Published online Oct 21, 2014. doi: 10.3748/wjg.v20.i39.14371
Revised: June 4, 2014
Accepted: July 11, 2014
Published online: October 21, 2014
Processing time: 207 Days and 23.9 Hours
The peritoneal stromal tissue which provides a rich source of growth factors and chemokines is a favorable environment for tumor proliferation. The pathophysiological mechanism of peritoneal carcinomatosis is an individual sequence consisting of genetic and environmental factors and remains controversial. The natural history of the disease reveals a poor median prognosis of approximately 6 mo; however aggressive surgery and multimodal treatment options can improve oncologic outcomes. Considering peritoneal carcinomatosis as though it is a locoregional disease but not a metastatic process, cytoreductive surgery and and intraperitoneal chemotherapy has been a curative option during recent years. Cytoreductive surgery implies a series of visceral resections and peritonectomy procedures. Although the aim of cytoreductive surgery is to eliminate all macroscopic disease, viable tumor cells may remain in the peritoneal cavity. At that point, intraperitoneal chemotherapy can extend the macroscopic disease elimination to microscopic disease elimination. The successful treatment of peritoneal carcinomatosis requires a comprehensive management plan including proper patient selection, complete resection of all visible disease, perioperative intraperitoneal chemotherapy and postoperative systemic chemotherapy. Surgical and oncologic outcomes are strictly associated with extent of the tumor, completeness of cytoreduction and patient-related factors as well as multidisciplinary management and experience of the surgical team. In this review, pathophysiology and current management of peritoneal carcinomatosis originating from gastrointestinal tumors are discussed according to the latest literature.
Core tip: In this review, pathophysiology and current management of peritoneal carcinomatosis originating from gastrointestinal tumors are discussed according to the latest literature.
- Citation: Terzi C, Arslan NC, Canda AE. Peritoneal carcinomatosis of gastrointestinal tumors: Where are we now? World J Gastroenterol 2014; 20(39): 14371-14380
- URL: https://www.wjgnet.com/1007-9327/full/v20/i39/14371.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i39.14371
The peritoneal surface is a failure site for patients with gastrointestinal tumors. Addition to lymphatic and hematogenous routes of dissemination, transcoelomic spread of tumor cells is a route that ends up to peritoneal carcinomatosis (PC). This condition was considered beyond curative intent treatment until cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) have been popularized during recent years. In this review pathophysiology and current management of PC originating from gastrointestinal tumors are discussed according to the latest literature.
There are two different ways to develop PC. First, via transversal growth, tumor cells can exfoliate from the primary tumor into the peritoneal cavity preoperatively (synchronous PC). Second, via intraperitoneal spread, surgical trauma can cause the release of tumor cells from transected lymph and blood vessels or by manipulation of the primary tumor (metachronous PC). Dissemination, adhesion, invasion and proliferation are crucial steps of the process from a free tumor to PC.
The most probable dissemination mechanism in gastrointestinal PC is the spontaneous exfoliation of malignant cells from T4 tumors. Independently of the mechanism, once liberated from their host tissue, tumor cells are free to disseminate around the peritoneal cavity. Several leukocyte-associated adhesion molecules such as CD44, integrin superfamily and selectins have been implicated in tumor-mesothelial interaction[1-3]. After adherence to submesothelial connective tissue, tumor cells penetrate the mesothelial monolayer and the process ending with PC begins.
The peritoneal stromal tissue, which provides a rich source of growth factors and chemokines, is a favorable environment for tumor proliferation. The effect of gravity, postoperative complications such as anastomotic leak, open surgical technique, non-curative procedures and even powdered gloves have been in charge of this pathological sequence[4]. However the pathophysiological mechanism of PC is an individual sequence which consists of genetic and environmental factors and remains controversial.
The first studies on the natural history of non-gynecologic PC reported that 54.6% of the patients had synchronous PC where ascites (34.9%) and bowel obstruction (24.3%) were the main symptoms[5,6]. Chu et al[5] reported the results of 100 patients (45 colorectal, 20 pancreas, 6 gastric, 4 small bowel, 2 appendix, 2 unknown primary and 21 miscellaneous) in 1989. The median survival was 6 mo for colorectal origin, 0.7 mo for pancreas origin and 1 mo for gastric origin.
The French EVOCAPE study including 370 patients (125 gastric, 118 colorectal, 58 pancreas, 4 small bowel, 3 liver, 12 pseudomyxoma, 7 mesothelioma and 43 unknown) revealed further details on the natural history of the disease[6]. The extent of PC was staged into 5 groups and 41.9% (155/370) patients presented with terminal stage disease (Stage 4). All 370 patients underwent surgery including resection of primary tumor, bypass and laparotomy with biopsy. Adjuvant systemic chemotherapy (CT) was administered to 26.2% of the patients. The mean and median overall survival were 6 mo (0.1-48) and 3.1 mo, respectively. For gastric cancer median survival was 3.1 mo. Initial pT stage, PC stage, presence of ascites and hepatic metastases were associated with overall survival. Median overall survival was 5.2 mo for colorectal origin and only the PC stage was associated with overall survival. Results for pancreatic carcinoma were very poor with a median overall survival of 1.5 mo. Presence of ascites was significantly related with survival rates in PC from pancreas origin where PC stage was not. These patients had the benefit of fluorouracil-based systemic chemotherapy, but the results were similar to those reported by Chu et al[5] a decade earlier.
In 2002, Jayne et al[7] performed a retrospective analysis of 349 PC out of 3019 colorectal cancer patients. Median survival was only 7 mo and affected by the extent of PC and stage of the primary tumor. A phase III trial by Brücher et al[8] revealed a median disease-specific survival of 12.6 mo with systemic CT in colorectal PC patients. Elias et al[9] reported improved survival to 23.9 mo in 48 patients with colorectal PC with recent systemic CT protocols based on oxaliplatin or irinotecan. In another study by Franko et al[10] median survival was reported as 16.8 mo in 38 patients with colorectal PC. A retrospective analysis of 2406 colorectal cancer patients compared treatment without CT, 5-Fluorouracil only and contemporary systemic CT[11]. Out of 256 (10.6%) PC patients, 141 (5.85%) were metachronous. Contemporary systemic CT did not improve survival rates significantly (17.9 mo vs 7 mo). In the same study the independent risk factors for the development of metachronous PC were younger age (< 62 year), N2 status, T4 status and the location of the primary tumor (left colon or appendix). However, a series of non-gynecologic PC has reported very poor prognosis with palliative care alone[5-12].
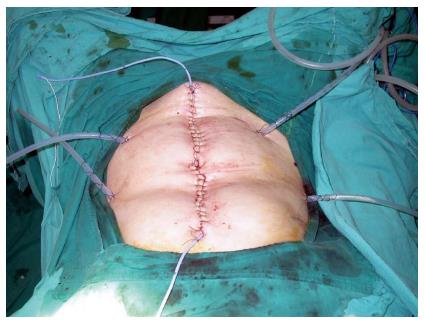
Since Sugarbaker[13] have asserted that management of PC should be performed as though it is a locoregional disease but not a metastatic process, CRS and intraperitoneal CT has been a curative option for PC patients. Cytoreductive surgery implies a series of visceral resections and peritonectomy procedures described by Sugarbaker[13]. Although the aim of CRS is to eliminate all macroscopic disease, viable tumor cells may remain in the peritoneal cavity. At that point, intraperitoneal chemotherapy extends the macroscopic disease elimination to microscopic disease elimination. By intraperitoneal administration, therapeutic concentrations in the peritoneal cavity can be reached with low doses of chemotherapeutics. This means a more effective therapy with less systemic side effects. Intraperitoneal administration of chemotherapeutics is shown to have superior pharmacokinetic activity than intravenous ways[14]. Hyperthermia, which has effects on peritoneal blood flow and the tumor microenvironment, enhances the efficiency of intraperitoneal chemotherapy[15]. Therefore, administration of intraperitoneal chemotherapy is recommended in a hyperthermic setting at 40-42 °C in which the thermal chemo sensitization is maximum[16]. Several techniques for HIPEC have been described[17], however there is no sufficient evidence in the literature confirming the superiority of one technique over the others in terms of outcome, morbidity and safety. In the closed abdominal technique which we prefer in our institution, after completion of CRS and anastomoses and/or stoma, closed suction drains and temperature probes are placed through the abdominal wall and the abdomen is closed (Figure 1). Perfusion of the chemotherapeutics at a constant intraperitoneal temperature of 41-42 °C is performed with a hyperthermic perfusion system. The duration of perfusion varies by chemotherapeutic agent between 30-120 min. The theoretical advantages of this method includes quicker reach and stabilization of constant target temperature, increased penetration of chemotherapeutics with high intra-abdominal pressure, safety of staff in the operation theater and easier application than open methods. After HIPEC, temperature probes are removed and drains are left in the abdomen for 2-3 d.
Hyperthermic intraperitoneal CT is highly recommended for the treatments of PMP, colorectal PC with limited extension and peritoneal mesotheliomas and in the evaluation phase for gastric PC and ovarian PC and neuroendocrine tumors[18,19]. The role of HIPEC in the prophylactic setting to prevent PC in patients with high risk of peritoneal metastases and the “second look” approach is promising[20]. In the treatment of sarcomatosis from GIST and small round-cell tumors, clinical trials are limited and HIPEC is not currently indicated. Cytoreductive surgery and HIPEC can be considered as a new standard of care in PC patients. There are many studies suggesting the survival benefit of CRS and HIPEC. Moreover, the lack of clinical evidence reporting better oncologic results with alternative treatment options indicates CRS and HIPEC as the only curative treatment of PC as of yet[21].
The successful treatment of PC requires a comprehensive management plan including proper patient selection, complete resection of all visible disease, perioperative intraperitoneal chemotherapy and postoperative systemic chemotherapy. Mesenteric root infiltration, massive involvement of retroperitoneum, massively infiltrated pancreatic capsule, expected small bowel resection for more than one-third of the whole length and unresectable liver metastases are widely accepted absolute exclusion criteria for radical CRS[8,22]. Before final decision of radical cytoreduction, patient should be fully examined in terms of resectability of the tumor as well as perioperative risks. The reliability of radiologic findings on computed tomography, positron emission tomography and/or magnetic resonance imaging is limited for preoperative clinical staging of PC. Intraoperative staging is recommended to select proper patients for radical treatment[23].
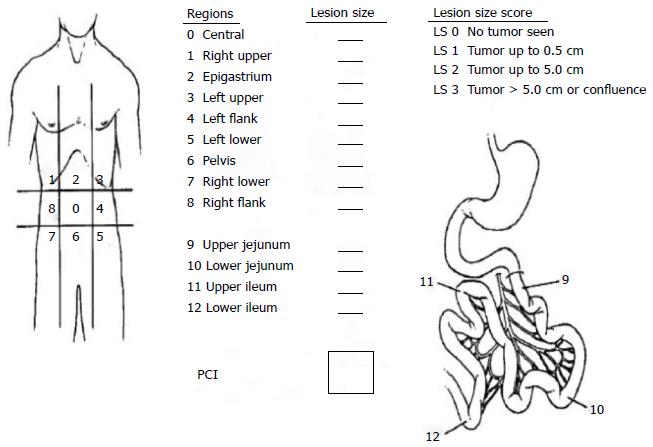
The peritoneal carcinomatosis index (PCI) is a useful classification of disease extensity for both prognosis and operability[17,24]. This classification system divides abdomen into 9 sectors and the small bowel into 4 more sectors. The total score is calculated according to lesion size scores of each sector (Figure 2). Sugarbaker[17] recommends palliative interventions for the patients with a PCI score greater than 20.
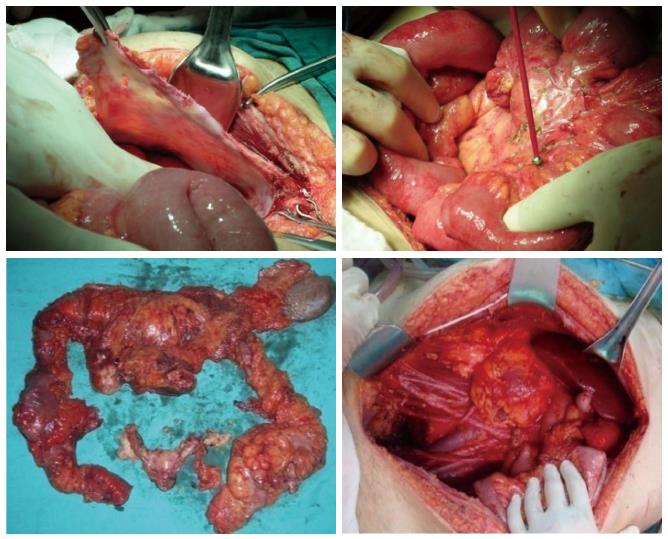
The completeness of cytoreduction (CC) score which is closely associated with PCI score is a major prognostic factor for survival in PC patients[25]. This classification responds to the need of target criteria for resectability in CRS, as R0 resection is not a realistic aim for the majority of multivisceral resections. Besides, CRS and HIPEC procedures can eliminate some microscopic disease especially in mucinous tumors, therefore the prerequisite for curative intent in PC patients is not absolute R0 resection. According to this residual tumor classification, CC-0 surgery is defined as no visible peritoneal seeding after CRS. Persisting nodules less than 0.25 cm after CRS indicates CC-1, nodules between 0.25 and 2.5 cm indicates CC-2 and nodules greater than 2.5 cm indicates CC-3. The aim of CRS and HIPEC should be complete cytoreduction which is determined as CC-0 and CC-1[26]. The R0 resection refers to CC-0 and CC-1 status in patients with mucinous pseudomyxoma peritonei, where only CC-0 status is equivalent to R0 resection in colorectal or gastric carcinomas (Figure 3). Patients with CC-0/CC-1 resections have significantly superior oncologic results[27,28]. The possibility of complete cytoreduction is the major determinant for estimating potential benefits of CRS and HIPEC treatment.
Patients without signs of unresectable systemic metastases and/or mesenteric root infiltration in preoperative radiologic examination are candidates for surgical exploration. Diagnostic laparoscopy can give valuable information for staging almost equivalent to laparotomy. Imaging-based PCI is still considered the main staging method; however diagnostic laparoscopy allows assessment of the true PCI and a correct selection of patients who can benefit from CRS and HIPEC[22]. Palliative HIPEC for the treatment of malign ascites can be performed in the same laparoscopic session after staging[29].
Candidates for CRS and HIPEC treatment usually have the history of multiple previous operations and several cycles of CT, some of those present in a poor condition. The potential side effects of multivisceral resections and HIPEC are additional factors that make PC patients a potentially high-risk population. Systemic effects of HIPEC can be life-threatening after a complex and prolonged surgical procedure[30,31]. Literature reveals an overall morbidity ranging from 12% to 56% and mortality from 0% to 12%[28,31-35]. The extent of carcinomatosis and cytoreduction, age, number of resected organs and blood loss as well as the length of the operation are factors associated with increased morbidity rate[34,35]. Particularly, infectious complications have been indicated as the leading cause of increased morbidity[36]. A standard perioperative care protocol to prevent complications is recommended for PC patients. As well as patient and tumor-related factors, the experience of the surgical team plays an important role on surgical complications. In a recent study, the significance of the learning curve in CRS and HIPEC was demonstrated and a minimum number of 200 procedures was recommended before optimization in surgical outcome[37]. However, some studies from tertiary or community care hospitals have reported morbidity and mortality rates comparable to other surgical procedures of similar extent[38].
Regarding high early and late perioperative complication rates, candidates for CRS and HIPEC should be considered as a special group of patients. As well as early surgical and infectious complications, HIPEC related toxicity and side effects which may present even after discharge from hospital, should be monitored closely[31].
Patients with colorectal cancer present with PC in approximately 10% of all cases[35]. Forty percent of patients with recurrent colorectal cancer have peritoneal metastases without other distant organ invasion to the peritoneum[39]. Half of those are synchronous PC with advanced stage of the primary tumor and the median survival rate with palliative management is very poor[6]. Despite M1 clinical stage, these patients indicate a special disease pattern considered as a locoregional disease limited to the abdominal cavity.
The first randomized trial on CRS and HIPEC revealed a 2-times shorter median survival (12.6 mo) in patients with systemic CT alone than in the CRS and HIPEC group (P = 0.03)[12]. Recent targeted therapies such as bevacizumab or cetuximab enhanced median survival by 3 to 6 mo[40]. Modern CT agents have prolonged median survival up to 19 mo in patients with synchronous colorectal PC; however the results of systemic CT alone is still poorer than CRS and HIPEC treatment[41].
The success of CRS and HIPEC in PC from colorectal carcinoma is strongly related with the clinical status of the patient and resectability[42]. Completeness of cytoreduction and PCI scores are quantitative prognostic indicators of long-term results[8]. A French multicenter study, including 523 patients, revealed that patients who were not amenable to complete cytoreduction have similar median survival (9 mo) with systemic CT alone[42]. In another study of 43 patients with unresectable PC reported that R2 resection resulted in a median survival of only 6.3 mo[43]. The results of the Erlangen group also confirmed that an incomplete resection does not afford any benefit in colorectal PC[44]. However, complete cytoreduction can be achieved in approximately 80% of the patients[42]. A comparative study questioning the effect of HIPEC on survival reported that 5-year overall survival was significantly superior in patients who underwent complete cytoreduction and HIPEC than the patients in complete cytoreduction alone group (51% vs 13%)[9]. Median survival of 523 patients treated with CRS and HIPEC in a French study was 33 mo[42]. In their prospective study, Quenet et al[45] obtained 42% 5-year overall survival in 143 patients after CRS and HIPEC with oxaliplatin. In this series, definitive cure was achieved in 16% of the patients who survived more than 5 years. Improved survival of HIPEC was shown by some other studies[46,47]. A meta-analysis of 47 studies on CRS and HIPEC in PC of colorectal origin showed that CRS plus HIPEC had a statistically significant survival benefit over control groups[48]. Some large series are summarized in Table 1.
| Ref. | Study design | Number of patients | Complete CRS | Intraperitoneal chemotherapy | Median follow-up (mo) | Median survival (mo) |
| Verwaal et al[12] | Randomized | 105 | NA | HIPEC | 21.6 | 22.3 |
| Glehen et al[47] | Retrospective | 506 | 53.5% | HIPEC/EPIC | 53.0 | 19.2 |
| Levine et al[46] | Retrospective | 133 | NA | HIPEC | 55.4 | 16.4 |
| Elias et al[9] | Retrospective | 48 | NA | HIPEC | 63.0 | 62.7 |
| Elias et al[42] | Retrospective | 523 | 84% | HIPEC/EPIC | 45.0 | 30.1 |
Currently, there are two main approaches to HIPEC in colorectal PC: The use of mytomycin C for 60-90 min at 41 °C and oxaliplatin for 30 min at 43 °C. In the literature results on survival and morbidity in HIPEC series for the two agents are comparable[49].
Currently, the absolute contraindications for CRS and HIPEC in colorectal PC are poor general status, the presence of extraabdominal metastases and > 3 liver metastases[50]. Resection of < 4 liver metastases can be performed[50,51]. For determining clear indications and contraindications, more randomized trials are needed. However, the use of HIPEC in prophylaxis has emerged recently. The argument that early PC cannot be detected with clinical, biological or radiologic signs revealed the necessity of defining some high risk criteria for metachronous PC. Management of high risk patients includes close follow up and a planned “second look” surgery. Elias et al[52] described a high risk population as completely resected primary tumor and a few synchronous nodules, ovarian metastasis and perforated primary tumor. They recommended adjuvant systemic chemotherapy for 6 mo after prior surgery and a second look surgery at the end of the first year. Sugarbaker[20] has also developed an algorithm for the management of high risk patients. He stated that high risk patients should have second look surgery immediately in case of any suspicious findings in physical examination, computed tomography or CEA levels. Independently of symptoms and findings during the follow up, he recommends planned laparotomy at the end of the first year for asymptomatic patients. If second look exploration reveals any recurrent disease CRS and HIPEC is performed, where omentectomy, oophorectomy and HIPEC is recommended in patients without recurrence.
The terminology “pseudomyxoma peritonei” refers to a syndrome which characterized with gelatinous ascites and peritoneal implants secreting mucin and commonly used for PC originating from appendiceal tumors. Treatment of PC from appendiceal origin with CRS and intraperitoneal CT was first described by the Basingstoke group in 1987[53]. This management which enhanced survival from months to decades can be accepted as a revolution for the treatment. There are three widely accepted types of PMP which Ronnett et al[54] reported significantly different prognosis for each; disseminated peritoneal adenomucinosis (DPAM), peritoneal mucinous carcinomatosis (PMCA) and PMCA with intermediate or discordant features. In this study the most significant prognostic factor was achieving CC-0/CC-1 cytoreduction. Similar results with an overall 10-year survival in patients who underwent CC-0/CC-1 CRS were reported by Deraco et al[55]. The 10-year overall survival rate was 67% in patients with DPAM and 40.7% in patients with PMCA. A new classification of PC from appendiceal cancer tiers patients into two groups regarding histopathological features. According to Wake Forest Classification cases formerly classified as DPAM, well differentiated mucinous carcinomatosis and low-grade mucinous appendiceal neoplasms are named “low-grade mucinous carcinoma peritonei”, while moderately or poorly differentiated adenocarcinomas, PMCA and cases with signet-ring cell component are classified as high-grade mucinous carcinoma peritonei[56]. In a retrospective study including 134 patients treated with CRS and HIPEC 5-year overall survival rates were 62.5% for low-grade and 37.7% for high-grade mucinous carcinoma peritonei[57]. In subgroup analysis of high grade mucinous patients who underwent complete CRS, 5-year overall survival of patients with PCI > 20 was poorer (45% vs 66%). The results of 301 patients treated with CRS and HIPEC was reported by a multi-institutional European study, the 5-year disease free survival rate was 56%[58]. A multi-institutional retrospective analysis of 2296 patients from 16 centers revealed a 10-year overall survival of 63%, a median overall survival of 196 mo and a median disease free survival of 98 mo. In this trial, mean PCI was 20 and 83% of the procedures achieved CC-0/CC-1 CRS. Perioperative mortality and major morbidity rates were 2% and 24%, respectively. Previous CT, major complications, CC-2/CC-3 CRS, age were found as independent prognostic factors for overall survival, while CRS without HIPEC and high PCI score were negative prognostic factors for disease free survival in addition to the factors stated above[59]. Tumor markers such as CEA, CA-19-9 and CA-125 were shown as predictors of incomplete CRS and valuable prognostic factors in several studies[60,61]. Nodal involvement also has prognostic significance but does not refer a contra-indication if complete CRS is possible[62]. Eligibility for CRS and HIPEC can be determined by contrast-enhanced computed tomography[63]. The probability of incomplete resection is 88% for patients who have segmental obstruction of small bowel or tumor nodules greater than 5 cm on the small bowel in radiology, while complete CRS can be achieved in 92% of those without these radiologic findings[64]. Patients with ECOG performance score 2 and 3 have poorer survival compared to patients with ECOG score 1[65], however poor performance is not an absolute contra-indication for surgery. The role of laparoscopy in staging is controversial[63]. On the contrary, with other origins high PCI score is not an exclusion criterion for PMP, however tumor grade, age, mesenteric invasion and liver metastases should be considered together with PCI.
Currently, quantitative prognostic factors for PC from appendiceal origin are histopathological type, complete CRS and tumor markers. High-grade nonmucinous appendiceal primaries such as appendiceal adenocarcinoma, goblet cell and carcinoid tumors have less benefit from CRS and HIPEC with a 3-year overall survival of 15%[66]. Complete cytoreduction and HIPEC is the standard of care in PMP. Unlike other gastrointestinal primaries of PC, resection of all peritoneal surfaces is highly recommended[67]; however this issue is controversial due to the lack of randomized trials comparing limited and complete peritonectomy in PMP.
Peritoneal metastasis is present in 5%-30% of the patients undergoing potentially curative surgery for gastric cancer[68]. Unlike the position with distant metastasis of gastric cancer, systemic CT does not provide an improvement on survival in PC from gastric origin; the median survival of these patients has been reported as 1-3 mo[69]. The benefit of CRS and HIPEC in PC of gastric cancer is controversial. Glehen et al[70] published the results of 150 patients from 15 centers in 2010. The overall median survival and 5-year overall survival rates were 9.2 mo and 13%, respectively. The only independent prognostic factor was CC score in this retrospective analysis. In 2011, Yang et al[71] performed the first prospective randomized phase III clinical trial on CRS and HIPEC in 68 patients with PC from gastric cancer. Median survival was significantly better in the CRS and HIPEC group than the CRS only group (11 mo vs 6.5 mo) with an increase to 13.5 mo after complete CRS. A review of 10 studies including 441 patients who underwent CRS and HIPEC for gastric PC reported an overall median survival of 7 mo which was improved to 15 mo when complete CRS was achieved[68]. Peritoneal lavage followed by intraperitoneal CT was shown to improve 5-year survival in advanced gastric cancer[60]. Recently, three meta-analyses demonstrated the prophylactic effect of HIPEC[72-74].
Regarding the literature, quantitative prognostic factors for PC from gastric cancer are completeness of cytoreduction and presence of ascites. Currently, selected patients with potential complete cytoreduction are considered to benefit from CRS and HIPEC. Eligibility for HIPEC can be determined with laparoscopic staging in patients with PC and peritoneal lavage in advanced gastric cancer.
Data about PC from the small bowel is very limited as small bowel adenocarcinoma is a rare diagnosis with a poor median survival ranging from 9 to 20 mo[75-77]. Approximately 25% of the patients present with synchronous PC[13]. After palliative treatment, median survival was reported as 3.1 mo by Sadeghi et al[6]. Chua et al[78] reported median disease free and overall survival rates of 12 mo and 25 mo in 7 patients who underwent complete CRS and HIPEC or early postoperative intraperitoneal chemotherapy. Despite a limited number of patients they concluded that cases with signet-cell morphology, lymphovascular invasion and poor differentiation had worse oncologic outcomes, where lymph node metastasis did not influence survival. Other results from Marchettini et al[79] and Jacks et al[80] showed improved median survival with CRS and intraperitoneal chemotherapy (12 mo and 30.1 mo, respectively). Complete CRS and HIPEC can be an option for these patients, however large series and randomized trials are needed.
Peritoneal carcinomatosis is a potentially curable disease with CRS and HIPEC. The proper indications of the treatment and patient selection directly influence the oncologic outcomes. Regardless of origin, some factors such as patient performance, tumor burden, extra-abdominal metastases and completeness of CRS should be considered carefully to achieve good outcomes, minimal mortality and morbidity. In conclusion, these procedure have promising results with early diagnosis and proper patient selection when performed in experienced centers.
P- Reviewer: Rutegard J S- Editor: Ding Y L- Editor: O’Neill M E- Editor: Wang CH
| 1. | Jonjić N, Peri G, Bernasconi S, Sciacca FL, Colotta F, Pelicci P, Lanfrancone L, Mantovani A. Expression of adhesion molecules and chemotactic cytokines in cultured human mesothelial cells. J Exp Med. 1992;176:1165-1174. [PubMed] |
| 2. | Klein CL, Bittinger F, Skarke CC, Wagner M, Köhler H, Walgenbach S, Kirkpatrick CJ. Effects of cytokines on the expression of cell adhesion molecules by cultured human omental mesothelial cells. Pathobiology. 1995;63:204-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 38] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 3. | Müller J, Yoshida T. Interaction of murine peritoneal leukocytes and mesothelial cells: in vitro model system to survey cellular events on serosal membranes during inflammation. Clin Immunol Immunopathol. 1995;75:231-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 4. | Jayne D. Molecular biology of peritoneal carcinomatosis. Cancer Treat Res. 2007;134:21-33. [PubMed] |
| 5. | Chu DZ, Lang NP, Thompson C, Osteen PK, Westbrook KC. Peritoneal carcinomatosis in nongynecologic malignancy. A prospective study of prognostic factors. Cancer. 1989;63:364-367. [PubMed] |
| 6. | Sadeghi B, Arvieux C, Glehen O, Beaujard AC, Rivoire M, Baulieux J, Fontaumard E, Brachet A, Caillot JL, Faure JL. Peritoneal carcinomatosis from non-gynecologic malignancies: results of the EVOCAPE 1 multicentric prospective study. Cancer. 2000;88:358-363. [PubMed] |
| 7. | Jayne DG, Fook S, Loi C, Seow-Choen F. Peritoneal carcinomatosis from colorectal cancer. Br J Surg. 2002;89:1545-1550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 649] [Cited by in RCA: 598] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 8. | Brücher BL, Piso P, Verwaal V, Esquivel J, Derraco M, Yonemura Y, Gonzalez-Moreno S, Pelz J, Königsrainer A, Ströhlein M. Peritoneal carcinomatosis: cytoreductive surgery and HIPEC--overview and basics. Cancer Invest. 2012;30:209-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 77] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 9. | Elias D, Lefevre JH, Chevalier J, Brouquet A, Marchal F, Classe JM, Ferron G, Guilloit JM, Meeus P, Goéré D. Complete cytoreductive surgery plus intraperitoneal chemohyperthermia with oxaliplatin for peritoneal carcinomatosis of colorectal origin. J Clin Oncol. 2009;27:681-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 618] [Cited by in RCA: 664] [Article Influence: 41.5] [Reference Citation Analysis (0)] |
| 10. | Franko J, Ibrahim Z, Gusani NJ, Holtzman MP, Bartlett DL, Zeh HJ. Cytoreductive surgery and hyperthermic intraperitoneal chemoperfusion versus systemic chemotherapy alone for colorectal peritoneal carcinomatosis. Cancer. 2010;116:3756-3762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 234] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 11. | Kerscher AG, Chua TC, Gasser M, Maeder U, Kunzmann V, Isbert C, Germer CT, Pelz JO. Impact of peritoneal carcinomatosis in the disease history of colorectal cancer management: a longitudinal experience of 2406 patients over two decades. Br J Cancer. 2013;108:1432-1439. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 90] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 12. | Verwaal VJ, van Ruth S, de Bree E, van Sloothen GW, van Tinteren H, Boot H, Zoetmulder FA. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol. 2003;21:3737-3743. [PubMed] |
| 13. | Sugarbaker PH. Peritonectomy procedures. Surg Oncol Clin N Am. 2003;12:703-727, xiii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 32] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Markman M. Intraperitoneal chemotherapy in the management of malignant disease. Expert Rev Anticancer Ther. 2001;1:142-148. [PubMed] |
| 15. | Kampinga HH, Dynlacht JR, Dikomey E. Mechanism of radiosensitization by hyperthermia (& gt; or = 43 degrees C) as derived from studies with DNA repair defective mutant cell lines. Int J Hyperthermia. 2004;20:131-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 76] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 16. | Issels RD. Hyperthermia adds to chemotherapy. Eur J Cancer. 2008;44:2546-2554. [PubMed] |
| 17. | Sugarbaker PH. Surgical responsibilities in the management of peritoneal carcinomatosis. J Surg Oncol. 2010;101:713-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 18. | Elias D, Goéré D, Dumont F, Honoré C, Dartigues P, Stoclin A, Malka D, Boige V, Ducreux M. Role of hyperthermic intraoperative peritoneal chemotherapy in the management of peritoneal metastases. Eur J Cancer. 2014;50:332-340. [PubMed] |
| 19. | Tabrizian P, Shrager B, Jibara G, Yang MJ, Romanoff A, Hiotis S, Sarpel U, Labow DM. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for peritoneal carcinomatosis: outcomes from a single tertiary institution. J Gastrointest Surg. 2014;18:1024-1031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 67] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 20. | Sugarbaker PH. Early intervention for treatment and prevention of colorectal carcinomatosis: a plan for individualized care. Surg Oncol Clin N Am. 2012;21:689-703. [PubMed] |
| 21. | Sugarbaker PH. Evolution of cytoreductive surgery and perioperative intraperitoneal chemotherapy for peritoneal carcinomatosis: are there treatment alternatives? Am J Surg. 2011;201:157-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Valle M, Federici O, Garofalo A. Patient selection for cytoreductive surgery and hyperthermic intraperitoneal chemotherapy, and role of laparoscopy in diagnosis, staging, and treatment. Surg Oncol Clin N Am. 2012;21:515-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 23. | Esquivel J, Elias D, Baratti D, Kusamura S, Deraco M. Consensus statement on the loco regional treatment of colorectal cancer with peritoneal dissemination. J Surg Oncol. 2008;98:263-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 152] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 24. | Elias D, Quenet F, Goéré D. Current status and future directions in the treatment of peritoneal dissemination from colorectal carcinoma. Surg Oncol Clin N Am. 2012;21:611-623. [PubMed] |
| 25. | Harmon RL, Sugarbaker PH. Prognostic indicators in peritoneal carcinomatosis from gastrointestinal cancer. Int Semin Surg Oncol. 2005;2:3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 189] [Cited by in RCA: 237] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 26. | Sugarbaker PH. Management of peritoneal-surface malignancy: the surgeon’s role. Langenbecks Arch Surg. 1999;384:576-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 199] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 27. | Sugarbaker PH. Successful management of microscopic residual disease in large bowel cancer. Cancer Chemother Pharmacol. 1999;43 Suppl:S15-S25. [PubMed] |
| 28. | Sugarbaker PH, Chang D. Results of treatment of 385 patients with peritoneal surface spread of appendiceal malignancy. Ann Surg Oncol. 1999;6:727-731. [PubMed] |
| 29. | Facchiano E, Risio D, Kianmanesh R, Msika S. Laparoscopic hyperthermic intraperitoneal chemotherapy: indications, aims, and results: a systematic review of the literature. Ann Surg Oncol. 2012;19:2946-2950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 30. | Di Miceli D, Alfieri S, Caprino P, Menghi R, Quero G, Cina C, Pericoli Ridolfini M, Doglietto GB. Complications related to hyperthermia during hypertermic intraoperative intraperitoneal chemiotherapy (HIPEC) treatment. Do they exist? Eur Rev Med Pharmacol Sci. 2012;16:737-742. [PubMed] |
| 31. | Canda AE, Sokmen S, Terzi C, Arslan C, Oztop I, Karabulut B, Ozzeybek D, Sarioglu S, Fuzun M. Complications and toxicities after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Ann Surg Oncol. 2013;20:1082-1087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 32. | Roviello F, Marrelli D, Neri A, Cerretani D, de Manzoni G, Pedrazzani C, Cioppa T, Nastri G, Giorgi G, Pinto E. Treatment of peritoneal carcinomatosis by cytoreductive surgery and intraperitoneal hyperthermic chemoperfusion (IHCP): postoperative outcome and risk factors for morbidity. World J Surg. 2006;30:2033-2040; discussion 2041-2042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 33. | Chua TC, Yan TD, Saxena A, Morris DL. Should the treatment of peritoneal carcinomatosis by cytoreductive surgery and hyperthermic intraperitoneal chemotherapy still be regarded as a highly morbid procedure?: a systematic review of morbidity and mortality. Ann Surg. 2009;249:900-907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 387] [Cited by in RCA: 420] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 34. | Kusamura S, Younan R, Baratti D, Costanzo P, Favaro M, Gavazzi C, Deraco M. Cytoreductive surgery followed by intraperitoneal hyperthermic perfusion: analysis of morbidity and mortality in 209 peritoneal surface malignancies treated with closed abdomen technique. Cancer. 2006;106:1144-1153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 196] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 35. | Glehen O, Osinsky D, Cotte E, Kwiatkowski F, Freyer G, Isaac S, Trillet-Lenoir V, Sayag-Beaujard AC, François Y, Vignal J. Intraperitoneal chemohyperthermia using a closed abdominal procedure and cytoreductive surgery for the treatment of peritoneal carcinomatosis: morbidity and mortality analysis of 216 consecutive procedures. Ann Surg Oncol. 2003;10:863-869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 273] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 36. | Capone A, Valle M, Proietti F, Federici O, Garofalo A, Petrosillo N. Postoperative infections in cytoreductive surgery with hyperthermic intraperitoneal intraoperative chemotherapy for peritoneal carcinomatosis. J Surg Oncol. 2007;96:507-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 37. | Andréasson H, Lorant T, Påhlman L, Graf W, Mahteme H. Cytoreductive surgery plus perioperative intraperitoneal chemotherapy in pseudomyxoma peritonei: aspects of the learning curve. Eur J Surg Oncol. 2014;40:930-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 38. | Kerscher AG, Mallalieu J, Pitroff A, Kerscher F, Esquivel J. Morbidity and mortality of 109 consecutive cytoreductive procedures with hyperthermic intraperitoneal chemotherapy (HIPEC) performed at a community hospital. World J Surg. 2010;34:62-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 39. | Cintron JR, Pearl RK. Colorectal cancer and peritoneal carcinomatosis. Semin Surg Oncol. 1996;12:267-278. [PubMed] |
| 40. | Saltz LB, Clarke S, Díaz-Rubio E, Scheithauer W, Figer A, Wong R, Koski S, Lichinitser M, Yang TS, Rivera F. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol. 2008;26:2013-2019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2302] [Cited by in RCA: 2267] [Article Influence: 133.4] [Reference Citation Analysis (0)] |
| 41. | Klaver YL, Lemmens VE, Creemers GJ, Rutten HJ, Nienhuijs SW, de Hingh IH. Population-based survival of patients with peritoneal carcinomatosis from colorectal origin in the era of increasing use of palliative chemotherapy. Ann Oncol. 2011;22:2250-2256. [PubMed] |
| 42. | Elias D, Gilly F, Boutitie F, Quenet F, Bereder JM, Mansvelt B, Lorimier G, Dubè P, Glehen O. Peritoneal colorectal carcinomatosis treated with surgery and perioperative intraperitoneal chemotherapy: retrospective analysis of 523 patients from a multicentric French study. J Clin Oncol. 2010;28:63-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 685] [Cited by in RCA: 731] [Article Influence: 45.7] [Reference Citation Analysis (0)] |
| 43. | Hompes D, Boot H, van Tinteren H, Verwaal V. Unresectable peritoneal carcinomatosis from colorectal cancer: a single center experience. J Surg Oncol. 2011;104:269-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 44. | Mulsow J, Merkel S, Agaimy A, Hohenberger W. Outcomes following surgery for colorectal cancer with synchronous peritoneal metastases. Br J Surg. 2011;98:1785-1791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 45. | Quenet F, Goéré D, Mehta SS, Roca L, Dumont F, Hessissen M, Saint-Aubert B, Elias D. Results of two bi-institutional prospective studies using intraperitoneal oxaliplatin with or without irinotecan during HIPEC after cytoreductive surgery for colorectal carcinomatosis. Ann Surg. 2011;254:294-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 137] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 46. | Levine EA, Stewart JH, Russell GB, Geisinger KR, Loggie BL, Shen P. Cytoreductive surgery and intraperitoneal hyperthermic chemotherapy for peritoneal surface malignancy: experience with 501 procedures. J Am Coll Surg. 2007;204:943-953; discussion 953-955. [PubMed] |
| 47. | Glehen O, Kwiatkowski F, Sugarbaker PH, Elias D, Levine EA, De Simone M, Barone R, Yonemura Y, Cavaliere F, Quenet F. Cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for the management of peritoneal carcinomatosis from colorectal cancer: a multi-institutional study. J Clin Oncol. 2004;22:3284-3292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 860] [Cited by in RCA: 880] [Article Influence: 41.9] [Reference Citation Analysis (0)] |
| 48. | Cao C, Yan TD, Black D, Morris DL. A systematic review and meta-analysis of cytoreductive surgery with perioperative intraperitoneal chemotherapy for peritoneal carcinomatosis of colorectal origin. Ann Surg Oncol. 2009;16:2152-2165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 189] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 49. | Hompes D, D’Hoore A, Van Cutsem E, Fieuws S, Ceelen W, Peeters M, Van der Speeten K, Bertrand C, Legendre H, Kerger J. The treatment of peritoneal carcinomatosis of colorectal cancer with complete cytoreductive surgery and hyperthermic intraperitoneal peroperative chemotherapy (HIPEC) with oxaliplatin: a Belgian multicentre prospective phase II clinical study. Ann Surg Oncol. 2012;19:2186-2194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 88] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 50. | Elias D, Benizri E, Pocard M, Ducreux M, Boige V, Lasser P. Treatment of synchronous peritoneal carcinomatosis and liver metastases from colorectal cancer. Eur J Surg Oncol. 2006;32:632-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 108] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 51. | Maggiori L, Goéré D, Viana B, Tzanis D, Dumont F, Honoré C, Eveno C, Elias D. Should patients with peritoneal carcinomatosis of colorectal origin with synchronous liver metastases be treated with a curative intent? A case-control study. Ann Surg. 2013;258:116-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 69] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 52. | Elias D, Honoré C, Dumont F, Ducreux M, Boige V, Malka D, Burtin P, Dromain C, Goéré D. Results of systematic second-look surgery plus HIPEC in asymptomatic patients presenting a high risk of developing colorectal peritoneal carcinomatosis. Ann Surg. 2011;254:289-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 167] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 53. | Sugarbaker PH, Kern K, Lack E. Malignant pseudomyxoma peritonei of colonic origin. Natural history and presentation of a curative approach to treatment. Dis Colon Rectum. 1987;30:772-779. [PubMed] |
| 54. | Ronnett BM, Yan H, Kurman RJ, Shmookler BM, Wu L, Sugarbaker PH. Patients with pseudomyxoma peritonei associated with disseminated peritoneal adenomucinosis have a significantly more favorable prognosis than patients with peritoneal mucinous carcinomatosis. Cancer. 2001;92:85-91. [PubMed] |
| 55. | Deraco M, Kusamura S, Laterza B, Favaro M, Fumagalli L, Costanzo P, Baratti D. Cytoreductive surgery and hyperthermic intra-peritoneal chemotherapy (HIPEC) in the treatment of pseudomyxoma peritonei: ten years experience in a single center. In Vivo. 2006;20:773-776. [PubMed] |
| 56. | Bradley RF, Stewart JH, Russell GB, Levine EA, Geisinger KR. Pseudomyxoma peritonei of appendiceal origin: a clinicopathologic analysis of 101 patients uniformly treated at a single institution, with literature review. Am J Surg Pathol. 2006;30:551-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 244] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 57. | El Halabi H, Gushchin V, Francis J, Athas N, Macdonald R, Nieroda C, Studeman K, Sardi A. The role of cytoreductive surgery and heated intraperitoneal chemotherapy (CRS/HIPEC) in patients with high-grade appendiceal carcinoma and extensive peritoneal carcinomatosis. Ann Surg Oncol. 2012;19:110-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 76] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 58. | Elias D, Gilly F, Quenet F, Bereder JM, Sidéris L, Mansvelt B, Lorimier G, Glehen O. Pseudomyxoma peritonei: a French multicentric study of 301 patients treated with cytoreductive surgery and intraperitoneal chemotherapy. Eur J Surg Oncol. 2010;36:456-462. [PubMed] |
| 59. | Bollinger DJ, Wick MR, Dehner LP, Mills SE, Swanson PE, Clarke RE. Peritoneal malignant mesothelioma versus serous papillary adenocarcinoma. A histochemical and immunohistochemical comparison. Am J Surg Pathol. 1989;13:659-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 98] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 60. | Kuramoto M, Shimada S, Ikeshima S, Matsuo A, Yagi Y, Matsuda M, Yonemura Y, Baba H. Extensive intraoperative peritoneal lavage as a standard prophylactic strategy for peritoneal recurrence in patients with gastric carcinoma. Ann Surg. 2009;250:242-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 185] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 61. | van Ruth S, Hart AA, Bonfrer JM, Verwaal VJ, Zoetmulder FA. Prognostic value of baseline and serial carcinoembryonic antigen and carbohydrate antigen 19.9 measurements in patients with pseudomyxoma peritonei treated with cytoreduction and hyperthermic intraperitoneal chemotherapy. Ann Surg Oncol. 2002;9:961-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 62. | Halabi HE, Gushchin V, Francis J, Athas N, Macdonald R, Nieroda C, Studeman K, Sardi A. Prognostic significance of lymph node metastases in patients with high-grade appendiceal cancer. Ann Surg Oncol. 2012;19:122-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 63. | Moran B, Baratti D, Yan TD, Kusamura S, Deraco M. Consensus statement on the loco-regional treatment of appendiceal mucinous neoplasms with peritoneal dissemination (pseudomyxoma peritonei). J Surg Oncol. 2008;98:277-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 168] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 64. | Jacquet P, Jelinek JS, Chang D, Koslowe P, Sugarbaker PH. Abdominal computed tomographic scan in the selection of patients with mucinous peritoneal carcinomatosis for cytoreductive surgery. J Am Coll Surg. 1995;181:530-538. [PubMed] |
| 65. | Stewart JH, Shen P, Levine EA. Intraperitoneal hyperthermic chemotherapy for peritoneal surface malignancy: current status and future directions. Ann Surg Oncol. 2005;12:765-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 90] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 66. | Stewart JH, Shen P, Russell GB, Bradley RF, Hundley JC, Loggie BL, Geisinger KR, Levine EA. Appendiceal neoplasms with peritoneal dissemination: outcomes after cytoreductive surgery and intraperitoneal hyperthermic chemotherapy. Ann Surg Oncol. 2006;13:624-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 128] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 67. | Sugarbaker PH. New standard of care for appendiceal epithelial neoplasms and pseudomyxoma peritonei syndrome? Lancet Oncol. 2006;7:69-76. [PubMed] |
| 68. | Gill RS, Al-Adra DP, Nagendran J, Campbell S, Shi X, Haase E, Schiller D. Treatment of gastric cancer with peritoneal carcinomatosis by cytoreductive surgery and HIPEC: a systematic review of survival, mortality, and morbidity. J Surg Oncol. 2011;104:692-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 69. | Saito H, Kihara K, Kuroda H, Matsunaga T, Tatebe S, Ikeguchi M. Surgical outcomes for gastric cancer patients with intraperitoneal free cancer cell, but no macroscopic peritoneal metastasis. J Surg Oncol. 2011;104:534-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 70. | Glehen O, Gilly FN, Arvieux C, Cotte E, Boutitie F, Mansvelt B, Bereder JM, Lorimier G, Quenet F, Elias D. Peritoneal carcinomatosis from gastric cancer: a multi-institutional study of 159 patients treated by cytoreductive surgery combined with perioperative intraperitoneal chemotherapy. Ann Surg Oncol. 2010;17:2370-2377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 340] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 71. | Yang XJ, Huang CQ, Suo T, Mei LJ, Yang GL, Cheng FL, Zhou YF, Xiong B, Yonemura Y, Li Y. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy improves survival of patients with peritoneal carcinomatosis from gastric cancer: final results of a phase III randomized clinical trial. Ann Surg Oncol. 2011;18:1575-1581. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 398] [Cited by in RCA: 491] [Article Influence: 35.1] [Reference Citation Analysis (0)] |
| 72. | Huang JY, Xu YY, Sun Z, Zhu Z, Song YX, Guo PT, You Y, Xu HM. Comparison different methods of intraoperative and intraperitoneal chemotherapy for patients with gastric cancer: a meta-analysis. Asian Pac J Cancer Prev. 2012;13:4379-4385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 73. | Sun J, Song Y, Wang Z, Gao P, Chen X, Xu Y, Liang J, Xu H. Benefits of hyperthermic intraperitoneal chemotherapy for patients with serosal invasion in gastric cancer: a meta-analysis of the randomized controlled trials. BMC Cancer. 2012;12:526. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 73] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 74. | Janunger KG, Hafström L, Nygren P, Glimelius B. A systematic overview of chemotherapy effects in gastric cancer. Acta Oncol. 2001;40:309-326. [PubMed] |
| 75. | Dabaja BS, Suki D, Pro B, Bonnen M, Ajani J. Adenocarcinoma of the small bowel: presentation, prognostic factors, and outcome of 217 patients. Cancer. 2004;101:518-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 357] [Cited by in RCA: 349] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 76. | Howe JR, Karnell LH, Menck HR, Scott-Conner C. The American College of Surgeons Commission on Cancer and the American Cancer Society. Adenocarcinoma of the small bowel: review of the National Cancer Data Base, 1985-1995. Cancer. 1999;86:2693-2706. [PubMed] |
| 77. | Locher C, Malka D, Boige V, Lebray P, Elias D, Lasser P, Ducreux M. Combination chemotherapy in advanced small bowel adenocarcinoma. Oncology. 2005;69:290-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 73] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 78. | Chua TC, Koh JL, Yan TD, Liauw W, Morris DL. Cytoreductive surgery and perioperative intraperitoneal chemotherapy for peritoneal carcinomatosis from small bowel adenocarcinoma. J Surg Oncol. 2009;100:139-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 79. | Marchettini P, Sugarbaker PH. Mucinous adenocarcinoma of the small bowel with peritoneal seeding. Eur J Surg Oncol. 2002;28:19-23. [PubMed] |
| 80. | Jacks SP, Hundley JC, Shen P, Russell GB, Levine EA. Cytoreductive surgery and intraperitoneal hyperthermic chemotherapy for peritoneal carcinomatosis from small bowel adenocarcinoma. J Surg Oncol. 2005;91:112-117; discussion 118-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |