Published online Oct 14, 2014. doi: 10.3748/wjg.v20.i38.13973
Revised: June 4, 2014
Accepted: June 25, 2014
Published online: October 14, 2014
Processing time: 230 Days and 16.1 Hours
AIM: To establish the feasibility of simultaneous modulated accelerated radiation therapy (SMART) in esophageal cancer (EC).
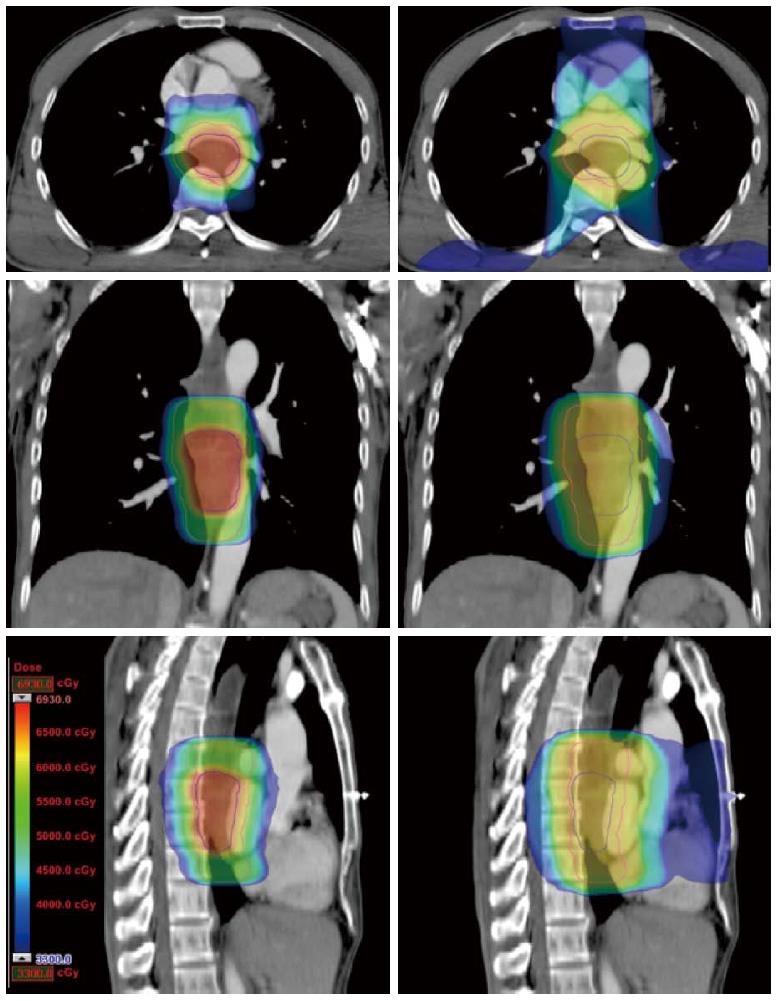
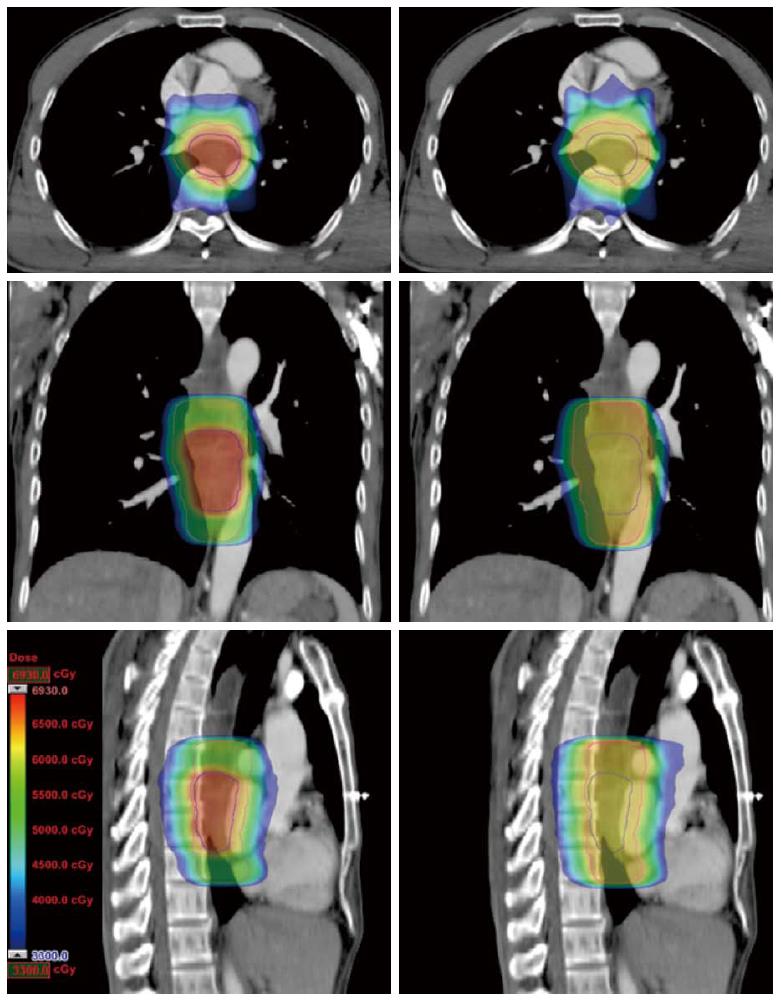
METHODS: Computed tomography (CT) datasets of 10 patients with upper or middle thoracic squamous cell EC undergoing chemoradiotherapy were used to generate SMART, conventionally-fractionated three-dimensional conformal radiotherapy (3DCRT) and intensity-modulated radiation therapy (cf-IMRT) plans, respectively. The gross target volume (GTV) of the esophagus, positive regional lymph nodes (LN), and suspected lymph nodes (LN±) were contoured for each patient. The clinical target volume (CTV) was delineated with 2-cm longitudinal and 0.5- to 1.0-cm radial margins with respect to the GTV and with 0.5-cm uniform margins for LN and LN(±). For the SMART plans, there were two planning target volumes (PTVs): PTV66 = (GTV + LN) + 0.5 cm and PTV54 = CTV + 0.5 cm. For the 3DCRT and cf-IMRT plans, there was only a single PTV: PTV60 = CTV + 0.5 cm. The prescribed dose for the SMART plans was 66 Gy/30 F to PTV66 and 54 Gy/30 F to PTV54. The dose prescription to the PTV60 for both the 3DCRT and cf-IMRT plans was set to 60 Gy/30 F. All the plans were generated on the Eclipse 10.0 treatment planning system. Fulfillment of the dose criteria for the PTVs received the highest priority, followed by the spinal cord, heart, and lungs. The dose-volume histograms were compared.
RESULTS: Clinically acceptable plans were achieved for all the SMART, cf-IMRT, and 3DCRT plans. Compared with the 3DCRT plans, the SMART plans increased the dose delivered to the primary tumor (66 Gy vs 60 Gy), with improved sparing of normal tissues in all patients. The Dmax of the spinal cord, V20 of the lungs, and Dmean and V50 of the heart for the SMART and 3DCRT plans were as follows: 38.5 ± 2.0 vs 44.7 ± 0.8 (P = 0.002), 17.1 ± 4.0 vs 25.8 ± 5.0 (P = 0.000), 14.4 ± 7.5 vs 21.4 ± 11.1 (P = 0.000), and 4.9 ± 3.4 vs 12.9 ± 7.6 (P = 0.000), respectively. In contrast to the cf-IMRT plans, the SMART plans permitted a simultaneous dose escalation (6 Gy) to the primary tumor while demonstrating a significant trend of a lower irradiation dose to all organs at risk except the spinal cord, for which no significant difference was found.
CONCLUSION: SMART offers the potential for a 6 Gy simultaneous escalation in the irradiation dose delivered to the primary tumor of EC and improves the sparing of normal tissues.
Core tip: The feasibility of simultaneous modulated accelerated radiotherapy (SMART) in the treatment of upper or middle thoracic esophageal cancer is evaluated in this study. Computed tomography datasets of 10 patients were used to generate SMART, conventionally-fractionated three-dimensional conformal radiotherapy (3DCRT) and intensity-modulated radiotherapy (cf-IMRT) plans, respectively. The prescribed dose for the SMART plans was 66 Gy/30 F to the gross tumor and 54 Gy/30 F to subclinical diseases. The dose for both the 3DCRT and cf-IMRT was 60 Gy/30 F to a single target volume. The results demonstrate that SMART can offer the potential for a 6 Gy simultaneous escalation in the dose delivered to the primary tumor of esophageal cancer and improve normal tissue sparing compared with 3DCRT and cf-IMRT.
- Citation: Zhang WZ, Chen JZ, Li DR, Chen ZJ, Guo H, Zhuang TT, Li DS, Zhou MZ, Chen CZ. Simultaneous modulated accelerated radiation therapy for esophageal cancer: A feasibility study. World J Gastroenterol 2014; 20(38): 13973-13980
- URL: https://www.wjgnet.com/1007-9327/full/v20/i38/13973.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i38.13973
Based on the results of the Radiation Therapy Oncology Group (RTOG) phase III intergroup trials RTOG 85-01 and 94-05, the standard therapy for patients with localized esophageal cancer (EC) selected for nonsurgical treatment is radiation therapy (RT) plus concurrent chemotherapy with a standard radiation dose of 50.4 Gy[1-3]. Although there is significant variation in the range of acceptable doses (50-66 Gy) and in the acceptable chemotherapeutic regimens, local-regional failure after chemoradiation remains a significant clinical problem.
The techniques for radiation planning, tumor imaging, and radiation delivery have advanced rapidly over the past several decades. The benefits of simultaneous radiation dose escalation for tumors at other anatomic sites in terms of improved local control and survival have been demonstrated, and dose escalation could logically be expected to also apply to EC[4-6]. A study including distal EC conducted at the M.D. Anderson Cancer Center demonstrated that using a simultaneous integrated boost (SIB) technique could increase the dose to the gross target volume (GTV) while simultaneously reducing the dose to adjacent critical structures[7].
Since EC is one of the most common malignant diseases in China (with an estimated incidence and mortality of approximately 22.4 and 16.8 per 100000 inhabitants, respectively, in 2009), this issue is of a particular importance[8]. In contrast to reports from other countries, squamous cell carcinoma (SCC) is the predominant pathology for EC diagnosed in China. In addition, this malignant disease commonly occurs in the upper or middle thoracic esophagus in China, whereas it is more often found in the distal esophagus in western countries. Therefore, investigating different treatment paradigms for SCC of the esophagus arising from the upper or middle thoracic esophagus is essential and meaningful.
In order to evaluate the potential clinical benefit of SMART in EC, we initiated a phase II clinical trial (NCT01670409)[9]. This study represents the preliminary results of said trial, aiming to establish the feasibility of SMART in EC and compare it dosimetrically with conventionally-fractionated three-dimensional radiation therapy (3DCRT) and intensity-modulated radiation therapy (cf-IMRT).
The computer tomography (CT) datasets of 10 patients with pathologically confirmed upper or middle thoracic esophageal squamous cell carcinoma treated with chemoradiation at the Cancer Hospital of Shantou University Medical College were identified. This retrospective study was approved by the institutional ethics committee. Informed consent was obtained from all subjects prior to the study. The CT datasets were acquired using a 16-slice CT scanner (Philips Brilliance CT Big Bore Oncology Configuration, Cleveland, OH, United States) for all patients. Immobilization was achieved using a thermoplastic shell with arms resting at both sides of the body in the supine position. The CT images were delivered to the Eclipse 10.0 treatment planning system (Varian Medical Systems, Palo Alto, CA, United States) for target volume, organs at risk (OAR) contouring, and subsequent treatment planning.
The GTV of the EC, positive regional lymph nodes (LN), and suspected lymph nodes [LN(±)] were contoured by the attending radiation physician for each patient. The GTV was defined using CT images, endoscopic report, and barium swallow fluoroscopy. The mediastinal or supraclavicular lymph node with the shortest axis ≥ 10 mm was defined as positive, and the paraesophageal or tracheoesophageal groove lymph node (the shortest axis < 10 mm but ≥ 5 mm) was defined as LN(±). The clinical target volume (CTV) was delineated with 2-cm longitudinal and 0.5- to 1.0-cm radial margins with respect to the GTV for all patients, and with 0.5-cm uniform margins for LN and LN(±), edited from the surrounding vessels, lung, and bone. Planning target volumes (PTVs) were derived from the GTV + LN or CTV plus a uniform 0.5-cm margin. For the SMART plans, there were two PTVs: PTV66 = (GTV + LN) + 0.5 cm and PTV54 = CTV + 0.5 cm. For the 3DCRT and cf-IMRT plans, there was only a single PTV: PTV60 = CTV + 0.5 cm. The clinical information and target volume are shown in Table 1. OAR contours were created for the spinal cord (planning risk volume or SC-PRV), lungs, and heart.
| No. | Site | Stage1 | V-GTV (cc) | V-LN (cc) | V-PTV66 (cc) | V-PTV60/54 (cc) |
| 1 | Upper | T2N1M1b | 11.5 | 11 | 80 | 206.3 |
| 2 | Middle | T3N0M0 | 18.1 | 0 | 49.1 | 151.2 |
| 3 | Upper | T3N1M1a | 38.8 | 3 | 102.1 | 253.3 |
| 4 | Upper | T3N1M0 | 25.4 | 1.3 | 75 | 191.6 |
| 5 | Upper | T3N2M0 | 16.9 | 1 | 47.8 | 196.4 |
| 6 | Middle | T3N1M0 | 24.6 | 6 | 86.8 | 197.6 |
| 7 | Middle | T4N1M1b | 32.1 | 2.7 | 82.5 | 214.4 |
| 8 | Middle | T3N0Mo | 15.8 | 0 | 42.5 | 149.9 |
| 9 | Middle | T3N0Mo | 14.9 | 0 | 41 | 144.6 |
| 10 | Upper | T3N0Mo | 16.1 | 0 | 45.5 | 161.9 |
The prescribed dose for the SMART plans was 66 Gy/30 F to PTV66 (delivered in 2.2-Gy fractions) and 54 Gy/30 F to PTV54 (delivered in 1.8-Gy fractions). The dose prescription to the PTV60 for both the 3DCRT and cf-IMRT plans was set to 60 Gy/30 F at 2 Gy/fraction.
For each patient, SMART, 3DCRT, and cf-IMRT plans were generated on the Eclipse 10.0 treatment planning system with the same goals and objectives. The planning objectives for PTV were 100% of the PTV volume receiving the prescribed dose, with V107% < 5% and V93% < 1% (Vn% = percentage of the PTV covered by n% of the prescribed dose). The planning objectives for OARs were defined as follows: spinal cord, Dmax (maximum dose) < 45 Gy; spinal cord planning risk volume (SC-PRV), Dmax < 50 Gy; heart, V40 (Vm = % of the whole OAR receiving ≤ mGy) < 100%, V45 < 67% and V50 < 33%; lungs, V20 < 30%, V10 < 50% and V5 < 60%. In the optimization process, fulfillment of the dose criteria for the PTVs received the highest priority, followed by the spinal cord, heart, and lungs. Only when the criteria for PTVs were fulfilled was the system considered sparing of OARs.
The SMART and cf-IMRT plans were generated using a sliding window dynamic delivery with 5 coplanar beams (angles: 210°/300°/0°/60°/150°). The beam arrangements of the 3DCRT plans were selected and optimized based on the PTV location, shape, extension, and relationship to relevant OARs, included the anterior beam + two posterior oblique fields in 6 cases, 4-field box in 2 cases, and parallel-opposed anteroposterior-posteroanterior portals followed by parallel-opposed oblique fields in 2 cases.
All plans were designed to be delivered using 6-MV photon beams from a Varian Truebeam linear accelerator (Varian Medical Systems, Palo Alto, CA, United States) equipped with an MLC with 120 leaves (spatial resolution of 5 mm at the isocenter for the central 20 cm and 10 mm for the outer 20 cm). Both SMART and IMRT plans were optimized by selecting a maximum DR of 600 MU/min. Plan optimization in both the SMART and cf-IMRT cases was completely disentangled from the dose calculation and performed with the IMRT Dose Volume optimizer (DVO), which was implemented in Eclipse 10.0. The maximum number of iterations was set at 1000, with the maximum time set at 100 min. The goal of the optimization was considered to be reached and the process terminated when the value of the objective function approached a minimum and showed no further decrease in value. The dose calculation was performed using the Anisotropic Analytical Algorithm (AAA, version 8.6.02) using a grid of 2.5 mm.
Quantitative evaluation of the plans was performed using the standard dose-volume histogram (DVH). For the PTVs, the parameters included the mean dose, V93%, V95%, V100%, and V107%. For the OARs, the analysis included the mean dose, maximum dose, and a set of appropriate Vm values. The average cumulative DVH for the PTVs and OARs was constructed from the individual DVHs. These histograms were obtained by averaging the corresponding volumes for the entire patient cohort for each dose bin of 0.05 Gy.
The Statistical Package for Social Sciences (SPSS, version 19.0, Chicago, IL) was used for statistical analysis in the present study. Comparisons of the DVHs were performed using paired, two-tailed Student’s t-test. A Wilcoxon matched-pair signed-rank test was alternatively used when the data did not follow a normal distribution. The results were considered statistically significant for P-values less than 0.05.
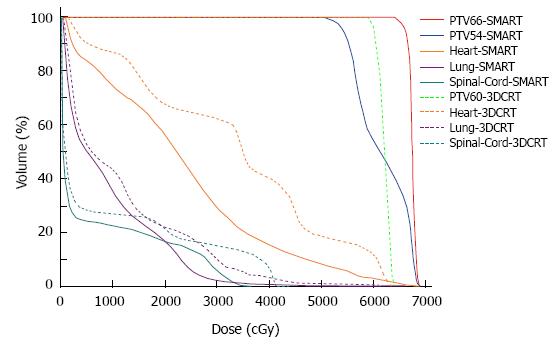
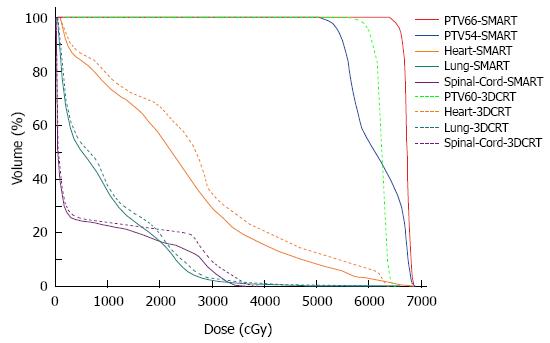
Clinically acceptable plans were achieved for all the SMART, cf-IMRT, and 3DCRT plans. Table 2 shows the numerical findings from the DVH analysis of the PTV and OARs. The data are presented as averages over the investigated patients, with errors indicating inter-patient variability at one standard deviation level.
| SMART | IMRT | 3DCRT | P value | |||
| SMART vs IMRT | SMART vs 3DCRT | |||||
| Cord | Dmax | 38.5 ± 2.0 | 39.5 ± 0.9 | 44.7 ± 0.8 | 0.660 | 0.0021 |
| D1cc | 35.6 ± 2.2 | 36.3 ± 1.2 | 41.6 ± 1.6 | 0.266 | 0.000 | |
| SC-PRV | Dmax | 44.5 ± 1.9 | 47.1 ± 2.7 | 59.3 ± 2.2 | 0.0101 | 0.0021 |
| D1cc | 40.8 ± 2.5 | 42.5 ± 2.2 | 52.9 ± 3.8 | 0.0271 | 0.0021 | |
| Heart | Dmean | 14.4 ± 7.5 | 16.2 ± 8.5 | 21.4 ± 11.1 | 0.000 | 0.000 |
| V20 | 34.1 ± 18.9 | 38.1 ± 21.9 | 42.7 ± 22.4 | 0.005 | 0.000 | |
| V30 | 18.2 ± 10.0 | 21.8 ± 11.6 | 38.0 ± 21.0 | 0.001 | 0.001 | |
| V40 | 9.4 ± 5.7 | 12.2 ± 7.2 | 25.3 ± 16.3 | 0.001 | 0.0021 | |
| V50 | 4.9 ± 3.4 | 7.0 ± 4.6 | 12.9 ± 7.6 | 0.001 | 0.000 | |
| Lung | Dmean | 9.0 ± 1.7 | 9.9 ± 1.8 | 12.4 ± 2.1 | 0.000 | 0.000 |
| V5 | 47.6 ± 9.7 | 50.4 ± 10.2 | 51.0 ± 9.9 | 0.000 | 0.017 | |
| V10 | 34.7 ± 7.4 | 37.6 ± 7.6 | 40.6 ± 8.1 | 0.000 | 0.002 | |
| V13 | 27.3 ± 5.9 | 30.2 ± 6.1 | 34.7 ± 7.3 | 0.000 | 0.000 | |
| V20 | 17.1 ± 4.0 | 20.2 ± 4.3 | 25.8 ± 5.0 | 0.000 | 0.000 | |
| V30 | 3.6 ± 1.3 | 4.7 ± 1.6 | 13.4 ± 4.8 | 0.000 | 0.000 | |
Compared with the 3DCRT plans, the SMART plans increased the dose delivered to the primary tumor (66 Gy vs 60 Gy), with improved sparing of normal tissues in all patients. The Dmax of the spinal cord, V20 of the lungs, and Dmean and V50 of the heart for the SMART and 3DCRT plans were as follows: 38.5 ± 2.0 vs 44.7 ± 0.8 (P = 0.002), 17.1 ± 4.0 vs 25.8 ± 5.0 (P = 0.000), 14.4 ± 7.5 vs 21.4 ± 11.1 (P = 0.000), and 4.9 ± 3.4 vs 12.9 ± 7.6 (P = 0.000), respectively. The dose distributions and corresponding DVHs for the SMART and 3DCRT plans in one patient are shown in Figures 1 and 2.
In contrast to the cf-IMRT plans, the SMART plans permitted a simultaneous dose escalation (6 Gy) to the primary tumor while demonstrating a significant trend of a lower irradiated dose to all organs at risk except the spinal cord, for which no significant difference was found. The dose distributions and corresponding DVHs for the SMART and cf-IMRT plans in one patient are shown in Figures 3 and 4.
The most widely accepted radiation technique for EC is 3DCRT, in which the GTV and a margin are typically treated with a uniform radiation dose of 50-60 Gy in 25-30 fractions. However, in our approach described in the current study, we hypothesize that the GTV and CTV should receive different radiation intensities because these areas have different densities of cancer cells[10]. Furthermore, the fractionated schedule for EC has remained 1.8-2.0 Gy per fraction for several decades, although the radiation techniques and imaging modalities have advanced significantly during this time. Therefore, investigating the application of modern radiotherapy techniques in EC with multiple target volumes, depending on the risk of disease, is important.
There is increasing evidence that tumor clonogen proliferation during conventional radiotherapy is a significant factor responsible for relapse in SCC of the upper respiratory and digestive tracts[11,12]. The accelerated repopulation of tumor cells occurs as the tumor shrinks, resulting in a more favorable microenvironment that allows tumor cells to proliferate. These factors may be an important aspect leading to local failure after radiotherapy. Nishimura et al[13] analyzed the local control and survival of patients with EC treated with radical radiation therapy, with a particular emphasis on the total treatment time and dose per fraction. They reported a 2.3% decrease in local control per day of delay in tumors treated with radiotherapy. Kajanti et al[14] reported a similar finding in an analysis of 353 EC SCC patients treated with radical radiotherapy. They also found that the overall decrease in treatment time was beneficial for the treatment of EC. Thus, avoiding a prolonged overall treatment time and increasing the radiation biologically equivalent dose (BED) are critical components for improving the locoregional control of EC.
SMART is a novel radiation technique that has been widely applied in head and neck cancer, non-small cell lung cancer, and prostate cancer. Using this technique, the gross tumor and sites of subclinical disease are treated with different doses depending on the clinical risk. Although a traditional fractionation schedule typically uses the same daily radiation dose, it is implicit with SMART that dose acceleration is present from the beginning of treatment, thus reducing the effect of accelerated tumor repopulation. In addition, a growing body of evidence now suggests that tumor recurrence is associated with failure to eradicate cancer stem cells. These cells, which are tumorigenic and are capable of self-renewal, are thought to be relatively radioresistant, and a high dose per fraction may be required for effective cell killing[15-17].
In this study, we report a comparison among SMART, 3DCRT, and cf-IMRT in EC patients to determine the feasibility of applying the SMART technique in EC patients (cases located in the distal esophagus were excluded in the current study). We found that SMART can offer the potential for a 6 Gy simultaneous escalation in doses delivered to the primary tumor and can improve normal tissue sparing compared with 3DCRT and cf-IMRT.
Previously, we performed a dosimetric study showing that cf-IMRT has a more conformal distribution of dose and better spinal cord sparing than 3DCRT, and can reduce the volume of the lung that receives a dose of 10 Gy or higher[18]. The current study also demonstrates that SMART could provide a significant and clinically relevant dosimetric benefit in greater sparing of OARs than 3DCRT. This result is consistent with other recently published reports[19,20].
Our data also suggest that SMART allows a 6 Gy dose escalation for the GTV, with a significant trend of a lower irradiated dose to all OARs except the spinal cord, in which no significant difference was found. One explanation could be that the dose prescribed to the subclinical disease was reduced from 60 Gy to 54 Gy, allowing more freedom to spare the neighboring tissues. Dogan et al[21] compared different IMRT boost delivery methods regarding target coverage and normal-tissue sparing in 15 patients with head-and-neck, lung, and prostate cancer. The results indicated that SIB-IMRT could markedly reduce doses to critical structures. The conformity of the SIB-IMRT plans was also superior to that obtained with both sequential-IMRT techniques. Welsh et al[7] reported a planning study in distal EC using the SIB technique to deliver a boost dose of radiation to GTV. In that study, the CTV and PTV received a standard IMRT dose of 50.4 Gy (1.8 Gy per fraction), and the dose to the GTV was simultaneously escalated to 64.8 Gy (28 fractions at 2.3 Gy per faction). Their results suggested that the use of SIB-IMRT allowed an increased dose to the GTV while simultaneously reducing the dose to the normal heart, lung, and liver. In the present report, we demonstrate similar findings for patients with cervical, upper, or middle thoracic EC, using the SMART technique.
Clinical data obtained in the treatment of lung cancer with external beam radiotherapy to very high doses have shown that severe esophagitis is rare, indicating that the esophagus may be more tolerant to external beam radiotherapy than common models have suggested[22]. The recent Quantitative Analyses of Normal Tissue Effects in the clinic review concluded that multiple volumetric parameters predict esophageal toxicity with conventional fractionation and suggests that up to 74 Gy may be tolerated by small volumes[23,24]. In the present study, the volume of the normal esophagus that received a dose greater than 60 Gy was minimal. Further radiotherapy dose escalation approaches should be carefully evaluated in prospective clinical trials, particularly when combining the technique with various systemic chemotherapies.
In the current study, nodal drainage regions were not routinely included in the CTV for prophylactic irradiation unless in the presence of positive lymph nodes or suspected lymph nodes of high risk. However, improved imaging techniques, such as positron emission tomography-computed tomography, allow for a more precise delineation of both the primary tumor and the involved lymph nodes[25-28]. Advances in image-guided techniques will continue to increase the accuracy of radiotherapy[29-31]. Additional studies have shown that the 3D image-guided radiation therapy technique can effectively detect setup errors of patients with EC undergoing RT, thereby reducing PTV margins. These advances will further reduce the radiation dose to critical organs and may translate into lower treatment-related toxicities[32,33].
In summary, this study demonstrates that SMART can offer the potential for a 6 Gy simultaneous escalation in the dose delivered to the primary tumor of EC and improve normal tissue sparing compared with 3DCRT and cf-IMRT. Further studies are warranted to evaluate the clinical benefits of SMART.
We thank Prof. Albert Koong from the Department of Radiation Oncology, Stanford University for his constructive comments and language editing.
Esophageal cancer (EC) is one of the most common malignant diseases in China. In contrast to reports from other countries, squamous cell carcinoma is the predominant pathology for EC diagnosed in China. Furthermore, this malignant disease commonly occurs in the upper or middle thoracic esophagus in China, whereas it is more often found in the distal esophagus in western countries. The current standard therapy for patients with localized EC selected for nonsurgical treatment is radiation therapy plus concurrent chemotherapy with a radiation dose of 50-60 Gy. Local-regional failure after treatment remains the most significant clinical problem.
The techniques for radiation planning, tumor imaging, and radiation delivery have advanced rapidly over the past several decades. The benefits of simultaneous radiation dose escalation for tumors at other anatomic sites in terms of improved local control and survival have been demonstrated, and dose escalation could logically be expected to also apply to EC.
The most widely accepted radiation technique for EC is three-dimensional conformal radiotherapy (3DCRT), in which the gross target volume (GTV) and a margin is typically treated with a uniform radiation dose of 50-60 Gy in 25-30 fractions. In this study, we hypothesize that the GTVs and clinical target volume should receive different radiation dose intensities because these areas have different densities of cancer cells. Furthermore, an accelerated treatment schedule with moderate hypo-fractionation may improve the tumor locoregional control. Simultaneous modulated accelerated radiation therapy (SMART) is a novel radiation technique that has been widely applied in head and neck cancer, non-small cell lung cancer, and prostate cancer. Using this technique, the gross tumor and sites of subclinical disease are treated with different doses depending on the clinical risk. The authors report a comparison among SMART, 3DCRT, and conventionally-fractionated intensity-modulated radiation therapy (cf-IMRT) in EC patients to determine the feasibility of applying the SMART technique in EC patients. The authors found that SMART can offer the potential for a 6 Gy simultaneous escalation in dose delivered to the primary tumor and can improve normal tissue sparing compared with 3DCRT and cf-IMRT.
The study results suggest that SMART can offer the potential for a 6 Gy simultaneous escalation in the dose delivered to the primary tumor of EC and improve normal tissue sparing compared with 3DCRT and cf-IMRT. Further studies are warranted to evaluate the clinical benefits of SMART.
Since esophageal cancer is still one of the most difficult clinical problems, all research done in this field is of a great importance. Radiotherapy in the treatment of esophageal cancer remains significant. This research leads to implementation of advanced radiotherapy techniques in esophageal cancer treatment and thus better control of the disease.
P- Reviewer: Arsenijevic T S- Editor: Nan J L- Editor: Rutherford A E- Editor: Liu XM
| 1. | Cooper JS, Guo MD, Herskovic A, Macdonald JS, Martenson JA, Al-Sarraf M, Byhardt R, Russell AH, Beitler JJ, Spencer S. Chemoradiotherapy of locally advanced esophageal cancer: long-term follow-up of a prospective randomized trial (RTOG 85-01). Radiation Therapy Oncology Group. JAMA. 1999;281:1623-1627. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1261] [Cited by in F6Publishing: 1321] [Article Influence: 52.8] [Reference Citation Analysis (0)] |
| 2. | Minsky BD, Pajak TF, Ginsberg RJ, Pisansky TM, Martenson J, Komaki R, Okawara G, Rosenthal SA, Kelsen DP. INT 0123 (Radiation Therapy Oncology Group 94-05) phase III trial of combined-modality therapy for esophageal cancer: high-dose versus standard-dose radiation therapy. J Clin Oncol. 2002;20:1167-1174. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 478] [Cited by in F6Publishing: 487] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 3. | Smith TJ, Ryan LM, Douglass HO, Haller DG, Dayal Y, Kirkwood J, Tormey DC, Schutt AJ, Hinson J, Sischy B. Combined chemoradiotherapy vs. radiotherapy alone for early stage squamous cell carcinoma of the esophagus: a study of the Eastern Cooperative Oncology Group. Int J Radiat Oncol Biol Phys. 1998;42:269-276. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 193] [Cited by in F6Publishing: 203] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 4. | Lee N, Harris J, Garden AS, Straube W, Glisson B, Xia P, Bosch W, Morrison WH, Quivey J, Thorstad W. Intensity-modulated radiation therapy with or without chemotherapy for nasopharyngeal carcinoma: radiation therapy oncology group phase II trial 0225. J Clin Oncol. 2009;27:3684-3690. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 480] [Cited by in F6Publishing: 513] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 5. | Pollack A, Zagars GK, Starkschall G, Antolak JA, Lee JJ, Huang E, von Eschenbach AC, Kuban DA, Rosen I. Prostate cancer radiation dose response: results of the M. D. Anderson phase III randomized trial. Int J Radiat Oncol Biol Phys. 2002;53:1097-1105. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1096] [Cited by in F6Publishing: 1129] [Article Influence: 51.3] [Reference Citation Analysis (0)] |
| 6. | Kong FM, Ten Haken RK, Schipper MJ, Sullivan MA, Chen M, Lopez C, Kalemkerian GP, Hayman JA. High-dose radiation improved local tumor control and overall survival in patients with inoperable/unresectable non-small-cell lung cancer: long-term results of a radiation dose escalation study. Int J Radiat Oncol Biol Phys. 2005;63:324-333. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 370] [Cited by in F6Publishing: 369] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 7. | Welsh J, Palmer MB, Ajani JA, Liao Z, Swisher SG, Hofstetter WL, Allen PK, Settle SH, Gomez D, Likhacheva A. Esophageal cancer dose escalation using a simultaneous integrated boost technique. Int J Radiat Oncol Biol Phys. 2012;82:468-474. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 76] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 8. | Chen W, He Y, Zheng R, Zhang S, Zeng H, Zou X, He J. Esophageal cancer incidence and mortality in China, 2009. J Thorac Dis. 2013;5:19-26. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 65] [Reference Citation Analysis (0)] |
| 9. | Chen CZ. Study of Simultaneous Modulated Accelerated Radiation Therapy Concurrent With Chemotherapy to Treat Esophageal Cancer. May 2013. Available from: http://clinicaltrials.gov/ct2/show/NCT01670409. [Cited in This Article: ] |
| 10. | Whyte RI, Orringer MB. Surgery for Carcinoma of the Esophagus: The Case for Transhiatal Esophagectomy. Semin Radiat Oncol. 1994;4:146-156. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 11. | Withers HR, Taylor JM, Maciejewski B. The hazard of accelerated tumor clonogen repopulation during radiotherapy. Acta Oncol. 1988;27:131-146. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1234] [Cited by in F6Publishing: 1160] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 12. | Taylor JM, Withers HR, Mendenhall WM. Dose-time considerations of head and neck squamous cell carcinomas treated with irradiation. Radiother Oncol. 1990;17:95-102. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 108] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 13. | Nishimura Y, Ono K, Tsutsui K, Oya N, Okajima K, Hiraoka M, Abe M. Esophageal cancer treated with radiotherapy: impact of total treatment time and fractionation. Int J Radiat Oncol Biol Phys. 1994;30:1099-1105. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 46] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Kajanti M, Kaleta R, Kankaanranta L, Muhonen T, Holsti L. Effect of overall treatment time on local control in radical radiotherapy for squamous cell carcinoma of esophagus. Int J Radiat Oncol Biol Phys. 1995;32:1017-1023. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Baumann M, Krause M, Hill R. Exploring the role of cancer stem cells in radioresistance. Nat Rev Cancer. 2008;8:545-554. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 614] [Cited by in F6Publishing: 618] [Article Influence: 38.6] [Reference Citation Analysis (0)] |
| 16. | Dingli D, Michor F. Successful therapy must eradicate cancer stem cells. Stem Cells. 2006;24:2603-2610. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 183] [Cited by in F6Publishing: 194] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 17. | Baumann M, Krause M, Thames H, Trott K, Zips D. Cancer stem cells and radiotherapy. Int J Radiat Biol. 2009;85:391-402. [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 54] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 18. | Zhang WZ, Chen ZJ, Li DR, Lin ZX, Li DS, Chen CZ. [Dosimetric comparison between intensity-modulated radiotherapy and conformal radiotherapy for upper thoracic esophageal carcinoma]. Ai Zheng. 2009;28:1127-1131. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 19. | Fenkell L, Kaminsky I, Breen S, Huang S, Van Prooijen M, Ringash J. Dosimetric comparison of IMRT vs. 3D conformal radiotherapy in the treatment of cancer of the cervical esophagus. Radiother Oncol. 2008;89:287-291. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 111] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 20. | Kole TP, Aghayere O, Kwah J, Yorke ED, Goodman KA. Comparison of heart and coronary artery doses associated with intensity-modulated radiotherapy versus three-dimensional conformal radiotherapy for distal esophageal cancer. Int J Radiat Oncol Biol Phys. 2012;83:1580-1586. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 97] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 21. | Dogan N, King S, Emami B, Mohideen N, Mirkovic N, Leybovich LB, Sethi A. Assessment of different IMRT boost delivery methods on target coverage and normal-tissue sparing. Int J Radiat Oncol Biol Phys. 2003;57:1480-1491. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 104] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 22. | Armstrong J, Raben A, Zelefsky M, Burt M, Leibel S, Burman C, Kutcher G, Harrison L, Hahn C, Ginsberg R. Promising survival with three-dimensional conformal radiation therapy for non-small cell lung cancer. Radiother Oncol. 1997;44:17-22. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 176] [Cited by in F6Publishing: 175] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 23. | Werner-Wasik M, Yorke E, Deasy J, Nam J, Marks LB. Radiation dose-volume effects in the esophagus. Int J Radiat Oncol Biol Phys. 2010;76 Suppl 3:S86-S93. [DOI] [Cited in This Article: ] [Cited by in Crossref: 184] [Cited by in F6Publishing: 183] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 24. | Emami B, Lyman J, Brown A, Coia L, Goitein M, Munzenrider JE, Shank B, Solin LJ, Wesson M. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys. 1991;21:109-122. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3227] [Cited by in F6Publishing: 2988] [Article Influence: 90.5] [Reference Citation Analysis (0)] |
| 25. | Mamede M, El Fakhri G, Abreu-e-Lima P, Gandler W, Nosé V, Gerbaudo VH. Pre-operative estimation of esophageal tumor metabolic length in FDG-PET images with surgical pathology confirmation. Ann Nucl Med. 2007;21:553-562. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 51] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 26. | Zhong X, Yu J, Zhang B, Mu D, Zhang W, Li D, Han A, Song P, Li H, Yang G. Using 18F-fluorodeoxyglucose positron emission tomography to estimate the length of gross tumor in patients with squamous cell carcinoma of the esophagus. Int J Radiat Oncol Biol Phys. 2009;73:136-141. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 76] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 27. | Barber TW, Duong CP, Leong T, Bressel M, Drummond EG, Hicks RJ. 18F-FDG PET/CT has a high impact on patient management and provides powerful prognostic stratification in the primary staging of esophageal cancer: a prospective study with mature survival data. J Nucl Med. 2012;53:864-871. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 85] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 28. | Yu W, Fu XL, Zhang YJ, Xiang JQ, Shen L, Chang JY. A prospective evaluation of staging and target volume definition of lymph nodes by 18FDG PET/CT in patients with squamous cell carcinoma of thoracic esophagus. Int J Radiat Oncol Biol Phys. 2011;81:e759-e765. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 29. | Li R, Mok E, Han B, Koong A, Xing L. Evaluation of the geometric accuracy of surrogate-based gated VMAT using intrafraction kilovoltage x-ray images. Med Phys. 2012;39:2686-2693. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 30. | Li R, Mok E, Chang DT, Daly M, Loo BW, Diehn M, Le QT, Koong A, Xing L. Intrafraction verification of gated RapidArc by using beam-level kilovoltage X-ray images. Int J Radiat Oncol Biol Phys. 2012;83:e709-e715. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 31. | Xing L, Thorndyke B, Schreibmann E, Yang Y, Li TF, Kim GY, Luxton G, Koong A. Overview of image-guided radiation therapy. Med Dosim. 2006;31:91-112. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 315] [Cited by in F6Publishing: 277] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 32. | Chen YJ, Han C, Liu A, Schultheiss TE, Kernstine KH, Shibata S, Vora NL, Pezner RD, Wong JY. Setup variations in radiotherapy of esophageal cancer: evaluation by daily megavoltage computed tomographic localization. Int J Radiat Oncol Biol Phys. 2007;68:1537-1545. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 33. | Hawkins MA, Aitken A, Hansen VN, McNair HA, Tait DM. Set-up errors in radiotherapy for oesophageal cancers--is electronic portal imaging or conebeam more accurate? Radiother Oncol. 2011;98:249-254. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |