INTRODUCTION
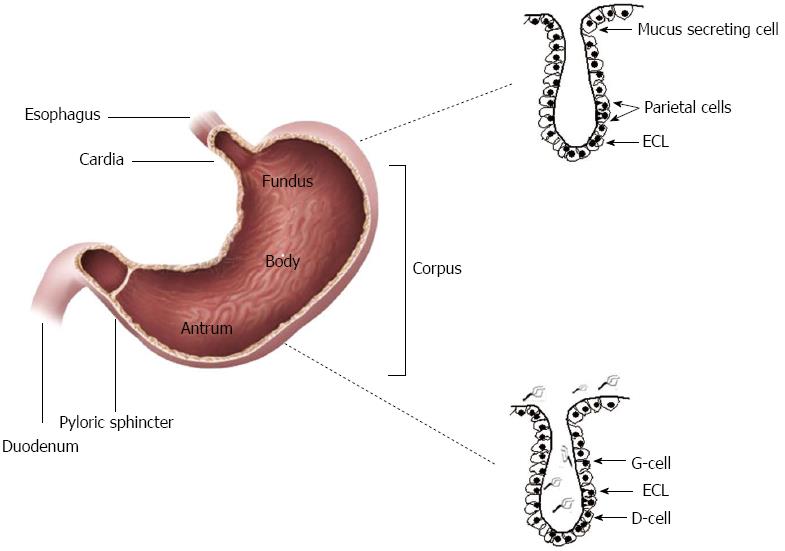
The gastric epithelium consists of a monolayer of cells covered by mucus and that invaginate in order to form functional gastric glands or pits, which can be cardiac, oxyntic and pyloric (Figure 1). Cardiac glands are located closest to the esophagus and lined mostly by mucus secreting cells. The oxyntic glands in the fundus and corpus of the stomach have (1) chief cells that produce pepsinogen; (2) parietal cells, which release hydrochloric acid and intrinsic factor; and (3) enterochromaffine-like cells, responsible for histamine release. Pyloric glands in the antrum contain G and D cells responsible for gastrin and somatostatin production, respectively.
Figure 1 Helicobacter pylori colonization the antrum of the stomach.
The gastric epithelium consists of a single layer of cells that invaginate in order to form cardiac, oxyntic or/and pyloric gland. Cardiac glands are located closest to the esophagus, while the oxyntic glands located in the fundus and corpus of the stomach and contain chief cells, parietal cells and enterochromaffine-like cells (ECL). Pyloric glands which located in the antrum contain G and D cells. H. pylori colonization is only limited to the antrum of the stomach.
Helicobacter pylori (H. pylori) colonization is mainly limited to the antrum of the stomach, devoid of parietal cells. Although gastric epithelial cells are responsible for digestive processes, it is not their only function. A crucial function of mucosal epithelial cells is to protect the underlying tissue from pathogenic microorganisms that may access the lumen[1]. H. pylori is a gram-negative bacterium that selectively colonizes the human gastric epithelium of more than half of the world’s population. The most common consequence of H. pylori infection is chronic gastritis; however, chronic infection may lead to clinically significant gastro-duodenal diseases[2]. In order to survive and maintain the chronic infection, H. pylori employs an assortment of mechanisms that aid its adaptation to the harsh environment of the stomach. Different studies have shown the multiple effects that H. pylori has on gastric epithelial cells, among which are induction of apoptosis, cell proliferation, and destruction of epithelial cell junctions[3]. The objective of this review is to discuss the strategies that H. pylori uses to perturb the gastric epithelial barrier that range from interactions of H. pylori with the cell surface to virulence products that are either translocated into the host cell cytosol or that are secreted by H. pylori and activate signaling processes that in turn promote specific responses by gastric epithelial cells.
MECHANISMS OF H. PYLORI ADHESION TO THE GASTRIC EPITHELIUM
An essential step in the colonization by H. pylori and its ability to mediate effects on the gastric epithelium is its selective tissue tropism leading to the establishment of intimate interactions with the epithelial surface. These interactions are largely mediated via outer membrane proteins (OMPs) that serve as adhesins. The H. pylori genome has more than 30 genes which encode OMPs that are divided into Hop (Helicobacter outer membrane proteins) and Hor (hop-related) subgroups. The Hop group of proteins contains H. pylori adhesion molecules such as BabA, SabA, AlpA/B, HopZ and OipA[4].
BabA
The blood group antigen-binding adhesion BabA was the first H. pylori adhesin discovered[5]. It facilitates the adherence of H. pylori to Lewisb antigens (Leb), an ABO blood group antigen that is expressed on the gastric mucosa. Binding of H. pylori to Leb on the epithelial surfaces via BabA enhances the type 4 secretion system (T4SS)’s ability to exert the pathogencity of H. pylori that includes triggering production of proinflammatory cytokines[6], a well-established response of the epithelium to the infection. Therefore, the expression of BabA adhesion is closely associated with the onset of T4SS-related host cell responses, since it increases the delivery of H. pylori virulence factors and promotes inflammation[4].
SabA
H. pylori infection leads to an increase in gastric epithelial expression of sialyl-dimeric-Lewisx glycosphingolipid. This molecule serves as a receptor for the bacteria, which uses sialic acid binding adhesion protein (SabA), an outer membrane protein, to bind to sialyl-LewisX[7]. In the early stages of infection, binding of BabA to Leb/ABO is essential, however, with increased inflammation, the expression sialyl-Lewisx antigen (sLex) also increases; thus, H. pylori SabA enhances the adherence to the inflamed gastric mucosa. SabA expression switches “on” or “off”, which may suggest that SabA up-regulation, may be determined by the shifting conditions of the stomach environment. Interestingly, SabA expression is closely associated with development of intestinal metaplasia, gastric atrophy and gastric cancer in United States[8].
AlpA/B
AlpA and AlpB are two homologous genes that encode OMPs involved in H. pylori adhesion[9]. Both AlpA and AlpB proteins have been found to adhere to mouse laminin in an in vitro study[10], but no other adhesion receptor for AlpA/B has been recognized. Our studies with human gastric epithelial cells confirmed by flow cytometry that AlpA/B were involved in cellular adhesion and modulate proinflammatory intracellular signaling cascades[11]. This was confirmed when alpAB deletion mutants poorly colonized the gastric mucosa of C57BL/6 mice and induced lower mucosal levels of KC and IL-6.
HopZ
The hopZ gene product is another H. pylori adhesin protein that is located at the bacterial surface[4]. HopZ was described as a bacterial adhesin protein since the hopZ mutant strain reduced adherence to AGS cells. Similar to AlpA/B, the host binding receptor for HopZ is still unknown. The hopZ gene is phase variable as a result of a CT dinucleotide repeat in the region that codes for the signal sequence[12]. The hopZ gene is also transcriptionally regulated by changing pH[13] and by contact with gastric epithelial cells[14].
OipA
The outer inflammatory protein A (OipA) is a 34kDa cell surface protein encoded by the hopH gene and was initially described as a promoter for IL-8 production in T4SS-independent manner and increased inflammation[15]. OipA expression was shown to be controlled by a slipped-strand repair mechanism related to the number of CT dinucleotide repeats at the 5′ end of the gene and is correlated with severe clinical outcomes, including duodenal ulcer and gastric cancer[15,16]. Subsequent studies showed that oipA mutant strains have reduced adherence to gastric cancer cell lines, AGS and Kato-III although the host receptor for the OipA adhesion has not been identified[15].
H. PYLORI VIRULENCE FACTORS THAT AFFECT THE GASTRIC EPITHELIUM
Among the armamentarium of virulence factors expressed by H. pylori, in addition to the adhesins described above, there are bacterial products that are translocated into host epithelial cells via a type 4 secretion system (T4SS) encoded within the cag pathogenicity island (cag PAI) as well as factors that are secreted by H. pylori and exert their effects independently of the intact bacteria interacting with the epithelium. In fact, it has been estimated that at any given time only 20% of the colonizing bacteria are in direct interaction with the epithelium[17].
Cag PAI
The H. pylori cag pathogenicity island (PAI) is perhaps the most studied virulence factor. Cag PAI consists of a cluster of 31 genes, most of which code for a T4SS. The T4SS is in essence a needle-like structure that penetrates the epithelial cell membrane and translocates H. pylori products into epithelial cells. One of the products injected is the effector protein CagA[18], which is encoded at one end of cagPAI and does not seem to have homologues in other bacterial species. CagA is perhaps the most virulent factor associated with H. pylori and its presence in an infecting strain is regarded as a risk factor for peptic ulcer disease and gastric cancer. Once CagA is delivered into host epithelial cells, it undergoes tyrosine phosphorylation at Glu-Pro-Ile-Tyr-Ala (EPIYA) motifs in the C terminus by Src and Abl kinases[19-21]and initiate signaling events described below in detail. There are four major types of EPIYA motifs (A, B, C, and D) based on the specific amino acid sequence bordering the EPIYA motif on both sides[22]. EPIYA-A, EPIYA-B and EPIYA-C motifs in tandem are found in “western strains” while CagA with EPIYA-A, EPIYA-B and EPIYA-D motifs are found in “East-Asian strains”[23]. It should be pointed out that these motifs contribute to the polymorphism in the C-terminus of the protein and are found as tandem repeats ranging in number from one to seven[24]. The quantity of EPIYA motifs is proportional to the levels of phosphorylation and the effects seen in epithelial cells[25], which will be described in detail below. These properties of CagA and its role in pathogenesis are well known and accepted by experts in the field.
In addition to the CagA protein, bacterial cell wall components such as peptidoglycan or muropeptides are also translocated via the T4SS into host epithelial cells. H. pylori peptidoglycan is, in turn, recognized by NOD1, an intracellular pathogen-associated molecular pattern (PAMP) recognition receptor that senses peptidoglycan which induces NF-κB activation and upregulation of proinflammatory immune responses[26].
Recently, various reports have brought to light the function of CagL, another cag PAI encoded protein[27-30]. Early researches defined CagL as a gene that is necessary for CagA delivery, therefore it was considered as a component of T4SS. More recently, it was identified as one component of pilus structures that develop at the interface between gastric epithelial cells and H. pylori[28]. Importantly, CagL enhances the binding of the T4SS to a5β1 integrin receptor on gastric epithelial cells[27,30]. Other Cag proteins, such as CagY, CagI and CagA can also bind to integrin[31]. This binding result in cellular alterations, some of which are described below, such as, cell spreading, formation of focal adhesion, and activation of tyrosine kinases[28]. In light of this important properties by these more recently described products of cag PAI, they represent interesting targets for translational studies in the development of a vaccine.
VacA
The vacuole-inducing toxin (VacA) is major H. pylori secreted protein without a known homologue in other bacterial species. VacA is initially made as a 140 kDa pro-toxin containing an N-terminal signal peptide, a central region that forms the toxin and a C-terminal domain responsible for transport function. After processing, the central region (about 88 kDa), which is the mature virulent form of the toxin, is secreted and further processed into two subunits of 33 (A subunit) and 55 kDa (B subunit), respectively. It has been suggested that the p33 form is the pore forming subunit, while the p55 form was initially regarded as the cell binding component[32,33]. However, now both subunits are known to contribute to both binding and vacuole formation[34,35]. The exact mechanism of entry is still in question as multiple receptors have been proposed, but binding to sphingomyelin appears to aid in the process[36].
Although all strains of H. pylori have the vacA gene, there is a great deal of diversity in the gene, which includes three regions: signal (s), mid-(m) and intermediate (i)-regions. There are two allelic types for each region and most virulent strains have the s1, i1, and m1 alleles, which are associated with the highest risk of gastric adenocarcinoma[37,38].
Urease
H. pylori urease is possibly the most abundant protein expressed by the bacteria as it represents 10% of the total protein by weight[39]. Urease is a high molecular weight multisubunit enzyme made of two subunits A and B that have molecular weights of 29.5 and 66 kDa, respectively[40,41]. The enzyme is actually a dodecameric aggregate containing six of each of the subunits resulting in a molecular weight of close to 600 kDa. Urease is best known for its enzymatic activity leading to the hydrolysis of urea into ammonia and bicarbonate, which help buffer the pH in the local microenvironment.
Urease also acts as an adhesion as it binds directly to both class II major histocompatibility complex (MHC) molecules and CD74[42,43]. Additional support for the role of urease as a bacterial adhesin was obtained by independent studies showing that urease mutants could not colonize even in hypochloridic conditions where gastric pH was maintained at neutrality[44]. Yet another study showed that as much as 50% of the urease activity is recovered from the surface of the bacteria where it could serve as an adhesin[45]. Because of these properties, urease has been a target for vaccine design that has been included in clinical trials, which unfortunately had disappointing results.
High temperature requirement A
HtrA is a recently described secreted product of H. pylori that has enzymatic activity[46]. HtrA has been shown to possess serine protease activity and one of the substrates on which it acts is on the adhesion molecule E-cadherin[46,47]. It cleaves the ectodomain of E-cadherin and proteolysis of E-cadherin contributes to disruption of adherence junctions. Thus, HtrA disrupts epithelial barrier integrity and permits H. pylori to invade the intercellular space between epithelial cells. Interestingly, the E-cadherin ectodomain that is released is a key prognostic indicator in gastric cancer[48], but more work is warranted to better understand this virulence factor and its role in disease and as target for intervention. Although a substantial body of work on the adhesins described above and the virulence factors listed has led to a greater understanding on the pathogenesis of H. pylori, much work is still needed on these and other yet uncharacterized virulence factors in order to develop a, thus far elusive, anti-H. pylori vaccine.
EPITHELIAL CELL SIGNALING INDUCED BY H. PYLORI
One of the outcomes of infection with H. pylori subsequent to its interaction either directly or indirectly via its soluble products is the activation of an array of epithelial cell signaling pathways. The various signaling pathways that are deregulated or activated by H. pylori will be described in this section with a brief introduction on their consequences for the host cell, and the corresponding cellular responses will be described in detail in the last section of this review.
Perhaps the best studied signaling pathways affected by H. pylori correspond to those affected by cag PAI positive strains of H. pylori, since those strains are more virulent and induce significant inflammatory responses. CagA as well as muropeptides are translocated into the host epithelium via the T4SS. They then trigger several intracellular signaling pathways that result in epithelial cell gene expression as well as the production of proinflammatory cytokines and chemokines. A central mediator in the expression of these cytokines/chemokines is NF-kB, a transcription factor that is the convergence point for multiple pathways activated by H. pylori. In addition to its role in inducing proinflammatory cytokines/chemokines, one of its target genes Bcl-XL can suppress the mitochondrial apoptosis pathway which may lead to unregulated proliferation. Crabtree and Naumenn summarized the overall effects of cagPAI translocated products in the sequential activation of the IKK complex, JNK, p38 kinase, NF-kB, and AP-1 in gastric epithelial cells[49].
After CagA is transported into the GEC cytoplasm and undergoes phosphorylation at the EPIYA motifs by Src-family kinases. It then deregulates multiple signaling pathways that affect host cell shape and adhesion, as well as cell transformation. The type of EPIYA motif phosphorylated appears to determine the molecule that is then bound. CagA phosphorylated at EPIYA-C or -D binds to and activates SHP-2 phosphatase[50]. SHP-2, in turn, activates the mitogen-activated protein kinase (MAPK) and extracellular signal regulated kinase (ERK) pathway as well as dephosphorylates and inactivates focal adhesion kinase, FAK. This tyrosine kinase regulates the turnover of focal adhesion spots[51] and its inhibition leads to the elongated cell shape typical of the hummingbird cell phenotype. Interestingly, phosphorylation of EPIYA-A and EPIYA-B motifs leads to CagA binding to and activation of Csk kinase[52]. Activation of Csk, in turn, inhibits Src in a negative feedback loop. The EPIYA-B motif when phosphorylated can bind phosphatidylinositol 3-kinase (PI3K) whose activation results in stimulation of phosphatidylinositol-dependent kinase 1 that phosphorylates and activates AKT[53,54]
The unphosphorylated form of CagA is able interact with a number of intracellular proteins and those associations exert an array of responses ranging from pro-inflammatory, mitogenic responses, disruption of cell-to-cell junctions and loss of gastric epithelial cell polarity. Perhaps the first interaction partner of unphosphorylated CagA is the adapter protein growth factor receptor bound 2 (Gbr2)[55], which in turn complexes with SOS to promote Ras-GTP formation and activation of MAPK/ERK signaling pathway. Unphosphorylated CagA can damage the barrier function of the epithelium by interacting with the scaffolding protein ZO-1 and the junctional adhesion protein (JAM), which are essential components of tight junctions, by mechanisms that will be detailed below. By disrupting the tight junctions H. pylori gains access to epidermal growth factor receptors (EGFR) as well as Her2/Neu on the basolateral membranes. CagA may also activate the calcium-dependent serine/threonine phosphatase calcineurin, which promotes mobilization of the nuclear factor of activated T cells (NFAT) from the cytoplasm into the nucleus of gastric epithelial cells. NFAT in turn activates a number of genes, such as p21Cip1, a cyclin-dependent kinase inhibitor[56]. CagA also induces cell scattering, also referred to as the “motogenic response”, by binding to the hepatocyte growth factor/scatter factor receptor, c-Met[57]. This mechanism is mediated via cag PAI-dependent activation of Rho-GTPases Rac1 and Cdc42. In addition, this response involves ERK1/2 and MEK1/2 activation via extracellular H. pylori signaling[58,59]. Downstream effects of binding to the c-Met receptor include tyrosine phosphorylation of Grb2-associated binder 1 (Gab1), which leads to activation of several intracellular signals like Grb2, PI3-K, PLCγ, and SHP-2. An additional signaling response that appears to depend on CagA is β-catenin translocation[60]. β-catenin is a proto-oncogene that has been shown to have numerous binding partners and plays multiple roles in cellular functions.
Since cells must undergo a highly regulated process to grow, an important question lies in the interaction of H. pylori and its mediators with those proteins that regulate the cell cycle. In vitro studies showed that cyclin D1, which controls the transition between G1 and S in the cell cycle in combination with CDK4 or CDK6 (2), is induced by H. pylori in a partially cag PAI-dependent manner (1). This then leads to increased proliferation of gastric epithelial cells. As will be described in detail below, another important effect of H. pylori infection is the ability of cag PAI+ strains to affect the balance between proliferation and apoptosis, particularly because of the role of cagPAI in triggering Fas-dependent apoptosis, reduction of Bax (pro-apoptotic factor), and overexpression of Bcl-2[61,62]
VacA referred above as a vacuolating toxin uses the 55 kDa B subunit to bind to PTP-β (aka Ptprz), a tyrosine phosphatase receptor, leading to phosphorylation of the G protein-coupled receptor kinase-interactor 1 (Git1). This has been shown to promote ulcer formation in mice. Other studies have shown that VacA binds both PTPα and PTPβ and this results in enhanced vacuole formation[63]. Lastly, VacA can also lead to apoptosis via the caspase-3 pathway because VacA form channels that lead to release of cytochrome c release.
There are signaling processes initiated in gastric epithelial cells during infection with H. pylori that are not the direct result of a virulence factor, but that are triggered by the binding of H. pylori to receptors on the surface of gastric epithelial cells. Two notable receptors used by H. pylori that are known to deliver intracellular signals include CD74 and class II MHC molecules[42,43,64,65]. Class II MHC molecules, when engaged, have been shown to involve several intracellular signaling processes, ranging from activation of phospholipase C (PLC), the kinases PKC, Src and Syk, to the mitogen activated kinases p38 and Erk[66],and one of the outcomes is stimulation of proinflammatory cytokine secretion and delivery of proapoptotic signals. Recent studies suggest that class II MHC molecules crosstalk with TLR receptors by amplifying their signals following association of MHC class II with the costimulatory molecule CD40 and Btk, a tyrosine kinase, in the endosomes of TLR stimulated cells[67]. However, this has not been examined in the context of H. pylori infection. CD74-initiated signaling has been better studied and it has been shown to activate transcription mediated by the NF-κB p65/RelA homodimer and its coactivator, TAFII105[68]. In contrast to class II MHC, CD74 activates proliferation and prosurvival pathways[69], as it induces BCL-XL transcription. Our group has shown that H. pylori CagA induces gastric epithelial cell production of migration inhibitory factor (MIF)[61]and MIF, which uses CD74 as a receptor, induces the NF-κB, Erk1/2, and AP-1 signaling pathways[70-72]. Our studies showed that the H. pylori urease B subunit alone, after binding to CD74, induced NF-κB activation and IL-8 production[42]. Although preliminary, these are important insights that could be exploited in intervention against H. pylori infection. Thus, additional research in this area is warranted.
One last important outcome of epithelial cell signaling induced by H. pylori is the transactivation of EGFR in gastric epithelial cells. There are several proposed mechanisms by which this may occur. The first is via“extracellular transmembrane cleavage or proHB-EGF followed by signaling of HB-EGF.” Cleavage is achieved via ADAM family metalloproteinases that are zinc dependent. Another possible mechanism is via NADPH oxidase forming ROS, leading to EGFR transactivation. This transactivation occurs in both cag PAI positive and negative strains. In addition, H. pylori expresses a γ-glutamyltranspepetidase that may be “an upregulator of HB-EGF in gastric epithelial cells.” ADAM family proteins are also involved in EGFR transactivation pathways involving IL-8, PGE2, gastrin, and oxidative damage from tobacco smoke.
Understanding of the different signaling pathways activated during the infection with H. pylori is important not only in understanding disease mechanisms, but also in defining potential therapeutic targets as suggested above.
EFFECT OF H. PYLORI ON GASTRIC EPITHELIAL CELL
The induction of host epithelial signaling pathways by H. pylori attachment or via soluble mediators has multiple consequences on the gastric epithelium that range from epithelial barrier function to proinflammatory and procarcinogenic processes, some of which are described here in detail. Another major consequence of the deregulation/activation of epithelial signaling pathways is the subversion of mechanisms important in host immune responses and impaired immunity contributes to the chronicity, which is a hallmark feature of the infection and is reviewed elsewhere in this issue.
Disruption of epithelial cells barrier by H. pylori
Epithelial cells form a barrier between the lumen and interstitial space. Apical junctions are critical in maintaining essential epithelial cells functions, such as maintaining apical-basolateral cell polarity, cell-cell adhesion, cell proliferation, and cell movement[73]. The overall integrity of the epithelial barrier depends on cell-cell sealing, which is maintained by four types of junctions including tight junctions, desmosome junctions, gap junctions and adherens junctions. Tight junctions are multiprotein complexes and represent the most common type of junction in the lumen. They are important in regulating diffusion across the epithelium. Tight junctions consist of different scaffolding proteins such as zonula occludens (ZO)-1, junctional adhesion molecules (JAM)-1, claudin, and occludin (Figure 2). Tight junctions are the targets for several bacterial pathogens. Some bacteria open the cell-cell junctions, while other bacteria disorganize the epithelial cell polarity, expose the basolateral surface and cause leaks at the epithelial lining.
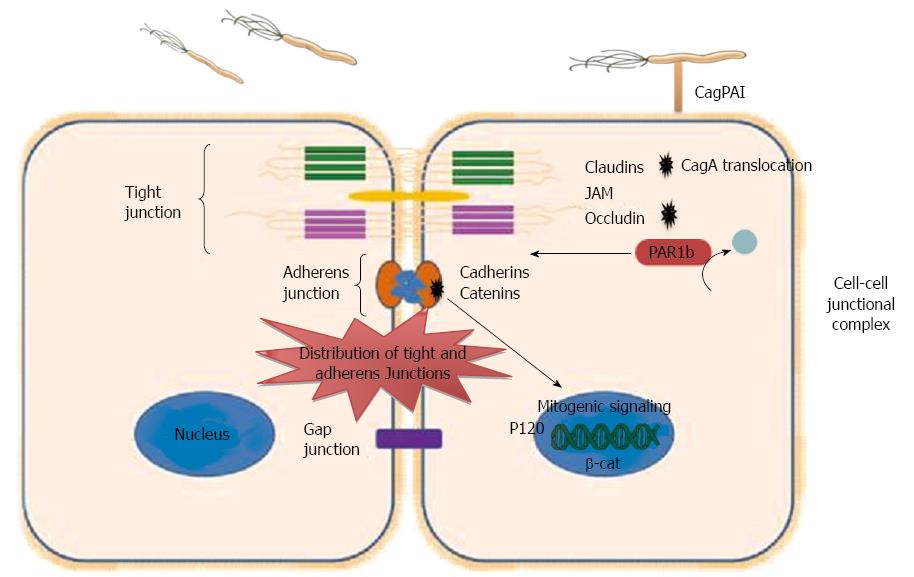
Figure 2 Dysregulation of epithelial junction by Helicobacter pylori.
Intercellular junctions include tight junctions, adherens junctions, and gap. Tight junction consists of important Integral membrane proteins, such as occludin, claudins, and junctional adhesion molecules (JAMs), while adheren junction consists of Catenins and Cadherins. Helicobacter pylori (H. pylori) interact with cell-cell junctions and disrupt the polarity of polarized epithelial cells. Translocated CagA binds to PAR1b and inhibits its activity and blocks its phosphorylation by atypical protein kinase C, eventually causing junctional and polarity defects. CagA interacts with E-cadherin leads to the release of E-cadherin from the E-cadherin/β-catenin adherent complex, results in buildup of β-catenin at the cytoplasm and nuclear translocation of β-catenin and p120, and activation of β-catenin-mediated signaling that induces transformation of gastric epithelial cells. CagPAI: Cag pathogenicity island.
H. pylori disrupts epithelial cell-cell junctions, cell polarity as well as cell proliferation via binding to the epithelial cell receptors and stimulation of different signaling pathways. H. pylori strains possessing cag PAI, and thus expressing T4SS, use the T4SS to translocate CagA which after phosphorylation by Src family kinases binds to and activates SHP-2 phosphatase leading to multiple cellular responses. Among the multiple effects that activated SHP-2 phosphatase has on gastric epithelial cells is the alteration of the tight junctions and adherens junctions of epithelial cells[74]. CagA contributes to these alterations through interactions with different junction proteins that include E-cadherin, ZO-1 and JAM, thus affecting the normal epithelial architecture and the normal function of both tight and adherent junctions[73]. β-catenin is localized at cell-cell junctions via interaction with E-cadherin, which is a transmembrane protein. The E-cadherin/β-catenin complex has an essential role in epithelial cell-cell interaction and maintaining the normal architecture of epithelial cell tissue. However, during H. pylori infection translocated CagA destabilizes the E-cadherin/β-catenin complex, in a phosphorylation independent manner[75]. The mechanism whereby CagA destabilizes the E-cadherin/β-catenin complex is still unclear, but it has been suggested that CagA may compete with β-catenin for binding with E-cadherin. Also, CagA may be connected with E-cadherin indirectly through another component of the apical-junction complex, and this connection disturbs the E-cadherin/β-catenin complex. Disturbance of the E-cadherin/β-catenin complex formation by the interaction of CagA with E-cadherin leads to the release of E-cadherin from the adherent complex, buildup of β-catenin at the cytoplasm and the nucleus, and activation of β-catenin-mediated signaling that induces transformation of gastric epithelial cells[75] (Figure 2). In addition, a study to localize the tight junction scaffolding protein ZO-1 in CagA-expressing cells indicated that translocated CagA was associated with mislocalization of ZO-1 to the basolateral membrane[76-78]. In addition to the inducing epithelial junctional defects, H. pylori CagA can also cause polarity defects via interaction with PAR1/MARK kinase, which preserves the polarity of epithelial cells. Partitioning-defective 1 kinases (PAR1) play an important function in sustaining the polarity of epithelial cells through phosphorylation of microtubule-associated proteins (MAPs). PAR1 has to be released from the tight junction to maintain the epithelial polarity. The dissociation of PAR1 from tight junctions occurs via atypical protein kinase C (aPKC), which binds and phosphorylates PAR1 at tight junctions. Thus, releasing of PAR1 from the membrane inhibits the penetration of PAR1 to the apical membrane. However, during H. pylori infection translocated CagA binds to PAR1b[79], which is one of four members of the PAR1 family of kinases, and inhibits its activity and its phosphorylation by aPKC, eventually causing junctional and polarity defects[78] (Figure 2). The resulting disruption in epithelial cell polarity appears to be a mechanism employed by H. pylori to enhance its survival and growth as it permits H. pylori to usurp the cell surface as a place for its growth[80].
Induction of gastric epithelial cell apoptosis by H. pylori
Continued and rapid turnover of epithelial cells is considered an innate host defense mechanisms provided by the epithelium as it contributes to reduced bacterial colonization. Since H. pylori inhabits the apical surfaces of gastric epithelial cells, to maintain a persistent infection H. pylori employs different mechanisms that favor its growth and survival. For instance, the imbalance between the epithelial turnover and proliferation could facilitate the H. pylori colonization and survival[81]. Several studies have shown that the development of H. pylori-associated gastric diseases during chronic infection involve redistribution of the equilibrium between the proliferation and the turnover of gastric epithelial cells[82]. Apoptosis is a highly conserved and regulated process in both healthy and inflamed tissue. It helps maintain tissue homeostasis by removing aged, damaged and infected cells from the tissues[83]. H. pylori elicits apoptosis of gastric epithelial cells in order to stimulate the cells proliferation as a compensatory mechanisms[84,85] and H. pylori was found to be able to regulate these processes in order to utilize the epithelial cells as a niche for their replication[82,86]. For instance, one study detailed below reported that H. pylori infection triggers pro-survival mechanisms (p-ERK) as well as an antiapoptotic protein (MCL1) in the gastric epithelium of infected gerbils in order to block apoptosis and thus enhance colonization by H. pylori[82].
As indicated above, enzymes (i.e., urease, phospholipases) and VacA that are released by H. pylori cause damage the gastric mucosa, and stimulate the inflammatory and immune responses that also contribute to epithelial cell apoptosis. The inflammatory response results in the production of a significant level of free radicals by neutrophils, which damage the DNA of epithelial cells while the immune response via production of TH1 cytokines (such as TNF-α and IFN-γ), which further damage the DNA of gastric epithelial cells, and induce apoptosis[3]. H. pylori express different adhesion molecules that aid their attachment to the epithelial surface and the overlying mucosa. As introduced earlier, one of those adhesions involves urease on the surface of H. pylori, which upon binding to class II major histocompatibility complex (MHC) molecules also stimulates apoptosis of gastric epithelial cells[43,64]. Interestingly, IFN-γ by Th1 cells in the infected gastric mucosa enhances apoptosis induction by increasing the density of class II MHC molecules on the surface of gastric epithelial cells to which H. pylori can bind and trigger apoptosis[64].
Paradoxically, H. pylori also has been shown to promote survival or anti-apoptotic mechanisms in gastric epithelial cells. After H. pylori translocates CagA via T4SS, CagA, through the signaling cascades activated inside gastric epithelial cells, induces activation of various transcription factors such as, NF-κB, NFAT, serum response factor, and T cell factor/lymphoid enhancer factor[73,74,87]. Stimulation of these transcription factors leads to an increase in Cyclin D1 production in epithelial cells leading to their proliferation[88]. As mentioned earlier, a study that used a Mongolian gerbil model of infection to elucidate the effect of H. pylori on gastric epithelial cells renewal showed that H. pylori could actually suppress apoptotic processes of gastric epithelial cells, which when uninfected are usually shed every 2-3 d, and in delaying this shedding colonization by H. pylori is favored. In that study, they orally administered etoposide, an inducer of epithelial cell apoptosis. The delivery of CagA was noted to up-regulate MCL1, an anti-apoptotic protein, and the pro-survival MAPK ERK1/2 in the gastric pits. These results gave insights on the role of CagA in suppression of epithelial cells turnover while also stimulating their proliferation, which is a key strategy used by H. pylori to persist[82]. Our group showed that another mechanism used by H. pylori to induce gastric epithelial cells proliferation involved the induction of macrophage migration inhibitory factor (MIF) production by gastric epithelial cells[42]. Gastric epithelial cell proliferation was induced as a result of MIF binding to CD74, whose expression increases during infection and is polarized toward the apical surface where H. pylori may engage CD74 molecules[65,89]. Interestingly, through the use of cagA- knockout H. pylori in conjuction with wild type strains, MIF production by infected gastric epithelial was found to depend on CagA. MIF, in turn led to decreased p53 phosphorylation as well as increased BCL-2 expression, both of which are anti-apoptotic processes[61]. Furthermore, a recent study showed that the N-terminus of CagA interacts with the tumor suppressor apoptosis-stimulating protein of p53 (ASPP2) and in doing so inhibits apoptosis[4]. In that study, it was observed that during H. pylori infection the N-terminus of translocated CagA interacts with ASPP2. After CagA hijacks ASPP2, it is altered and recruits and binds to p53, and eventually leads to proteasomal degradation of p53. Thus, this represents another mechanism whereby CagA causes the infected gastric epithelial cells to become more resistant to the natural cell turnover.
Cytokines secretion
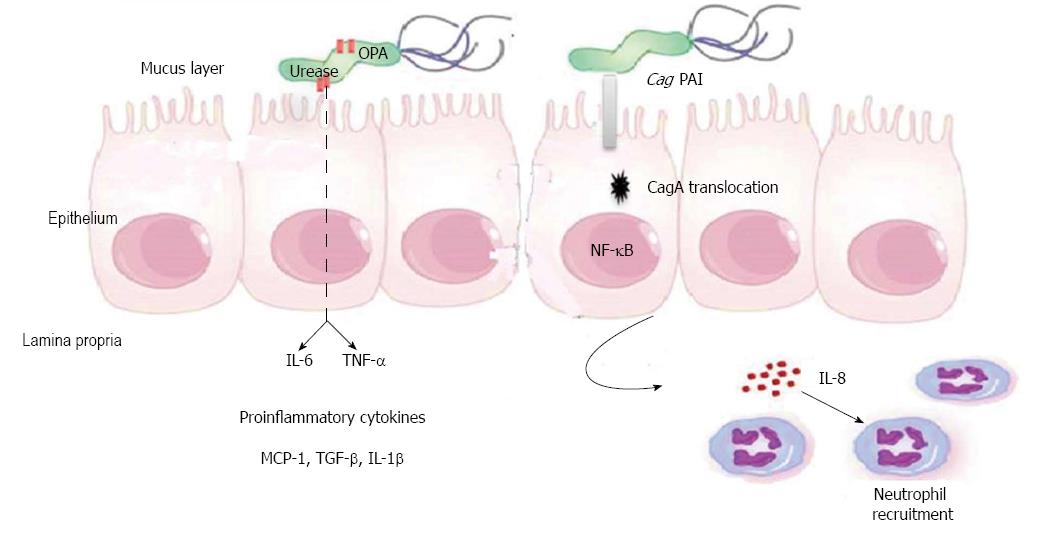
A hallmark of the infection of the gastric mucosa with H. pylori is the secretion of multiple proinflammatory cytokines, several of which are secreted by gastric epithelial cells. These cytokines, in turn, play significant roles in the development of gastroduodenal diseases associated with H. pylori infection. One of the earliest cytokines reported to be produced by the infected gastric epithelium is interleukin (IL)-8[90-93]. It is well accepted that H. pylori increases the IL-8 expression both in vivo and in vitro[91]. This production of IL-8 was linked to the expression of CagA by the infecting strains[90] and is associated with the recruitment of neutrophils. Other cytokines reported to be produced by the infected gastric epithelium include IL-6, tumor necrosis factor alpha (TNF-α)[94], IL-1β, IL-1α, granulocyte-macrophage colony-stimulating factor (GM-CSF), monocyte chemoattractant protein-1 (MCP-1)[95], MIF[61] and TGF-β[42] (Figure 3). While this may not be an exhaustive list, it shows the ample repertoire of cytokines that originates from infected gastric epithelial cells, which is a well-known response to the infection.
Figure 3 Cytokines production by gastric epithelial cell during Helicobacter pylori Infection.
Helicobacter pylori (H. pylori) leads to production of proinflammatory cytokines by gastric epithelial cells (GECs). Interactions between the translocated cagA and gastric epithelial cells lead to activation of nuclear factor (NF)-κB, alteration in gene transcription in the GECs, and secretion of IL-8 by GECs, which leads to recruitment of neutrophils. H. pylori urease induces the production of IL-6 and tumor necrosis factor alpha (TNF-α) by GECs. Other cytokines secreted by GECs during H. pylori infection such as, TNF-α, IL-1β, IL-1α, granulocyte-macrophage colony-stimulating factor, monocyte chemoattractant protein-1 (MCP-1), migration inhibitory factor and tumor growth factor (TGF)-α.
As the understanding of underlying mechanisms responsible for proinflammatory cytokine production during infection with H. pylori is key in gaining important insights into pathogenesis and potential targets for therapy, various efforts have been directed at characterizing the virulence factors that are linked to cytokine induction. Various studies have examined the response of human gastric epithelial cells to purified H. pylori urease, since it is the most abundant protein produced by the bacteria. One of those studies reported that H. pylori urease induces the production of IL-6 and TNF-α by gastric epithelial cells[94]. Interestingly, mucosal IL-6 and TNF-α had been found years earlier to be increased in patients suffering from H. pylori chronic gastritis[96]. In more detailed studies to map the domain on urease inducing IL-8, the urease B subunit alone was found to induce the activation of NF-κB and IL-8 production subsequent to binding to CD74 on gastric epithelial cells[42]. As CagA has multiple effects on signaling processes once it is translocated into the host epithelium, it is not surprising that it also has been shown to stimulate cytokine production. Early investigations correlated IL-8 production with CagA protein expression by H. pylori[90,97]. In a detailed comparison of H. pylori strains that lack CagA protein with those that express the protein, CagA positive strains were reported to induce higher levels of IL-8 mRNA and IL-8 protein expression by gastric epithelial cells[90]. In a more recent study the cag PAI and OipA were examined for their role in IL-6 secretion by gastric epithelial cells and both virulence factors were found to increase the levels of IL-6 production by MKN-28 gastric epithelial cells through different pathways[98]. Remarkably, in one recent careful study to examine cytokine secretion by gastric epithelial cells that, unlike monocytes or lymphocytes, are polarized, cytokine secretion was reported to be polarized to the basolateral compartment, which is where the cells that would be affected by the cytokines are located[99].
While some of the cytokines produced by the gastric epithelium are induced directly by H. pylori virulence factors, it is important to keep in mind that in vivo there is a complex network of interactions, some of which include factors from other cells that infiltrate the infected gastric mucosa. Initially, the adaptive response was considered as a polarized Th1 response; however, more recently other CD4+ T cell subsets have been reported in the infected mucosa. One of those subsets are Th17 cells, which are key in immune defense against extracellular bacteria, but are also commonly found in multiple inflammatory processes[100,101]. Interleukin-17 (IL-17) RNA transcription and IL-17 secretion have been found to increase in the human gastric mucosa during H. pylori infection and its expression is up-regulated in animal stomach after 3 wk of infection and its level elevated up to 12 mo post infection[102]. An animal study that used Mongolian gerbils as a model confirmed the in vivo increased expression of IL-17 mRNA, especially during the chronic phase of H. pylori infection[103]. Since gastric epithelial cells express IL-17 receptors, IL-17 interaction with its receptor stimulates the epithelial cells to produce IL-8; thus confirming that gastric epithelial cells serve as important contributors for IL-17 and IL-8 synthesis during H. pylori infection[102]. Furthermore, IL-21 and IL-23, which are cytokines that induce and sustain IL-17 production, are found to be upregulated in the gastric mucosa of patients infected with H. pylori. IL-23 expression does not appear to be associated with CagA protein, but it certainly plays a role in sustaining production of IL-17[102,104].
Pro-carcinogenic responses
One of the well accepted and significant clinical outcomes of H. pylori infection is gastric adenocarcinoma. Gastric cancer (GC) remains the second leading cause of cancer-related deaths and close to one million people succumb to GC on an annual basis. When diagnosed, the 5 year survival rate of GC patients is 15%[105]. Because of the clear evidence collected linking H. pylori to GC[106]., H. pylori is the first bacterium to have been classified as a class I carcinogen. As the infection typically results in chronic gastritis and inflammation has been linked to cancer development[107], this has been one of most accepted general mechanisms linking the infection to GC. However, not everyone who is infected develops GC. In fact, it has been reported that approximately 1%-3% of those who are infected develop GC while about 10% develop peptic ulcer disease[106]. There are two histologically distinct types of GC. The first is a diffuse type of GC comprised of individual infiltrating neoplastic cells that fail to form glandular structures. The second type is an intestinal type GC that results from the transition of normal mucosa to chronic superficial gastritis, followed by atrophic gastritis, intestinal metaplasia, and eventual dysplasia and adenocarcinoma[108]. Another neoplasia that is linked to infection with H. pylori is a non-Hodgkins type or mucosal associated tissue lymphoma (MALToma). Although H. pylori strains carrying cagA represent an important risk factor for GC development, GC development is thought to be the result of a multifactorial process that is orchestrated to include an array of etiological factors as well as several genetic and epigenetic modifications. As far as H. pylori virulence factors is concerned, CagA was reported to interact with E-cadherin and causing deregulation of β-catenin signaling and this stimulated intestinal transdifferentiation of gastric epithelial cells, a premalignant event from which gastric adenocarcinoma develops[75]. Also, a study in which CagA alone was transfected into human gastric epithelial cells showed that H. pylori CagA targets PAR1 and CagA-PAR1 interaction causes junctional and polarity deficits that free cells from growth-inhibitory cues and thus foster carcinogenesis[78]. A separate study by an independent group, using the transfection approach, also showed that CagA interacted with E-cadherin and induced cytoplasmic/nuclear accumulation of β-catenin by preventing its membrane association, in turn activating β-catenin signaling[75]. That same study showed that CagA-transfected cells expressed intestinal-specific molecules as a sign of intestinal transdifferentiation of gastric epithelial cells. Perhaps the most revealing study demonstrating the oncogenic potential of CagA was an in vivo study showing that genetically modified mice made to express CagA developed gastrointestinal and hematological malignancies[109]. Mice expressing wild type CagA developed gastric epithelial hyperplasia and some mice had gastric polyps as well as gastric and intestinal adenocarcinomas. Some of those CagA transgenic mice developed myeloid leukemias and B cell lymphomas. Interestingly, transgenic mice expressing phosphorylation-resistant CagA did not develop any of those pathologies, which highlight the importance of CagA phosphorylation in the deregulation of SHP-2 and subsequent cellular responses described above.