ascites/collection resulted negative in eight patients (Gram negative bacteria was isolated in an intra-abdominal collection/hematoma in one patient, Candida Albicans and Coagulase-negative Staphylococcus were isolated in ascites sampling in the other). Large spectrum antibiotherapy and antifungal treatment were administered to all patients; after massive repletion, owing to hypotension persistency, all patients received noradrenalin, without effect on arterial hypotension. Prior decision to discontinue the treatment, noradrenalin reached a median maximal dose of 3.5 mg/h (range 1-20 mg/h).
Late MOF and death: In all patients, hepatic failure associated with hepatic encephalopathy was observed as a pre-mortem step. Cardio-circulatory collapse was the etiology of death.
During the course of the study, in hospital postoperative mortality rate of liver resection reached 3.5% in the department. Among these deceased patients, 89% underwent major liver resection and 37% underwent major liver resection and did present without any underlying cause of liver disease except MDA. The observation of a recurrent sequence of events ending up with death in an unusually high number of patients compelled an analysis.
First, the cause of death in this small series is not clear. No bleeding, no infection/sepsis (after retrospective overview), no cardiac arrest, no pulmonary embolism or any other cause of death was reported as cause of death; all patients experienced the same course of events (Figure 2) starting with early renal failure associated with jaundice/ascites, then arterial hypotension refractory to volume expansion/inotropes administration, and finally MOF leading to death. Repeated abdominal imaging did not show biliary dilatation or obstruction (causing jaundice) or portal/hepatic vein thrombosis. Although jaundice/ascites was an early event, interestingly enough, patients did not present with any alteration of hepatic function until the very end-stage of the disease (Figure 2); PR was stable around 50% and no clinical symptom of hepatic failure was reported. Such a dissociation between the clinical picture (jaundice/ascitis) and liver function tests is not usual after major hepatic resection with fatal outcome[18]. Yet, jaundice does not explain such a sequence of events and mortality.
Although the whole course of events is evidently triggered by major liver resection, and the occurrence of early jaundice and late hepatic failure seems to point out liver as the responsible of fatal outcome, the pathogenic mechanism remains unclear. Indeed, considering demonstrated risk factors of perioperative mortality after major liver resection, none seems to be related to the observed mechanism: first, in no case remnant liver volume was inferior to 30%[20]; second, no patient presented with any underlying liver disease except NAFLD; third, only two patients out of ten were found to have cirrhosis (at surgery), whereas the others had no or marginal liver fibrosis (stage 0 to 2); fourth, liver parenchyma showed no specific histopathological alteration in the two patients who had preoperative chemotherapy. By exclusion, it seems that there is only one common, newly identified risk factor for major liver surgery[15]: MDA and MS.
Analyzing the timeline of the mechanism, renal failure occurred concomitantly to jaundice, as the fourth postoperative day was the median day of occurrence for both events (Table 2). No known renal disease was preoperatively identified and preoperative GFR was virtually normal (median 71.85 mL/min, in only one case below 50-45.1 mL/min). Interestingly, renal failure was interpreted as being caused by sepsis. As such, it was treated by volume expansion followed by inotropes’ administration. Since GFR worsened with time (and volume expansion), renal failure seems to meet the definition of hepato-renal syndrome (HRS)[21].
Since HRS is invariably associated with acute portal hypertension, we hypothesized that this latter may have had a pivotal role in determining the escalation from jaundice/ascitis to HRS to MOF and death, in patients presenting a MDA (and possibly NAFLD) undergoing major liver resection. This thesis may be even more intriguing when considering recent literature: on one hand, metabolic disorders and MS are associated with NAFLD[3-5], and NAFLD (steatosis) may be associated with portal hypertension without fibrosis/cirrhosis[22,23]. On the other hand, major liver resections may be associated to increased portal tension[24]. This report may suggest that performing a major resection of a liver exposed to multiple metabolic disorders may cumulatively increase the risk of developing portal hypertension, thus triggering the HRS.
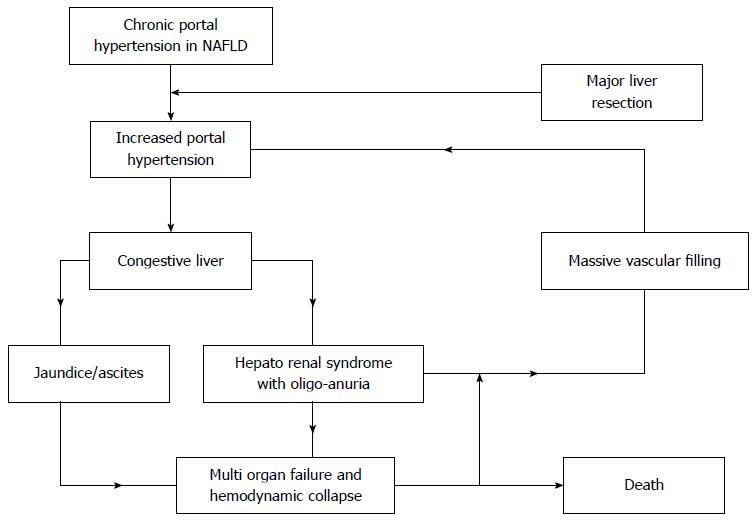
Moreover, interestingly, such an evolution of the clinical picture and laboratory tests of these patients may also remind another condition associated to “acute” portal hypertension: the post-liver transplantation “small-for-size syndrome”, typically occurring after partial liver grafting[25]. Due to the increased vascular inflow to the grafted liver (and a decreased vascular outflow), the transplanted liver is too small compared to the recipient, leading to liver congestion and portal hypertension, potentially resulting in graft failure[25]. Indeed, major liver resection in patients with MDA has the same trigger factors of “small-for-size-syndrome”: a recognized underlying liver alteration (NAFLD), an increased preoperative vascular inflow associated with chronic portal hypertension[22,23] and its postsurgical further increase[24], and a reduction of vascular outflow especially when one or two hepatic veins are removed[26]. Moreover, the fact that, the middle hepatic vein resection in patients affected by MDA undergoing right (extended-right) hepatectomy resulted as related to > 50% mortality[15], somehow corroborates the hypothesis that not only portal hypertension, but also a low outflow may be responsible of liver parenchyma congestion[26] and, secondarily, HRS. We propose an algorithm to resume the mechanism leading patients with MDA undergoing massive liver reduction to death (Figure 3).
Such a hypothesis has practical consequences. First, HRS misinterpretation may lead to massive vascular expansion, as in our series where hypotension was interpreted as resulting from intra-abdominal infection and treated consequently. In our series, the massive vascular expansion probably led to an increase of the vascular retention in the liver, so worsening the congestion and increasing the portal hypertension until HRS and liver failure developed. The most appropriate management should probably have been the depletion instead of the massive repletion, in order to interrupt the sequence of events leading to HRS and, ultimately, to death. Such a management was performed in an eleventh case. This patient presented with HRS (liver/renal failure) after right hepatectomy for colorectal metastases and finally survived. This otherwise healthy, 51-year-old, male patient (GFR of 104 mL/min and no liver alteration at preoperative labs) presented with MS (low HDL-cholesterolemia, arterial hypertension and increased waist circumference). BMI was 28.4 kg/m2. A preoperative chemotherapy (six cycles of fluorouracil + oxaliplatin + bevacizumab) was performed prior the surgical procedure. Intraoperative blood loss was 3100 mL owing to technical difficulty. Histology of the 1730 g-specimen showed stage 1 fibrosis, severe (90%) macrovacuolare steatosis, and an aspecific inflammation. Postoperatively (day 3, 5 and 7), PR was 53%, 73%, 78%, respectively, whereas STB increased (93, 138, 159 μmol/L, respectively). GFR was initially preserved (87 mL/min on day 3, 95.2 on day 5) then the patient developed oliguria. Differently from the other patients and also because he was hemodynamically stable, we opted for a daily hemofiltration with fluids depletion. This treatment was effective, jaundice and ascites decreased before the renal function recovered. After a 53-d hospital stay (including 41 d in ICU) the patient was discharged. At 2-year follow-up, the patient is going well. Although one case obviously does not set a rule, the case of the patient with ongoing HRS/jaundice after right hepatectomy successfully treated using fluids depletion seems to confirm our hypothesis and should be considered when deciding for the most appropriate treatment of such a severe condition.
From an etiological point of view, it is not clear why and how MDA predisposes liver to HRS when undergoing massive resection. Since steatosis and inflammation (and oxydative stress) are the only liver alterations found at histology, their possible role is worth a commentary. Steatosis, which is often associated to NAFLD, varied widely through the current series, as it was absent, mild, moderate, in one, six and one patient, respectively (in two patients it was not measured). As steatosis was not severe in the very majority of patients, the vascular/hepatocellular physiopathology leading to portal hypertension and HRS is still unclear. Such a dissociation between steatosis’ severity and the possible development of portal hypertension and fatal HRS after major liver resection does not support the hypothesis of a major role played by steatosis in the described sequence of events, and has the practical consequence to limit the interest of performing liver biopsy before surgery in order to identify patients at risk. Moreover, even if we admit that also mild steatosis may somehow be a risk factor of developing portal hypertension and HRS in patients presenting with MDA undergoing major liver resection, unfortunately, liver biopsy is ineffective to assess steatosis, as it may vary widely in liver parenchyma[27]. For all these reasons, identifying MDA when collecting patient’s personal history is, at the present state of art, the only possible screening able to identify patients at risk of developing a postoperative acute portal hypertension and HRS after major liver resection.
The relationship between portal hypertension, steatosis and inflammation, and the possible role of this latter in the sequence of events described in our series, may also be discussed. After the association between liver steatosis (including NAFLD) and chronic portal hypertension has been shown[23], this latter has very recently been reported to cause splanchnic and systemic low-grade inflammation in an experimental model[28]. Similarly, a vasculopathy caused by a low-grade, chronic inflammation caused by steatosis-related portal hypertension may be supposed to occur even in human patients (including our series). Such a chronic inflammation, characterized by oxidative stress, increased levels of proinflammatory cytokines and profibrogenic mediators, is seemingly not confined to portal-liver district, but rather related to a systemic vasculopathy, including aortopathy. As proposed by Aller et al[28], the increased aortic reduced-nicotinamide-adenine dinucleotide phosphate [NAD(P)H] oxidase activity could be associated with the production of reactive oxygen species promoting inflammation. If we also consider that oxidative stress mediated by NAD(P)H oxidase has been associated with risk factors for inflammation and atherosclerosis, we can have a rudimental model of how the inflammation process may develop from portal hypertension. Interestingly, in order to increase portal pressure in their experimental model, Aller et al[28] performed a triple portal ligation; this latter is comparable to the surgical portal ligation performed during major liver resection, which is also related to an increase of portal hypertension[24], somehow strengthening the idea that a correlation between the two models exists and that major liver resection may imply a further increase of portal hypertension and play a role in the sequence of events leading to HRS.
Our study actually presents the typical bias of a retrospective analysis performed in a small-sized population; moreover, the main hypothesis is based on the pivotal role played by portal hypertension. Indeed, its presence is deduced by the sudden development of HRS (which implies portal hypertension), but it has not been measured since the series is retrospective and no portal catheterism is routinely performed in our daily practice. Nevertheless, among the strengths of the paper, it should be remarked that the studied sample was highly selected (patients with ≥ 2 metabolic disorders undergoing major liver surgery and concomitantly not presenting any other possible etiology for liver-related disease) from a large series of patients treated in a Tertiary Level University Hospital specialized in hepato-biliary surgery. Also considering the extremely high death-rate of patients entering the sequence of events described above, we definitely believe that ethics compels us to share our findings in order to show a previously unknown risk factor for liver surgery, to identify a more appropriate management and to orient future research.
In conclusion, postoperative mortality after major liver resection in patients presenting with MDA seems to be related to a misunderstood hepatic mechanism, with portal hypertension playing a central role in a vicious circle leading to HRS and, ultimately, to death. When evaluating therapeutic options, patients with MDA should be considered to have a patent portal hypertension potentially leading to HRS when undergoing major liver resection, thus, being related to higher mortality rate. In the future, patients with multiple metabolic disorders undergoing major liver surgery should be considered at higher perioperative risk, portal pressure should be possibly measured and included in the preoperative work up and considered in prospective trials.
Authors studied patients who passed away during perioperative course of major liver resection and presenting with the association of ≥ 2 metabolic disorders without any other known cause of liver disorders were reviewed. The same four-consecutive-steps sequence of events occurred, including jaundice/ascites, renal failure, hemodynamic collapse with inotrope use and death. The analysis suggested a rapidly deteriorating congestive liver resulting in an increased portal hypertension leading to hepatorenal syndrome and lately to multiorgan failure as the mechanism leading to exitus. As fibrosis was marginal in liver specimens, cause of portal hypertension in patients with multiple metabolic disorders should be investigated with further studies.
Non-alcoholic fatty liver disease (NAFLD) and metabolic syndrome are contemporary concerns, their incidences reaching 25% in occidental population. Understanding an occult portal hypertension may have daily application.
The analysis suggested a rapidly deteriorating congestive liver resulting in an increased portal hypertension without major fibrosis leading to hepatorenal syndrome and lately to multiorgan failure as the mechanism leading to exitus. It is the first study demonstrating this clinical portal hypertension.
In spite of a small sample, this demonstration is of major interest in daily hepatology and in order to understand NAFLD.
In spite of a small sample, the topic of the manuscript is important. This demonstration is of major interest in daily hepatology and in order to understand NAFLD. This is an interesting report for the clinical practice. Overall the report appears to be carefully examined and data adequately discussed.
P- Reviewer: Riutta A, Sakabe K S- Editor: Gou SX L- Editor: A E- Editor: Liu XM
| 1. | Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith SC. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640-1645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8720] [Cited by in RCA: 10565] [Article Influence: 660.3] [Reference Citation Analysis (0)] |
| 2. | Fraser A, Longnecker MP, Lawlor DA. Prevalence of elevated alanine aminotransferase among US adolescents and associated factors: NHANES 1999-2004. Gastroenterology. 2007;133:1814-1820. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 267] [Cited by in RCA: 259] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 3. | El-Serag HB, Tran T, Everhart JE. Diabetes increases the risk of chronic liver disease and hepatocellular carcinoma. Gastroenterology. 2004;126:460-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 873] [Cited by in RCA: 893] [Article Influence: 42.5] [Reference Citation Analysis (0)] |
| 4. | Tolman KG, Fonseca V, Dalpiaz A, Tan MH. Spectrum of liver disease in type 2 diabetes and management of patients with diabetes and liver disease. Diabetes Care. 2007;30:734-743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 351] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 5. | El-Serag HB, Everhart JE. Diabetes increases the risk of acute hepatic failure. Gastroenterology. 2002;122:1822-1828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 111] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 6. | Paradis V, Zalinski S, Chelbi E, Guedj N, Degos F, Vilgrain V, Bedossa P, Belghiti J. Hepatocellular carcinomas in patients with metabolic syndrome often develop without significant liver fibrosis: a pathological analysis. Hepatology. 2009;49:851-859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 416] [Cited by in RCA: 423] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 7. | Savage AP, Malt RA. Elective and emergency hepatic resection. Determinants of operative mortality and morbidity. Ann Surg. 1991;214:689-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 63] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 8. | Fortner JG, Kim DK, Maclean BJ, Barrett MK, Iwatsuki S, Turnbull AD, Howland WS, Beattie EJ. Major hepatic resection for neoplasia: personal experience in 108 patients. Ann Surg. 1978;188:363-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 132] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 9. | Jarnagin WR, Gonen M, Fong Y, DeMatteo RP, Ben-Porat L, Little S, Corvera C, Weber S, Blumgart LH. Improvement in perioperative outcome after hepatic resection: analysis of 1,803 consecutive cases over the past decade. Ann Surg. 2002;236:397-406; discussion 406-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1148] [Cited by in RCA: 1080] [Article Influence: 47.0] [Reference Citation Analysis (0)] |
| 10. | Belghiti J, Hiramatsu K, Benoist S, Massault P, Sauvanet A, Farges O. Seven hundred forty-seven hepatectomies in the 1990s: an update to evaluate the actual risk of liver resection. J Am Coll Surg. 2000;191:38-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 800] [Cited by in RCA: 799] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 11. | Asiyanbola B, Chang D, Gleisner AL, Nathan H, Choti MA, Schulick RD, Pawlik TM. Operative mortality after hepatic resection: are literature-based rates broadly applicable? J Gastrointest Surg. 2008;12:842-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 125] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 12. | Karanjia ND, Lordan JT, Quiney N, Fawcett WJ, Worthington TR, Remington J. A comparison of right and extended right hepatectomy with all other hepatic resections for colorectal liver metastases: a ten-year study. Eur J Surg Oncol. 2009;35:65-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 13. | Capussotti L, Polastri R. Operative risks of major hepatic resections. Hepatogastroenterology. 1998;45:184-190. [PubMed] |
| 14. | Pol B, Campan P, Hardwigsen J, Botti G, Pons J, Le Treut YP. Morbidity of major hepatic resections: a 100-case prospective study. Eur J Surg. 1999;165:446-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 77] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 15. | Zarzavadjian Le Bian A, Costi R, Constantinides V, Smadja C. Metabolic disorders, non-alcoholic fatty liver disease and major liver resection: an underestimated perioperative risk. J Gastrointest Surg. 2012;16:2247-2255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 16. | Blumgart LH. Surgery of the Liver, Biliaru Tract, and Pancreas. 4th Edition. Elsevier: Saunders 2006; . |
| 17. | Hickling K, Begg E, Moore ML. A prospective randomised trial comparing individualised pharmacokinetic dosage prediction for aminoglycosides with prediction based on estimated creatinine clearance in critically ill patients. Intensive Care Med. 1989;15:233-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 18. | Paugam-Burtz C, Janny S, Delefosse D, Dahmani S, Dondero F, Mantz J, Belghiti J. Prospective validation of the “fifty-fifty” criteria as an early and accurate predictor of death after liver resection in intensive care unit patients. Ann Surg. 2009;249:124-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 152] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 19. | Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313-1321. [PubMed] |
| 20. | Guglielmi A, Ruzzenente A, Conci S, Valdegamberi A, Iacono C. How much remnant is enough in liver resection? Dig Surg. 2012;29:6-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 248] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 21. | Testino G, Ferro C. Hepatorenal syndrome: a review. Hepatogastroenterology. 2010;57:1279-1284. [PubMed] |
| 22. | Francque S, Wamutu S, Chatterjee S, Van Marck E, Herman A, Ramon A, Jung A, Vermeulen W, De Winter B, Pelckmans P. Non-alcoholic steatohepatitis induces non-fibrosis-related portal hypertension associated with splanchnic vasodilation and signs of a hyperdynamic circulation in vitro and in vivo in a rat model. Liver Int. 2010;30:365-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 23. | Francque S, Verrijken A, Mertens I, Hubens G, Van Marck E, Pelckmans P, Van Gaal L, Michielsen P. Noncirrhotic human nonalcoholic fatty liver disease induces portal hypertension in relation to the histological degree of steatosis. Eur J Gastroenterol Hepatol. 2010;22:1449-1457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 24. | Ueno S, Kobayashi Y, Kurita K, Tanabe G, Aikou T. Effect of prior portosystemic shunt on early hepatic hemodynamics and sinusoids following 84% hepatectomy in dogs. Res Exp Med (Berl). 1995;195:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 25. | Dahm F, Georgiev P, Clavien PA. Small-for-size syndrome after partial liver transplantation: definition, mechanisms of disease and clinical implications. Am J Transplant. 2005;5:2605-2610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 444] [Cited by in RCA: 473] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 26. | Yamamoto H, Maetani Y, Kiuchi T, Ito T, Kaihara S, Egawa H, Itoh K, Kamiyama Y, Tanaka K. Background and clinical impact of tissue congestion in right-lobe living-donor liver grafts: a magnetic resonance imaging study. Transplantation. 2003;76:164-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 27. | El-Badry AM, Breitenstein S, Jochum W, Washington K, Paradis V, Rubbia-Brandt L, Puhan MA, Slankamenac K, Graf R, Clavien PA. Assessment of hepatic steatosis by expert pathologists: the end of a gold standard. Ann Surg. 2009;250:691-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 239] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 28. | Aller MA, de las Heras N, Nava MP, Regadera J, Arias J, Lahera V. Splanchnic-aortic inflammatory axis in experimental portal hypertension. World J Gastroenterol. 2013;19:7992-7999. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 6] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |