Published online Oct 21, 2014. doi: 10.3748/wjg.v20.i39.14280
Revised: March 6, 2014
Accepted: May 28, 2014
Published online: October 21, 2014
Gastric cancer poses a significant public health problem, especially in the Far East, due to its high incidence in these areas. Surgical treatment and guidelines have been markedly different in the West, but nowadays this debate is apparently coming to an end. Laparoscopic surgery has been employed in the surgical treatment of gastric cancer for two decades now, but with controversies about the extent of resection and lymphadenectomy. Despite these difficulties, the apparent advantages of the laparoscopic approach helped its implementation in early stage and distal gastric cancer, with an increase on the uptake for distal gastrectomy for more advanced disease and total gastrectomy. Nevertheless, there is no conclusive evidence about the laparoscopic approach yet. In this review article we present and analyse the current status of laparoscopic surgery in the treatment of gastric cancer.
Core tip: The laparoscopic practice for gastric cancer is growing worldwide; primarily for distal early tumours and to a lesser extent for advanced and/or proximal lesions. The supporting evidence for the extent of the resection and the minimally invasive approach has yet to become conclusive. In view of these limitations and the future perspectives, the current status of laparoscopic surgery in the treatment of gastric cancer is presented herein.
- Citation: Antonakis PT, Ashrafian H, Isla AM. Laparoscopic gastric surgery for cancer: Where do we stand? World J Gastroenterol 2014; 20(39): 14280-14291
- URL: https://www.wjgnet.com/1007-9327/full/v20/i39/14280.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i39.14280
Gastric cancer has a long history since the first alleged case reports in the Ebers papyrus in 1600 Before Christ[1]. The modern era of gastric cancer treatment commenced in the late 18th century. The first successful subtotal gastrectomy for cancer was performed by Billroth in 1881, followed by the first total gastrectomy performed by Karl Schlatter in 1897[1]. Throughout the 20th century open gastrectomy (total or subtotal) became the cornerstone of gastric cancer therapy for non-metastatic disease in medically fit patients. In 1992 Kitano from Japan pioneered minimally invasive surgery for gastric cancer by performing the first laparoscopic assisted gastrectomy[2]. Since then, significant advances occurred in the application of minimally invasive approaches to gastric cancer. Our aim herein, is to review the current standards and trends of laparoscopic surgery in gastric cancer therapy.
During the 20th century mortality from gastric cancer improved worldwide because of its decreasing incidence[3], together with the improvement in surgical technique and patient care. Despite this, gastric cancer still remains one of the most important contributors to global cancer deaths. About one million new cases of stomach cancer were estimated to have occurred in 2008[4] (988000 cases, 7.8% of the total), making it currently the fourth most common malignancy worldwide. This disease demonstrates marked geographic variation of its incidence. More than 70% of cases occur in developing countries including Eastern Asia, Central and Eastern Europe and South America. East Asia (mainly China) by itself accounts for 50% of all global cases[4].
Due to these differences, Eastern health systems, especially those in Japan have employed a different approach to the disease. Mass screening in the East contributes to detection in earlier stages of the disease process, which results in better prognosis. This contrasting approach has conditioned almost every aspect of its treatment, including the staging and the extent of surgery (mainly lymphadenectomy), leading to an unfruitful/antagonistic “Eastern vs Western debate”. Fortunately, in the last decade, this debate seems to be coming to an end, following a better understanding in the West on tumour location and staging[5], minimizing the perception that gastric cancer in the East is a different disease to that in the West. Nonetheless gastric cancer lesions in the West are more advanced at diagnosis, located more proximally, and more commonly of the diffuse-type histology, compared to the ones in the East[6], so that overall outcomes are more similar than previously thought, when comparing the same gastric cancer subtypes[7]. This has led to the unification of the two previously divergent staging systems from the Japanese Gastric Cancer Association (JGCA)[8] and the Union for International Cancer Control (UICC/TNM)[9], which is supported by data from Eastern[10] and Western randomized controlled trials (RCTs)[11,12]. Consequently D2 lymphadenectomy has been implemented in the guidelines for the surgical treatment of gastric cancer in the West[13,14], and to the acceptance that no more than D2 is mandatory in the East[15].
This new, almost identical consideration of gastric cancer in the East and the West, has profound implications in the practice of laparoscopic surgery, since, for the first time, there is a common language for staging and treatment. This offers an opportunity for the standardization of both training and surgical techniques, presenting the potential for improved outcomes; even in Western countries with low case volumes[16]. Gastric cancer remains to have an overall prognosis with a 70% fatality-to-case ratio, and the inherent limitations in surgical practice and patient care in developing countries increase this figure by 5%-10%, resulting in a disappointing 75% for women and 81% for men in these areas[17].
Laparoscopic surgery for gastric cancer has evolved rapidly and has increased in popularity during the last two decades in both East and West; though at a slower pace and case load in the latter. Japan demonstrated a ten-fold increase in the use of laparoscopic surgery between 1991-2009[18] with an impressive 42% of laparoscopic operations for Stage I and II cancer in 2010[19]. In South Korea, laparoscopic gastric cancer operations increased from 6.6% in 2004 to 25.8% in 2009[20]. In the West the pace was much slower[21], but recent data suggest that the uptake of laparoscopic surgery for gastric cancer is slowly increasing, with a concurrent increase in case load, but not to the level of Asian countries. In Spain there were 245 laparoscopic operations for gastric cancer between 2005-2008[22], whereas in the Unites Kingdom 133 out of 747 operations for gastric cancer with a curative intent were performed laparoscopically (18%) between 2011-2012 (National Oesophago-Gastric Cancer Audit 2013, available at http://www.hscic.gov.uk/og). As a comparison Japanese surgeons performed 7341 laparoscopic distal and 1103 total gastrectomies in 2009[18], while in the same year Korean surgeons performed 3.783 laparoscopic procedures[20].
Despite the vast numbers of cases performed, laparoscopic gastrectomy is still considered as under review in most published guidelines, including the ones from the NCCN in the United States, from ESMO in Europe and from JGCA in Japan[13-15]. The rationale for this, is that large RCTs, providing conclusive evidence on the long term oncologic safety of laparoscopic gastric surgery, are still pending for the early, and are certainly lacking for the advanced gastric cancer.
These guidelines reflect the variation in Eastern and Western approaches to gastric cancer. The minimum requirements in the West are an R0 resection with adequate margins and a D1 or modified D2 lymphadenectomy (without pancreatectomy/splenectomy) of at least 15 lymph nodes[13,14]. One key difference is the margin of 4-5 cm required in Western guidelines that is not mandatory in the Japanese ones. Depending on tumour growth pattern and T status, “adequate margins” can range from 2 to 5 cm in the Japanese guidelines[15]. D1 lymphadenectomy in the West does not include the dissection of the nodes along the left gastric artery (station 7) and D2 lymphadenectomy is not required. This is in contrast to the Japanese guidelines where D1 lymphadenectomy includes the dissection of left gastric artery nodes and D2 is mandatory for T2-T4 tumours or even T1N+ disease[15].
These differences make it very difficult to standardise the universal role of laparoscopic surgery in gastric cancer, a disease for which optimal treatment has yet to be defined. This problem is also reflected in the results from a recent international expert consensus collaboration in 2010, trying to define the worldwide appropriate standards of care for gastric cancer[23]. The panel agreed on the appropriateness of laparoscopic distal gastrectomy (LDG) for T1-2 N0 distal tumours and on that of laparoscopic total gastrectomy (LTG) for T1 N0 proximal tumours. There was no consensus for all other stages. Conversely, the 1st St. Gallen EORTC Gastrointestinal Cancer consensus meeting with participants from 43 countries proposed a standard approach for both Europe and Asia. This consisted of a modified D2 procedure (gastrectomy with D2 lymph node dissection without routine splenectomy and without pancreatic tail resection)[24].
There are two main types of formal laparoscopic gastrectomy for gastric cancer, distal and total gastrectomy. For T1 tumours in the middle third of the stomach without lymph node involvement (according to the Japanese guidelines[15]) there is also the alternative of a pylorus-preserving procedure or a proximal gastrectomy, neither of which will be reviewed here further, on the basis of the low frequency that they are performed worldwide. There is however promising data on laparoscopic pylorous-preserving gastrectomy revealing improved nutrition status and lower percentage of gallstone formation during follow-up[25].
LDG is the most common curative minimally invasive procedure for early gastric cancer due to high disease incidence and distal tumour predominance in the East. The progress in advanced gastric cancer has been slower due to technical difficulty. Nevertheless, LDG currently has a small role in the surgical treatment of advanced gastric cancer in Japan and South Korea[26].
LDG includes the resection of the lower two-thirds of the stomach, along with adequate lymphadenectomy depending on T and N status. Western guidelines support an R0 resection of at least 4-5 cm[13,14], whereas for the JGCA smaller margins could be acceptable depending on tumour macroscopic features[15]. For non-infiltrating tumours (type 1 and 2) a 3 cm margin is required.
According to Western guidelines, the dissection of 15 lymph nodes is adequate (D2 lymphadenectomy is not required). Conversely, JGCA guidelines specify that D1 is only acceptable for differentiated tumours with a diameter less than or equal to 1.5 cm, not extending beyond the submucosa, and in the absence of preoperatively detected lymph node involvement (T1N0). Furthermore, the Japanese definition of N1 includes the perigastric nodes (stations 1, 3a, 3b, 4sb, 4d, 5, 6) alongside the ones on the left gastric artery (station 7). For the same stage (T1N0) if the tumour is undifferentiated and/or larger than 1.5 cm in diameter, a D1+ lymphadenectomy is advocated, which extends the dissection to include the lymph nodes along the anterior aspect of the common hepatic artery (station 8a) and the coeliac axis (station 9). For all other combinations of higher T and N status (excluding of course metastatic disease), D2 lymphadenectomy which includes the lymph node dissection of the proximal half of the splenic artery (station 11p) and the proper hepatic artery, from the pancreas to the bifurcation of the common bile duct (station 12a) is required.
This laparoscopically procedure can be performed either totally or partially. The usual terms for descriptive purposes are totally laparoscopic distal gastrectomy (TLDG) and laparoscopic assisted distal gastrectomy (LADG). LADG includes hand-assisted procedures. There is no prospective data in favour of either LADG or TLDG, and despite favourable results in case series[27,28] a clear-cut benefit for TLDG over LADG has not been established up to now yet[29]. By far, the most commonly performed technique is LADG without hand assistance, which is also the most frequently used procedure in the current literature and ongoing RCTs[30-37].
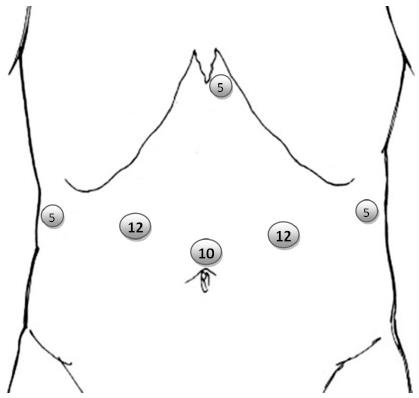
In LADG 4-6 trocars are used, one in the middle-line at or above the umbilicus for the 30 degree scope, and usually two on each side of the abdomen for dissection and retraction (Figure 1). A subxiphoid trocar can also be used instead of the far right trocar. This position is our own preference, since it allows for the introduction of a Nathanson® retractor for liver and gastric retraction during lymphadenectomy of the coeliac axis (Figure 1). The number of trocars may decrease with the expertise gained by the surgeon. In an impressive recently published Korean series of 528 laparoscopic gastrectomies, the authors performed all cases after the 331st with 4 trocars, instead of the initial 5[38].
The anastomosis can be hand-sewn or, more usually with a stapler. The reconstruction varies and is a matter of personal preference. We favour the side to side anastomosis with linear stapler and manual closure of the opening. The three usual types of reconstruction are Billroth I (gastroduodenostomy), Billroth II (loop gastrojejunostomy) and Roux-en-Y gastrojejunostomy. Billroth I is by far the most popular type of reconstruction in studies from Eastern countries, especially RCTs[30,31,33-36], while Billroth II and Roux-en-Y reconstruction are almost exclusively employed in the West[32,39-42]. This probably reflects the different perception of distal gastrectomy between the East and the West. Because of the more advanced stage and the higher percentage of diffuse histology found in western patients, it is not surprising that western surgeons tend to be more extensive in the portion of the stomach resected, and therefore are more easily inclined to resect the 3/4 or 4/5 of the stomach (i.e., subtotal gastrectomy), rendering a Billroth I anastomosis after such an extensive resection unlikely. On the other hand, Eastern surgeons supported by clear-cut JGCA guidelines for necessary resection margins, perform their operations on more distal, and more often non-diffuse lesions with a standardized Billroth I technique for LADG[26], that allows for the inspection and the marking of the lesion from the inside on the gastric wall.
TLDG differs from LADG mainly in three aspects. Firstly, there is a need for intraoperative localization of the tumour that will allow the correct marking of the resection line, in order to provide adequate margins. Intraoperative endoscopy[43] is one way to solve this problem. An alternative is clip placement in preoperative endoscopy, the position of which can be compared with that of laparoscopic clips placed in the greater and the lesser curve at the level of the planned resection line. This comparison is performed intraoperatively with a plain abdominal radiograph, taken after laparoscopic clip placement[44]. Secondly, in TLDG the anastomosis is performed intracorporeally, usually a delta shaped anastomosis[45]. Thirdly, the specimen extraction site is not necessarily situated in the upper abdominal wall. A Pfannenstiel incision can be employed, the extended umbilical port can be used for distal specimens and even a transvaginal route has been reported[46].
The literature is rich in retrospective studies, case series, and comparative studies and there is less wealth of RCTs and high quality meta-analyses. Unsurprisingly, most data on laparoscopic distal gastrectomy come from Eastern countries and are mainly related to early gastric cancer. Despite enthusiasm for LADG, and its popularity in the East, there are only a few noticeable published prospective RCTs[30-36,47]. All but one came from the East (Table 1).
| RegionCountry | East | West | ||||||
| Japan | South Korea | China | Italy | |||||
| Author | Kitano et al[30] | Hayashi et al[31] | Lee et al[33] | Takiguchi et al[36] | Kim et al[34,47] | Kim et al[35] | Cai et al[56] | Huscher et al[32] |
| Year | 2002 | 2002 | 2005 | 2013 | 2008, 2013 | 2010 | 2011 | 2005 |
| Patients | 28 | 28 | 47 | 40 | 164 | 342 | 123 | 59 |
| Reconstruction | B-I | B-I | B-I | B-I | B-I | B-I or B-II (mostly) | B-I or B-II | B-II or R-Y |
| Lymphadenectomy type | D1 + a | D1 + a | D2 | D1 + b or D2 | D1 + b or D2 | D1 + b or D2 | D2 | D1 or D2 |
| Blood Loss | ↓ | NS | NS | ↓ | ↓ | ↓ | ↓ | ↓ |
| Operation Time | ↑ | ↑ | ↑ | ↑ | ↑ | NR | ↑ | NS |
| Pain or analgesia consumption | ↓ | ↓ | NS | ↓ | ↓ | NR | NR | NR |
| Time to Oral Intake or First Flatus | NS | ↓ | NS | ↓ | ↓ | NR | NS | ↓ |
| Morbidity | NS | ↓ NC | NS | NS | ↓ | NS | NS | NS |
| Pulmonary complications | ↓ NC | ↓ NC | ↓ | NR | NS | 0% NS | ↓ | ↓ NC |
| Mortality | 0% NC | 0% NC | 0% NC | 0% NC | 0% NC | About 0% NS | 0% NC | NS |
| Length of Stay | NS | ↓ | NS | ↓ | ↓ | NR | NS | ↓ |
| Mean number of lymph nodes | ↓4.7-NS | NS | ↓6.3 - NS | NS | ↓6.1 P < 0.01 | NR | NS | ↓3.3 - NS |
| 5-yr survival | NR | NR | NR | 100% NC | NS | NR | NR | NS |
In the first ever published RCT Kitano et al[30] from Japan compared LADG and open distal gastrectomy (ODG) in 28 patients with early gastric cancer employing a D1+ lymphadenectomy and Billroth I reconstruction. Their laparoscopic approach proved advantageous in terms of blood loss, postoperative pain, and pulmonary function despite a longer operative time and fewer (but non-significant) lymph-node harvest (Table 1). Hayashi et al[31] published a similar-sized study using the same surgical technique as Kitano with no difference in blood loss or number of harvested lymph nodes, although identified earlier restoration of oral intake and a shorter length of stay by 6 d. Neither study identified any recurrences. The authors concluded that LADG is a safe and less invasive procedure than ODG. These studies reported that LADG is advantageous compared to ODG without compromising the short-term oncological result, although both suffered from a small number of patients and a short follow-up period.
The only RCT from a Western country by Huscher et al[32] compared LADG and ODG for early and non-early gastric cancer in 59 patients and reported on 5-year survival. They performed D1 (30%) or D2 (70%) gastrectomy and employed Roux-en-Y (80%) or Billroth II (20%) for reconstruction. Earlier resumption of oral intake and shorter hospital stay (by 4 d) were found in the LADG group with no significant differences in morbidity, mortality or 5-year overall survival. Lee et al[33] from South Korea reported their early results from the comparison of LADG versus ODG in 47 early gastric cancer patients where D2 lymphadenectomy and Billroth I reconstruction was utilised in all cases. LADG took longer to perform but was statistically advantageous for a lower incidence of pulmonary complications.
The first prospective RCT for LADG exceeding 100 patients came from South Korea in 2008. Kim et al[47] reported on 164 patients with early gastric cancer managed by D1+ or D2 lymphadenectomy and a primarily Billroth I reconstruction. Operating time was significantly longer and lymph node harvest was lower for LADG whilst 5-year disease free survival was not significantly different when compared to ODG. However blood loss, 3 mo quality of life (QoL) and hospital stay (decreased by 1.5 d) were more advantageous for LADG. Furthermore, 5-year disease free and overall survival and long-term QoL were not significantly different between the two groups[34]. The demonstration of earlier recovery of physical activity was also verified in another recently published small RCT[36], from Japan.
The small number of patients and the lack of convincing long-term results from these trials are obvious. In view of these limitations several noteworthy meta-analyses were published[48-52]. In order to compensate for the small number of available RCTs, three of these meta-analyses included high-quality nonrandomized studies[48,49,51], while three included not only early gastric cancer cases but also advanced ones[48,50,52]. Regardless of methodology or case mix, the results from all these studies point to the direction that LADG is a safe option for early gastric cancer. Despite of the prolonged operative time, LADG apparently compares favourably with its open counterpart in terms of perioperative results. In two meta-analyses[48,52], the smaller number of retrieved lymph nodes by 4 in the LADG group, raised authors’ concern about its oncological completeness. A common conclusion among the authors of all the aforementioned studies was, that long-term oncological non-inferiority to ODG needs to be documented, and that only large prospective randomized trials can substantiate the role of LADG.
The need for large well organized trials resulted in a step-wise Japanese approach. The Japan Clinical Oncology Group (JCOG) conducted a multicentre, phase II trial (JCOG0703) to confirm the safety of LADG for clinical Stage I gastric cancer[53], and then launched a multicentre prospective randomized trial (JCOG0912) in 2010 to compare LADG to ODG for clinical Stage I gastric cancer[37]. This study is planned to recruit 920 patients, in 33 centres, within 5 years. The primary endpoint is survival. Secondary endpoints include disease-free survival, perioperative results, and postoperative quality of life. A similar study to JCOG0912 was launched by the Korean Laparoendoscopic Gastrointestinal Surgery Study group (KLASS-01). Between 2006 and 2010, 1415 patients were enrolled, 704 in the LADG arm and 711 in the ODG arm[54]. The primary endpoint is also overall survival. The secondary endpoints are disease-free survival, morbidity, mortality, quality of life, inflammatory and immune responses, and cost-effectiveness. An interim report from KLASS-01 was published in 2010[35]. Based on the results from 342 patients, the authors reported that the differences in morbidity (about 5% in favour of LADG), and mortality (about 1% in favour of ODG) were not of statistical significance.
Both JCOG0912 and KLASS-01 are based on the sound principle that, only accredited surgeons, experienced with both laparoscopic (30 cases for each in JCOG and 50 for each in KLASS) and open techniques (50 cases), can participate in such trials, thereby limiting any learning curve effects in these studies.
JCOG0912 and KLASS-01 are also unique in design for another reason: patients with Stage I disease and not with early gastric cancer are included. This shift of focus from early gastric cancer (tumour up to submucosa i.e. up to T1b, regardless of lymph node involvement Nx) to early stage gastric cancer (Stage I: T1N0, T2N0 or T1N1) in study design, was an important step forward, towards a better understanding and communication between the East and the West. JCOG0912 and KLASS-01 trials expand the indication for LADG beyond early gastric cancer, for the first time in Japan and South Korea, in studies of such magnitude. This is because patients with T2N0 tumours (extending to the muscularis propria without lymph node involvement) are actually included in these trials, and are treated with D2 LADG. To avoid confusion it is very important to clarify here that both these studies were planned to include patients with T2a tumours, according to the previous (6th) version of the TNM, which in the latest version are denoted as T2. Survival results from both these trials will facilitate consideration by Western surgeons and oncologists as well.
Data for LADG in advanced (non-early) or advanced stage (Stage II and Stage III) gastric cancer are much less abundant in the literature. This is no surprise considering the technical difficulties a formal D2 lymphadenectomy entails, let alone a laparoscopic one. To make things even more complex, comparisons are more difficult to make with the use of different neoadjuvant or adjuvant therapies. For advanced gastric cancer, even in Japan and South Korea, the laparoscopic practice is limited and technical details are still evolving[20,26,55].
Apart from the aforementioned study from Italy[32] there is only one more published prospective RCT from China[56] on LADG for advanced gastric cancer, but without long-term survival data. Retrospective case series and comparative studies, along with the two aforementioned RCTs, either by themselves or included in later meta-analyses, are up to now the only available data for LADG in advanced (stage) gastric cancer. For instance, a recent meta-analysis from Spain and the Netherlands[57] concurred with other meta-analyses that LADG compared to ODG for advanced (stage) gastric cancer is a more time consuming procedure, with better perioperative outcome, allegedly without compromise of the oncologic result (lymph node yield). Another relatively standard conclusion was that multicentre prospective RCTs are necessary in order to clarify the short-term advantage and comparability of long-term survival for LADG to open techniques.
Once more these issues are expected to be addressed by high quality prospective RCTs from Japan and South Korea. The Japanese Laparoscopic Surgery study group launched in 2010 a multicentre phase II/III prospective randomized trial (JLSSG0901) in order to evaluate firstly safety (Phase II: incidence of anastomotic leakage or pancreatic fistula), and later on survival after LADG for early gastric cancer. Expert accredited Japanese surgeons will participate in this trial.
Following phase II trial evidence regarding the technical feasibility and safety of LADG in the treatment of advanced gastric cancer[58], the Korean KLASS group launched a multicentre prospective phase III RCT (KLASS-02-RCT) in 2011 with an estimated sample of 1050 patients and the primary end-point of 3-year disease free survival[20]. In a recent review[59], the flow chart of this RCT was made available along with a report on an about 30% completion of accrual within the first 18 mo.
An important lesson taken from these RCTs of laparoscopic distal and total gastrectomy (JCOG0912, JLSSG0901, KLASS-01 and KLASS-02) is their focus on surgical quality and the aforementioned focus on surgical experience. For example the 10 participating surgeons in the KLASS-01 trial visited and observed the other participating surgeons and watched recorded tapes or DVDs of LADGs, as well as standardized operative field photos of ODGs before entering the trial[35]. The KLASS group took standardisation and qualification of participating surgeons to the next level in the KLASS-02-RCT, by launching a surgical quality control trial (KLASS-02-QC), which allows an independent review of surgical performance during D2 LADG in action. Eligibility for participation in the KLASS-02-RCT includes only surgeons certified by KLASS-02-QC.
The planned quality of these 4 multicentre prospective RCTs for LADG, brings once more to the surface the consequences of the marked differences in gastric cancer incidence between the East and the West. The step-wise approach that was adopted both in Japan and South Korea with the organization of these large scale trials, firstly for early stage gastric cancer and secondly for more advanced disease, is a luxury that western surgeons simply can’t afford. In a recent review article, Y. Kodera astutely observed that Far East surgeons had a much greater chance to train themselves, due to the abundance of early stage gastric cancer in their areas, and ample opportunity to comfortably develop both the surgical skills and necessary instrumentation, while keeping patient-related consequences to a minimum[60]. This learning process was complete before launching the aforementioned large scale trials, initially for early and in the second round for advanced gastric cancer[60]. This has never been an option in the West, where low incidence prohibits the organization of such trials, let alone gaining vast experience outside them for individual surgeons.
Assembling high-quality data from well-organized multicentre prospective RCTs is a necessary step before worldwide recommendations for the use of LADG in the surgery of curable gastric cancer can be developed. Before the implementation of any results in western guidelines, all differences in data variables must be taken into account, including patient characteristics (higher age, BMI, lower ASA score, higher probability of having received neoadjuvant therapy), stage and pathology (more advanced stage, more proximal tumours, and higher percentage of diffuse histology type) as well as surgical experience/quality characteristics (higher complication rate, less number of harvested lymph nodes). These well documented differences are prevalent, even in the more recent comparative studies[6]. One cannot simply extrapolate results and issue guidelines for western patients, based on the results from trials, even of the highest quality, which are/were performed with the participation of patients and surgeons only from the Far East. The participation of western patients in high quality trials could possibly represent a solution to this issue[48], but due to low incidence rates in the West, actually this is rather impractical and unlikely to happen[60].
Laparoscopic total gastrectomy (LTG) has a quite different history from LDG. The intrinsic difficulties in the surgical approach of the upper third of the stomach and the OG junction, pose significant extra technical challenge, especially during the oesophago-jejunal anastomosis, even in the most experienced hands. Advanced technical skills are required for a safe and oncologically sound LTG. Increased difficulty combined with the low incidence of upper gastric cancer in the Far East set the slow pace of LTG uptake worldwide. In the West, LTG is only performed in highly specialized centres and in small numbers mainly for Stage II and III disease. In the East, the general indication is upper third early gastric cancer, and the procedure is slowly gaining popularity, even in Japan and South Korea. Nevertheless the collective eastern experience in LTG mainly from these two countries significantly outscores the western one. Only in 2009, 1103 patients in Japan[18] and 231 in South Korea[61] underwent LTG for gastric cancer, whereas in United Kingdom, the reported number of LTGs for 2012, in the 2013 edition of the annual audit of upper GI cancer cases, was only 53. LTG remains an investigational technique according to published guidelines worldwide[13-15].
The aim of LTG is an R0 total resection of the stomach. The rules for the extent of lymphadenectomy are different between the East and the West in line with those described for LDG. In the West, 15 or more lymph nodes suffice for staging purposes and D1+ or D2 lymphadenectomy are employed. The Japanese system is more complicated and the extent of necessary lymphadenectomy is derived from the T and N status as previously reported. D1+ according to the JGCA guidelines in total gastrectomy includes the nodes along the proximal half of the splenic artery (station 11p), which for distal gastrectomy are required only for D2. Furthermore, nodes at the splenic hilum (station 10) and along the distant part of the splenic artery (station 11d) are included in D2 total gastrectomy. Therefore, the Japanese approach is much more radical even for D1 gastrectomy and definitely for D2 total gastrectomy, mandating total clearance of spleen-related lymph nodes. The latter is the very essence of the long-standing debate of spleen-preservation vs splenectomy in total gastrectomy and consequently LTG. In the West, splenectomy is only indicated if there is direct involvement of the spleen or its hilum, whereas in Japanese guidelines this issue is considered unresolved pending further evidence from the JCOG0110 RCT[62].
From a technical point of view, whether splenectomy is performed or not, LTG presents the surgeon with a unique challenge: the oesophagojejunal (OJ) anastomosis. Whether intracorporeal in totally laparoscopic total gastrectomy (TLTG), or extracorporeal through a 5 cm minilaparotomy in laparoscopic assisted total gastrectomy (LATG), OJ anastomosis is the most technically challenging and morbidity causing surgical manoeuvre. Our practice is to introduce the circular stapler, already placed in the jejunal loop, through an small 6-7 cm midline incision, used to retrieve the specimen, where a hand-port has been placed and fitted around the stapler in order the keep it air tight. Anastomotic leak and morbidity/mortality attributed to this anastomosis, coupled with anastomotic stricture later on, are the most dreaded complications. For OJ anastomosis there is no accepted standard and various techniques have been used worldwide. Intracorporeal and extracorporeal hand-sewn, circular stapled end to side, linear stapled side to side anastomoses, and the use of various technical adjuncts like OrVil® (which allows for trans oral introduction of the circular stapler anvil and is our technique of choice) and EndoCameleon® (which allows better visualization of the surgical field from various angles), have all been tried by various accomplished surgical teams, but no technique is markedly outstanding or preferable[55,63]. As a result, in the largest ongoing multicentre prospective RCT from the KLASS group (KLASS-03), the type of OJ anastomosis is left to surgeon’s preference.
The use of LTG for gastric cancer is selective, even in highly specialized centres. This fact is reflected in the marked paucity of concrete data from prospective RCTs in the literature. Pioneer surgical teams in the field have published retrospective or prospective case series and comparative studies, from both eastern and western countries[64-68]. Dulucq et al[64] in 2005 and Huscher et al[65] in 2007 from Europe published 8 and 11 laparoscopic total gastrectomies respectively, showing the feasibility of the operation in the Western setting. Median operative time was approximately 3 h in the former study and 5 h in the latter, while perioperative mortality was 0% and 18% respectively. The median lymph node yield was 22 and 35 respectively. The reports coming from eastern populations are much larger in patient accrual. In their multicentre trial from South Korea, Jeong et al[66] published their collective experience from 131 laparoscopic assisted total gastrectomies in 2009. They showed that LATG is feasible and safe (morbidity 19%, mortality 0%) but a lengthy procedure (mean operative times 4.5 h), which allows for adequate lymph node dissection (mean number of harvested lymph nodes 35). There was an impressive 94% and 89% disease specific and overall survival respectively. Equivalent results were reported concurrently from Japan by Shinohara et al[67]. Their 31% morbidity and zero mortality rates along with a median number of 46 harvested lymph nodes, strongly suggested that D2 laparoscopic total gastrectomy was both safe and effective in their series of 55 cases. Despite these initial promising results the uptake of LTG in the West remains low. Even in the most contemporary western series coming from the most prestigious specialized upper GI cancer centres[68], the number of patients is too small to allow for conclusions on the safety and the efficacy of the procedure. On the other hand Eastern surgeons have continued publishing their results, thus allowing the comparison between laparoscopic and open gastrectomy by means of several large-sized recent contemporary meta-analyses[69-71]. These studies suggest that, in experienced hands, LTG (TLTG and LATG) are both feasible and safe, with an advantage over open total gastrectomy in the perioperative course (blood loss, pain, restoration of oral diet, length of stay) at the expense of a longer operative time. From an oncologic point of view, the available data point to the direction of non-inferiority of LTG compared to open surgery, in terms of lymph node yield and survival.
All these data are retrospective or prospectively collected, but there are no multicentre RCTs. Thus, an important conclusion from the published data on LTG, is the need for well-organized RCTs in the same manner with the ongoing ones in South Korea and Japan for distal gastrectomy. In this context, the KLASS group launched recently a phase II trial (KLASS-03) for LTG in patients with stage I gastric cancer to properly evaluate the perioperative morbidity and mortality of the procedure. The results of this trial are eagerly awaited.
Becoming an accomplished laparoscopic upper GI gastric cancer surgeon is difficult. Advanced laparoscopic skills and expertise in open gastric surgery are indispensable, and participation in a large number of operations is required, before one can embark as the operating surgeon. With the exception of Japan, where centralisation of gastric cancer treatment is not mandated by the health system, in all other developed countries laparoscopic gastric cancer surgery is practiced in only a few highly specialized centres. Residency followed by an upper GI fellowship is the typical training pathway. In an upper GI fellowship in Japan or South Korea one can initially assist, and after 3 to 6 mo, perform parts of LADG. This should be complemented with lab-based training and simulator systems[26]. For western health systems an important available educational pathway is the experience that can be gained in the context of a fellowship in a bariatric surgical unit. Bariatric surgery with all the surgical manoeuvres and anastomoses performed in the oesophagogastric junction area is probably the second best stepping stone for a future laparoscopic gastric cancer surgeon.
Dealing with the steep learning curve is a very important part of the training of a laparoscopic upper GI cancer surgeon. For LDG a number between 40 and 60 cases is considered to represent the learning curve, and that can be higher for D2 LDG[72-75]. This can be helped by a solid background in open surgery[76], and the completion of an organised fellowship; which will markedly accelerate the progress, without compromising patient safety. This is particularly salient in countries such as Japan where there is a high incidence and a corresponding case-load of the disease[77]. After the completion of the learning curve, and working in an environment with standardized techniques and within a multidisciplinary team, the effects of operating in low-volume hospitals on patient outcomes, can be alleviated[78]. Therefore, it is reasonable to believe that, with proper training, surgical quality can be satisfactory in laparoscopic surgery for gastric cancer, particularly in western countries, where low incidence limits case load. Of course, there is a number of cases per year below which, it is not prudent for a surgeon to practice laparoscopic surgery for gastric cancer. According to a recent study based in experts’ opinions, an annual case load of 20 cases is currently an appropriate minimum[79]. In the West, the problem of low volume due to low incidence has traditionally been dealt with centralization. Patients are referred to highly specialized centres in order to allow expert upper GI surgeons to overcome the limitations of the learning curve and provide the highest possible quality of care and outcomes. Clinical governance and centralization are suggested as the only possible mechanisms of modern health systems, in order to overcome the problems in quality of care, arising from the low incidence of gastric cancer[80]. In Eastern countries, where the incidence of gastric cancer is higher, surgical quality is easier to quantify. In recent years, the documentation of surgical quality and the accreditation of surgeons came into focus. A stringent accreditation system is currently employed both in South Korea[20] and Japan[55]. A recent study demonstrated the benefits of these advanced accreditation systems, where the authors found that the complication rate after LDG was significantly lower in the hands of accredited surgeons in Japan, compared to not accredited ones[81]. Such accreditation methodology is likely to represent the future direction of quality control in laparoscopic upper GI surgery.
Robotic assistance is one of the latest adjuncts to laparoscopic gastrectomy. The high cost and the increased operating times are the main drawbacks of the technique. Another potential disadvantage is the smaller view of the operating field, at least at the beginning of the robotic experience. The main advantages of robotic assistance are (1) the three dimensional view of an absolutely steady operating field, chosen by the surgeon and not by the camera assistant (the surgeon chooses when and what to see); (2) the 7 degrees of freedom that robotic instruments have, which allows for open-surgery-like manoeuvres; (3) the motion scaling and tremor elimination that provide extraordinary movement precision; (4) the improved ergonomics for the console surgeon, which allows less fatigue throughout the operation; and (5) that it potentially allows the application of future technologies including augmented reality for enhanced lymphadenectomy. These advantages are believed to ease the learning curve[82] and also to improve surgical competence, thus making an operation easier to perform for any given surgeon of definite surgical skills.
The aforementioned advantages are theoretically well suited for the special needs of the minimally invasive (MI) surgery for gastric cancer. Visualisation of a small and focused operating field, combined with enhanced dexterity and movement precision are of utmost importance in the dissection around vessels during lymphadenectomy, especially near the pancreas and the spleen, and definitely during the construction of the OJ anastomosis, in case of total gastrectomy. Furthermore, ergonomics and tremor filtering are definitely an advantage, when the expected operating time is more than 4 h, as in MI surgery for gastric cancer. Finally, robotic assistance could help minimize the steep learning curve of this kind of surgery, and allow upper GI surgeons without advanced laparoscopic skills, to perform it.
These potential benefits and applications to MI surgery for gastric cancer have sparkled worldwide enthusiasm. Several groups started reporting on feasibility, safety and initial experience, in terms of perioperative and later on long term results, from both western and eastern countries, for robotic assisted gastrectomy alone or in comparison to the open and/or laparoscopic approaches[42,83-88]. The initial results suggest that, robotic gastrectomy is a safe and feasible technique, with potential benefits in terms of blood loss and length of stay, without compromising the completeness of lymphadenectomy, at the expense of prolonged operating time. These are also in concert with a recent meta-analysis[89]. Despite these results, the potential benefits from robotic assistance need further validation with well organised RCTs. The KLASS group in South Korea launched in 2010 and finished accrual in 2012 of a multicentre prospective RCT. The aim of this study is to analyse the surgical outcomes of the robotic gastrectomy, focusing on the learning curve, cost-effectiveness, quality of life, and acute-inflammatory reaction in comparison with laparoscopic gastrectomy in a sample of 400 patients.
Laparoscopic upper GI cancer surgeons currently operate in an exciting era. The results from high quality trials that will define the role of the laparoscopic approach to all types of gastrectomy are awaited in the near future. In the meanwhile, the ongoing debate between the West and the East over gastric cancer in general, is apparently coming towards its conclusion for the first time in history, through a newly adopted common language of sound evidence for standardising every aspect of gastric cancer treatment, from diagnosis and staging to surgical training and accepted practice. On the other hand, whilst the old questions are still waiting to be answered, new ones arise about the benefits from new technical adjuncts. On occasion, it has proven difficult to establish international guidelines regarding contemporaneous laparoscopic gastric surgery due to the persistent introduction of ever-newer technologies and techniques, which constantly change the reference standards and evidence for disease treatment.
Laparoscopic surgery in the treatment of gastric cancer is a relatively new technique that is beginning to enjoy worldwide popularity. In the Far East, LADG is practiced on a large scale, owing mainly to the high incidence of early stage gastric cancer and the high percentage of distal gastric tumours in these geographical areas. Concrete evidence is now being acquired to support its advantages over its open counterpart and long-term oncologic safety are still pending. LADG for more advanced than Stage I tumours as well LTG require in-depth consideration before they become widely accepted and practiced to the same extent. Data for these techniques are also pending. Laparoscopic surgery for gastric cancer requires high surgical skills, if quality of care is to be kept up to the highest standards. Appropriate training with fellowships and practice within a multidisciplinary setting should be the aim. Technical advancements such as robotic surgery, that can decrease learning-curves with increased surgical efficiency are welcome, as long they prove themselves to be comparable in terms of outcome and cost-effective. The future of laparoscopic gastric cancer surgery remains an area of continued innovation and anticipation as it offers the enhanced management and outcomes to combat this unremitting global disease.
P- Reviewer: Kuper MA, Spinoglio G, Zheng B S- Editor: Ma YJ L- Editor: A E- Editor: Wang CH
| 1. | Santoro E. The history of gastric cancer: legends and chronicles. Gastric Cancer. 2005;8:71-74. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 2. | Kitano S, Iso Y, Moriyama M, Sugimachi K. Laparoscopy-assisted Billroth I gastrectomy. Surg Laparosc Endosc. 1994;4:146-148. [PubMed] [Cited in This Article: ] |
| 3. | Parkin DM, Pisani P, Ferlay J. Estimates of the worldwide incidence of eighteen major cancers in 1985. Int J Cancer. 1993;54:594-606. [PubMed] [Cited in This Article: ] |
| 4. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23762] [Cited by in F6Publishing: 25182] [Article Influence: 1937.1] [Reference Citation Analysis (3)] |
| 5. | Noguchi Y, Yoshikawa T, Tsuburaya A, Motohashi H, Karpeh MS, Brennan MF. Is gastric carcinoma different between Japan and the United States? Cancer. 2000;89:2237-2246. [PubMed] [Cited in This Article: ] |
| 6. | Strong VE, Song KY, Park CH, Jacks LM, Gonen M, Shah M, Coit DG, Brennan MF. Comparison of gastric cancer survival following R0 resection in the United States and Korea using an internationally validated nomogram. Ann Surg. 2010;251:640-646. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 250] [Cited by in F6Publishing: 278] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 7. | Strong VE, Song KY, Park CH, Jacks LM, Gonen M, Shah MA, Coit DG, Brennan MF. Comparison of disease-specific survival in the United States and Korea after resection for early-stage node-negative gastric carcinoma. J Surg Oncol. 2013;107:634-640. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 8. | Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14:101-112. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2390] [Cited by in F6Publishing: 2656] [Article Influence: 204.3] [Reference Citation Analysis (0)] |
| 9. | Sobin LH, Gospodarowicz MK, Wittekind C; International Union against Cancer. TNM classification of malignant tumours. 7th ed. West Sussex, UK: Chichester; Hoboken, NJ: Wiley-Blackwell 2010; . [Cited in This Article: ] |
| 10. | Sasako M, Sano T, Yamamoto S, Kurokawa Y, Nashimoto A, Kurita A, Hiratsuka M, Tsujinaka T, Kinoshita T, Arai K. D2 lymphadenectomy alone or with para-aortic nodal dissection for gastric cancer. N Engl J Med. 2008;359:453-462. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 698] [Cited by in F6Publishing: 720] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 11. | Hartgrink HH, van de Velde CJ, Putter H, Bonenkamp JJ, Klein Kranenbarg E, Songun I, Welvaart K, van Krieken JH, Meijer S, Plukker JT. Extended lymph node dissection for gastric cancer: who may benefit? Final results of the randomized Dutch gastric cancer group trial. J Clin Oncol. 2004;22:2069-2077. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 647] [Cited by in F6Publishing: 633] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 12. | Degiuli M, Sasako M, Ponti A; Italian Gastric Cancer Study Group. Morbidity and mortality in the Italian Gastric Cancer Study Group randomized clinical trial of D1 versus D2 resection for gastric cancer. Br J Surg. 2010;97:643-649. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 199] [Cited by in F6Publishing: 217] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 13. | Ajani JA, Bentrem DJ, Besh S, D’Amico TA, Das P, Denlinger C, Fakih MG, Fuchs CS, Gerdes H, Glasgow RE. Gastric cancer, version 2.2013: featured updates to the NCCN Guidelines. J Natl Compr Canc Netw. 2013;11:531-546. [PubMed] [Cited in This Article: ] |
| 14. | Okines A, Verheij M, Allum W, Cunningham D, Cervantes A. Gastric cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21 Suppl 5:v50-v54. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 208] [Cited by in F6Publishing: 247] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 15. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer. 2011;14:113-123. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1723] [Cited by in F6Publishing: 1844] [Article Influence: 141.8] [Reference Citation Analysis (0)] |
| 16. | Krijnen P, den Dulk M, Meershoek-Klein Kranenbarg E, Jansen-Landheer ML, van de Velde CJ. Improved survival after resectable non-cardia gastric cancer in The Netherlands: the importance of surgical training and quality control. Eur J Surg Oncol. 2009;35:715-720. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 17. | Guggenheim DE, Shah MA. Gastric cancer epidemiology and risk factors. J Surg Oncol. 2013;107:230-236. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 296] [Cited by in F6Publishing: 350] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 18. | Etoh T, Shiraishi N, Kitano S. Current trends of laparoscopic gastrectomy for gastric cancer in Japan. Asian J Endosc Surg. 2009;2:18-23. [DOI] [Cited in This Article: ] |
| 19. | Yasunaga H, Horiguchi H, Kuwabara K, Matsuda S, Fushimi K, Hashimoto H, Ayanian JZ. Outcomes after laparoscopic or open distal gastrectomy for early-stage gastric cancer: a propensity-matched analysis. Ann Surg. 2013;257:640-646. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 105] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 20. | Yang HK, Suh YS, Lee HJ. Minimally invasive approaches for gastric cancer-Korean experience. J Surg Oncol. 2013;107:277-281. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 21. | Strong VE, Devaud N, Karpeh M. The role of laparoscopy for gastric surgery in the West. Gastric Cancer. 2009;12:127-131. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 22. | Rodríguez Santiago JM, Clemares M, Roig-Garcia J, Asensio JI, Feliu X, Toscano E, Resa J, Targarona E, Ibáñez-Aguirre J, Castell J. [Gastric cancer and laparoscopy: analysis of data from the national register of laparoscopic gastric surgery]. Cir Esp. 2009;85:280-286. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 23. | Brar S, Law C, McLeod R, Helyer L, Swallow C, Paszat L, Seevaratnam R, Cardoso R, Dixon M, Mahar A. Defining surgical quality in gastric cancer: a RAND/UCLA appropriateness study. J Am Coll Surg. 2013;217:347-357.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 24. | Lutz MP, Zalcberg JR, Ducreux M, Ajani JA, Allum W, Aust D, Bang YJ, Cascinu S, Hölscher A, Jankowski J. Highlights of the EORTC St. Gallen International Expert Consensus on the primary therapy of gastric, gastroesophageal and oesophageal cancer - differential treatment strategies for subtypes of early gastroesophageal cancer. Eur J Cancer. 2012;48:2941-2953. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 96] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 25. | Suh YS, Han DS, Kong SH, Kwon S, Shin CI, Kim WH, Kim HH, Lee HJ, Yang HK. Laparoscopy-assisted pylorus-preserving gastrectomy is better than laparoscopy-assisted distal gastrectomy for middle-third early gastric cancer. Ann Surg. 2014;259:485-493. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 96] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 26. | Lee HJ, Shiraishi N, Kim HH, Hiki N, Uyama I, Choi SH, Yang HK, Kitano S. Standard of practice on laparoscopic gastric cancer surgery in Korea and Japan: experts’ survey. Asian J Endosc Surg. 2012;5:5-11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 27. | Kanaji S, Harada H, Nakayama S, Yasuda T, Oshikiri T, Kawasaki K, Yamamoto M, Imanishi T, Nakamura T, Suzuki S. Surgical outcomes in the newly introduced phase of intracorporeal anastomosis following laparoscopic distal gastrectomy is safe and feasible compared with established procedures of extracorporeal anastomosis. Surg Endosc. 2014;28:1250-1255. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 28. | Lee SW, Tanigawa N, Nomura E, Tokuhara T, Kawai M, Yokoyama K, Hiramatsu M, Okuda J, Uchiyama K. Benefits of intracorporeal gastrointestinal anastomosis following laparoscopic distal gastrectomy. World J Surg Oncol. 2012;10:267. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 29. | Kim DG, Choi YY, An JY, Kwon IG, Cho I, Kim YM, Bae JM, Song MG, Noh SH. Comparing the short-term outcomes of totally intracorporeal gastroduodenostomy with extracorporeal gastroduodenostomy after laparoscopic distal gastrectomy for gastric cancer: a single surgeon’s experience and a rapid systematic review with meta-analysis. Surg Endosc. 2013;27:3153-3161. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 30. | Kitano S, Shiraishi N, Fujii K, Yasuda K, Inomata M, Adachi Y. A randomized controlled trial comparing open vs laparoscopy-assisted distal gastrectomy for the treatment of early gastric cancer: an interim report. Surgery. 2002;131:S306-S311. [PubMed] [Cited in This Article: ] |
| 31. | Hayashi H, Ochiai T, Shimada H, Gunji Y. Prospective randomized study of open versus laparoscopy-assisted distal gastrectomy with extraperigastric lymph node dissection for early gastric cancer. Surg Endosc. 2005;19:1172-1176. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 251] [Cited by in F6Publishing: 251] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 32. | Huscher CG, Mingoli A, Sgarzini G, Sansonetti A, Di Paola M, Recher A, Ponzano C. Laparoscopic versus open subtotal gastrectomy for distal gastric cancer: five-year results of a randomized prospective trial. Ann Surg. 2005;241:232-237. [PubMed] [Cited in This Article: ] |
| 33. | Lee JH, Han HS, Lee JH. A prospective randomized study comparing open vs laparoscopy-assisted distal gastrectomy in early gastric cancer: early results. Surg Endosc. 2005;19:168-173. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 308] [Cited by in F6Publishing: 298] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 34. | Kim YW, Yoon HM, Yun YH, Nam BH, Eom BW, Baik YH, Lee SE, Lee Y, Kim YA, Park JY. Long-term outcomes of laparoscopy-assisted distal gastrectomy for early gastric cancer: result of a randomized controlled trial (COACT 0301). Surg Endosc. 2013;27:4267-4276. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 86] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 35. | Kim HH, Hyung WJ, Cho GS, Kim MC, Han SU, Kim W, Ryu SW, Lee HJ, Song KY. Morbidity and mortality of laparoscopic gastrectomy versus open gastrectomy for gastric cancer: an interim report--a phase III multicenter, prospective, randomized Trial (KLASS Trial). Ann Surg. 2010;251:417-420. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 562] [Cited by in F6Publishing: 627] [Article Influence: 44.8] [Reference Citation Analysis (0)] |
| 36. | Takiguchi S, Fujiwara Y, Yamasaki M, Miyata H, Nakajima K, Sekimoto M, Mori M, Doki Y. Laparoscopy-assisted distal gastrectomy versus open distal gastrectomy. A prospective randomized single-blind study. World J Surg. 2013;37:2379-2386. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 88] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 37. | Nakamura K, Katai H, Mizusawa J, Yoshikawa T, Ando M, Terashima M, Ito S, Takagi M, Takagane A, Ninomiya M. A phase III study of laparoscopy-assisted versus open distal gastrectomy with nodal dissection for clinical stage IA/IB gastric Cancer (JCOG0912). Jpn J Clin Oncol. 2013;43:324-327. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 108] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 38. | Lee J, Kim W. Clinical experience of 528 laparoscopic gastrectomies on gastric cancer in a single institution. Surgery. 2013;153:611-618. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 39. | Rosin D, Goldes Y, Bar Zakai B, Shabtai M, Ayalon A, Zmora O. Laparoscopic subtotal gastrectomy for gastric cancer. JSLS. 2009;13:318-322. [PubMed] [Cited in This Article: ] |
| 40. | Strong VE, Devaud N, Allen PJ, Gonen M, Brennan MF, Coit D. Laparoscopic versus open subtotal gastrectomy for adenocarcinoma: a case-control study. Ann Surg Oncol. 2009;16:1507-1513. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 144] [Cited by in F6Publishing: 153] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 41. | Grantcharov TP, Kehlet H. Laparoscopic gastric surgery in an enhanced recovery programme. Br J Surg. 2010;97:1547-1551. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 61] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 42. | Pugliese R, Maggioni D, Sansonna F, Costanzi A, Ferrari GC, Di Lernia S, Magistro C, De Martini P, Pugliese F. Subtotal gastrectomy with D2 dissection by minimally invasive surgery for distal adenocarcinoma of the stomach: results and 5-year survival. Surg Endosc. 2010;24:2594-2602. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 120] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 43. | Xuan Y, Hur H, Byun CS, Han SU, Cho YK. Efficacy of intraoperative gastroscopy for tumor localization in totally laparoscopic distal gastrectomy for cancer in the middle third of the stomach. Surg Endosc. 2013;27:4364-4370. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 44. | Kim HI, Hyung WJ, Lee CR, Lim JS, An JY, Cheong JH, Choi SH, Noh SH. Intraoperative portable abdominal radiograph for tumor localization: a simple and accurate method for laparoscopic gastrectomy. Surg Endosc. 2011;25:958-963. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 45. | Kanaya S, Kawamura Y, Kawada H, Iwasaki H, Gomi T, Satoh S, Uyama I. The delta-shaped anastomosis in laparoscopic distal gastrectomy: analysis of the initial 100 consecutive procedures of intracorporeal gastroduodenostomy. Gastric Cancer. 2011;14:365-371. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 114] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 46. | Jeong SH, Lee YJ, Choi WJ, Paik WY, Jeong CY, Park ST, Choi SK, Hong SC, Jung EJ, Joo YT. Trans-vaginal specimen extraction following totally laparoscopic subtotal gastrectomy in early gastric cancer. Gastric Cancer. 2011;14:91-96. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 47. | Kim YW, Baik YH, Yun YH, Nam BH, Kim DH, Choi IJ, Bae JM. Improved quality of life outcomes after laparoscopy-assisted distal gastrectomy for early gastric cancer: results of a prospective randomized clinical trial. Ann Surg. 2008;248:721-727. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 475] [Cited by in F6Publishing: 492] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 48. | Viñuela EF, Gonen M, Brennan MF, Coit DG, Strong VE. Laparoscopic versus open distal gastrectomy for gastric cancer: a meta-analysis of randomized controlled trials and high-quality nonrandomized studies. Ann Surg. 2012;255:446-456. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 277] [Cited by in F6Publishing: 297] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 49. | Zeng YK, Yang ZL, Peng JS, Lin HS, Cai L. Laparoscopy-assisted versus open distal gastrectomy for early gastric cancer: evidence from randomized and nonrandomized clinical trials. Ann Surg. 2012;256:39-52. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 155] [Cited by in F6Publishing: 164] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 50. | Jiang L, Yang KH, Guan QL, Cao N, Chen Y, Zhao P, Chen YL, Yao L. Laparoscopy-assisted gastrectomy versus open gastrectomy for resectable gastric cancer: an update meta-analysis based on randomized controlled trials. Surg Endosc. 2013;27:2466-2480. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 51. | Wang Y, Wang S, Huang ZQ, Chou WP. Meta-analysis of laparoscopy assisted distal gastrectomy and conventional open distal gastrectomy for EGC. Surgeon. 2014;12:53-58. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 52. | Memon MA, Khan S, Yunus RM, Barr R, Memon B. Meta-analysis of laparoscopic and open distal gastrectomy for gastric carcinoma. Surg Endosc. 2008;22:1781-1789. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 145] [Cited by in F6Publishing: 137] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 53. | Kurokawa Y, Katai H, Fukuda H, Sasako M; Gastric Cancer Surgical Study Group of the Japan Clinical Oncology Group. Phase II study of laparoscopy-assisted distal gastrectomy with nodal dissection for clinical stage I gastric cancer: Japan Clinical Oncology Group Study JCOG0703. Jpn J Clin Oncol. 2008;38:501-503. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 54. | Kim HH, Han SU, Kim MC, Hyung WJ, Kim W, Lee HJ, Ryu SW, Cho GS, Kim CY, Yang HK. Prospective randomized controlled trial (phase III) to comparing laparoscopic distal gastrectomy with open distal gastrectomy for gastric adenocarcinoma (KLASS 01). J Korean Surg Soc. 2013;84:123-130. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 90] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 55. | Etoh T, Inomata M, Shiraishi N, Kitano S. Minimally invasive approaches for gastric cancer-Japanese experiences. J Surg Oncol. 2013;107:282-288. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 56. | Cai J, Wei D, Gao CF, Zhang CS, Zhang H, Zhao T. A prospective randomized study comparing open versus laparoscopy-assisted D2 radical gastrectomy in advanced gastric cancer. Dig Surg. 2011;28:331-337. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 119] [Cited by in F6Publishing: 130] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 57. | Martínez-Ramos D, Miralles-Tena JM, Cuesta MA, Escrig-Sos J, Van der Peet D, Hoashi JS, Salvador-Sanchís JL. Laparoscopy versus open surgery for advanced and resectable gastric cancer: a meta-analysis. Rev Esp Enferm Dig. 2011;103:133-141. [PubMed] [Cited in This Article: ] |
| 58. | Lee JH, Son SY, Lee CM, Ahn SH, Park do J, Kim HH. Morbidity and mortality after laparoscopic gastrectomy for advanced gastric cancer: results of a phase II clinical trial. Surg Endosc. 2013;27:2877-2885. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 59. | Lee HJ, Yang HK. Laparoscopic gastrectomy for gastric cancer. Dig Surg. 2013;30:132-141. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 60. | Kodera Y. Surgery for gastric cancer: has the East versus West issue been solved? Dig Surg. 2013;30:92-95. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 61. | Jeong O, Park YK. Clinicopathological features and surgical treatment of gastric cancer in South Korea: the results of 2009 nationwide survey on surgically treated gastric cancer patients. J Gastric Cancer. 2011;11:69-77. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 185] [Cited by in F6Publishing: 185] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 62. | Sano T, Yamamoto S, Sasako M; Japan Clinical Oncology Group Study LCOG 0110-MF. Randomized controlled trial to evaluate splenectomy in total gastrectomy for proximal gastric carcinoma: Japan clinical oncology group study JCOG 0110-MF. Jpn J Clin Oncol. 2002;32:363-364. [PubMed] [Cited in This Article: ] |
| 63. | Kitano S. Laparoscopic Gastrectomy for Cancer: Standard Techniques and Clinical Evidences. Tokyo: Springer Japan 2012; . [Cited in This Article: ] |
| 64. | Dulucq JL, Wintringer P, Perissat J, Mahajna A. Completely laparoscopic total and partial gastrectomy for benign and malignant diseases: a single institute’s prospective analysis. J Am Coll Surg. 2005;200:191-197. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 53] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 65. | Huscher CG, Mingoli A, Sgarzini G, Brachini G, Binda B, Di Paola M, Ponzano C. Totally laparoscopic total and subtotal gastrectomy with extended lymph node dissection for early and advanced gastric cancer: early and long-term results of a 100-patient series. Am J Surg. 2007;194:839-44; discussion 844. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 90] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 66. | Jeong GA, Cho GS, Kim HH, Lee HJ, Ryu SW, Song KY. Laparoscopy-assisted total gastrectomy for gastric cancer: a multicenter retrospective analysis. Surgery. 2009;146:469-474. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 92] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 67. | Shinohara T, Kanaya S, Taniguchi K, Fujita T, Yanaga K, Uyama I. Laparoscopic total gastrectomy with D2 lymph node dissection for gastric cancer. Arch Surg. 2009;144:1138-1142. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 97] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 68. | LaFemina J, Viñuela EF, Schattner MA, Gerdes H, Strong VE. Esophagojejunal reconstruction after total gastrectomy for gastric cancer using a transorally inserted anvil delivery system. Ann Surg Oncol. 2013;20:2975-2983. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 69. | Wang W, Li Z, Tang J, Wang M, Wang B, Xu Z. Laparoscopic versus open total gastrectomy with D2 dissection for gastric cancer: a meta-analysis. J Cancer Res Clin Oncol. 2013;139:1721-1734. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 70. | Haverkamp L, Weijs TJ, van der Sluis PC, van der Tweel I, Ruurda JP, van Hillegersberg R. Laparoscopic total gastrectomy versus open total gastrectomy for cancer: a systematic review and meta-analysis. Surg Endosc. 2013;27:1509-1520. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 131] [Cited by in F6Publishing: 138] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 71. | Chen K, Xu XW, Zhang RC, Pan Y, Wu D, Mou YP. Systematic review and meta-analysis of laparoscopy-assisted and open total gastrectomy for gastric cancer. World J Gastroenterol. 2013;19:5365-5376. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 59] [Cited by in F6Publishing: 67] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 72. | Kim MC, Jung GJ, Kim HH. Learning curve of laparoscopy-assisted distal gastrectomy with systemic lymphadenectomy for early gastric cancer. World J Gastroenterol. 2005;11:7508-7511. [PubMed] [Cited in This Article: ] |
| 73. | Jin SH, Kim DY, Kim H, Jeong IH, Kim MW, Cho YK, Han SU. Multidimensional learning curve in laparoscopy-assisted gastrectomy for early gastric cancer. Surg Endosc. 2007;21:28-33. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 116] [Cited by in F6Publishing: 106] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 74. | Kunisaki C, Makino H, Yamamoto N, Sato T, Oshima T, Nagano Y, Fujii S, Akiyama H, Otsuka Y, Ono HA. Learning curve for laparoscopy-assisted distal gastrectomy with regional lymph node dissection for early gastric cancer. Surg Laparosc Endosc Percutan Tech. 2008;18:236-241. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 79] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 75. | Yoo CH, Kim HO, Hwang SI, Son BH, Shin JH, Kim H. Short-term outcomes of laparoscopic-assisted distal gastrectomy for gastric cancer during a surgeon’s learning curve period. Surg Endosc. 2009;23:2250-2257. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 76. | Kang SY, Lee SY, Kim CY, Yang DH. Comparison of Learning Curves and Clinical Outcomes between Laparoscopy-assisted Distal Gastrectomy and Open Distal Gastrectomy. J Gastric Cancer. 2010;10:247-253. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 77. | Nunobe S, Hiki N, Tanimura S, Nohara K, Sano T, Yamaguchi T. The clinical safety of performing laparoscopic gastrectomy for gastric cancer by trainees after sufficient experience in assisting. World J Surg. 2013;37:424-429. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 78. | Ichikawa D, Komatsu S, Kubota T, Okamoto K, Deguchi K, Tamai H, Obayashi T, Kitagawa K, Soga K, Inoue K. Effect of hospital volume on long-term outcomes of laparoscopic gastrectomy for clinical stage I gastric cancer. Anticancer Res. 2013;33:5165-5170. [PubMed] [Cited in This Article: ] |
| 79. | Dixon M, Mahar A, Paszat L, McLeod R, Law C, Swallow C, Helyer L, Seeveratnam R, Cardoso R, Bekaii-Saab T. What provider volumes and characteristics are appropriate for gastric cancer resection? Results of an international RAND/UCLA expert panel. Surgery. 2013;154:1100-1109. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 80. | de Steur WO, Dikken JL, Hartgrink HH. Lymph node dissection in resectable advanced gastric cancer. Dig Surg. 2013;30:96-103. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 81. | Mori T, Kimura T, Kitajima M. Skill accreditation system for laparoscopic gastroenterologic surgeons in Japan. Minim Invasive Ther Allied Technol. 2010;19:18-23. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 122] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 82. | Heemskerk J, van Gemert WG, de Vries J, Greve J, Bouvy ND. Learning curves of robot-assisted laparoscopic surgery compared with conventional laparoscopic surgery: an experimental study evaluating skill acquisition of robot-assisted laparoscopic tasks compared with conventional laparoscopic tasks in inexperienced users. Surg Laparosc Endosc Percutan Tech. 2007;17:171-174. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 79] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 83. | Song J, Oh SJ, Kang WH, Hyung WJ, Choi SH, Noh SH. Robot-assisted gastrectomy with lymph node dissection for gastric cancer: lessons learned from an initial 100 consecutive procedures. Ann Surg. 2009;249:927-932. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 213] [Cited by in F6Publishing: 214] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 84. | Yoon HM, Kim YW, Lee JH, Ryu KW, Eom BW, Park JY, Choi IJ, Kim CG, Lee JY, Cho SJ. Robot-assisted total gastrectomy is comparable with laparoscopically assisted total gastrectomy for early gastric cancer. Surg Endosc. 2012;26:1377-1381. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 104] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 85. | Kim MC, Heo GU, Jung GJ. Robotic gastrectomy for gastric cancer: surgical techniques and clinical merits. Surg Endosc. 2010;24:610-615. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 154] [Cited by in F6Publishing: 154] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 86. | Anderson C, Ellenhorn J, Hellan M, Pigazzi A. Pilot series of robot-assisted laparoscopic subtotal gastrectomy with extended lymphadenectomy for gastric cancer. Surg Endosc. 2007;21:1662-1666. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 70] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 87. | Huang KH, Lan YT, Fang WL, Chen JH, Lo SS, Hsieh MC, Li AF, Chiou SH, Wu CW. Initial experience of robotic gastrectomy and comparison with open and laparoscopic gastrectomy for gastric cancer. J Gastrointest Surg. 2012;16:1303-1310. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 105] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 88. | D’Annibale A, Pende V, Pernazza G, Monsellato I, Mazzocchi P, Lucandri G, Morpurgo E, Contardo T, Sovernigo G. Full robotic gastrectomy with extended (D2) lymphadenectomy for gastric cancer: surgical technique and preliminary results. J Surg Res. 2011;166:e113-e120. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 67] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 89. | Marano A, Choi YY, Hyung WJ, Kim YM, Kim J, Noh SH. Robotic versus Laparoscopic versus Open Gastrectomy: A Meta-Analysis. J Gastric Cancer. 2013;13:136-148. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 93] [Article Influence: 8.5] [Reference Citation Analysis (0)] |