Published online Aug 14, 2014. doi: 10.3748/wjg.v20.i30.10425
Revised: March 19, 2014
Accepted: April 30, 2014
Published online: August 14, 2014
Metastatic colorectal cancer (mCRC) is still one of the tumor types with the highest incidence and mortality. In 2012, colorectal cancer was the second most prevalence cancer among males (9%) and the third among females (8%). In this disease, early diagnosis is important to improve treatment outcomes. However, at the time of diagnosis, about one quarter of patients already have metastases, and overall survival of these patients at 5-years survival is very low. Because of these poor statistics, the development of new drugs against specific targets, including the pathway of angiogenesis, has witnessed a remarkable increase. So, targets therapies through epidermal growth factor and its receptor and also KRAS pathways modulation acquired a main role whether in association with standard chemotherapy and radiotherapy. With the current knowledge in the field of molecular biology, including genetic mutations and polymorphisms, we know better why patients respond so differently to the same treatments. So, in the future we can develop increasingly personalized treatments to the patient and not the disease. This review aims to summarize some molecular pathways and their relation to tumor growth, as well as novel targeted developing drugs and recently approved for mCRC.
Core tip: Metastatic colorectal cancer (mCRC) treatment remains a challenge for clinicians worldwide. Recently, tumor molecular profile and tailored therapies are objects of great interest throughout the scientific community. Our manuscript will give the readers an interesting overview regarding the innovative drugs developing and recently approved for the treatment of mCRC.
- Citation: Marques AM, Turner A, de Mello RA. Personalizing medicine for metastatic colorectal cancer: Current developments. World J Gastroenterol 2014; 20(30): 10425-10431
- URL: https://www.wjgnet.com/1007-9327/full/v20/i30/10425.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i30.10425
Colorectal cancer (CRC) has high incidence and mortality worldwide. In 2012, CRC was the second most prevalent cancer type among males (9%) and the third among females (8%)[1]. Approximately 15%-25% of patients with CRC present with synchronous liver metastases, and 80%-90% of these have unresectable metastatic liver disease[2,3]. In addition to this, nearly 50% of patients who are initially diagnosed with localized disease ultimately develop metastases[4]. Despite recent advances in the treatment of advanced disease, the 5-year survival rates for patients with advanced CRC remain low[5]. Nowadays, in combination with standard therapy, two therapeutic strategies have demonstrated activity in metastatic CRC (mCRC) targeting the epidermal growth factor receptor (EGFR) and vascular endothelial growth factor (VEGF) both in first and second-line therapy[6,7]. Therefore, with developed in the knowledge and understanding of molecular pathways was possible to the identification of genetic markers existed in some patients. This can be used as a therapeutic target or may to explain why some patients do not respond to target therapy. For example, tumors that have a mutation in codon 12 or 13 of the KRAS gene are not sensitive to EGFR inhibitors. There is also some evidence that mutation in the BRAF gene conferring resistance to anti-EGFR therapy in the non-first-line setting of metastatic disease[8]. Thus, the role of molecular biology in the characterization of tumor is object of great interest in order to improve the systemic treatment for mCRC. This review will focus briefly on novel issues related to personalizing medicine for mCRC.
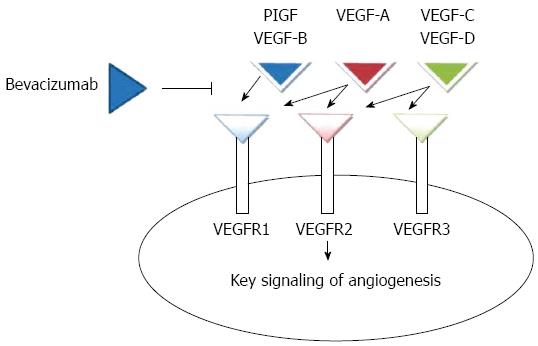
Angiogenesis is a complex process mediated by a number of intersecting pathways, including VEGF, angiopoietins, notch, and integrins (Figure 1). In normal tissues, there is a balance between proangiogenic and antiangiogenic factors[9]. The VEGF family comprises VEGF-A, VEGF-B, VEGF-C, VEGF-D, and placental growth factor (PlGF), 3 receptors [VEGF receptor (VEGFR)-1 (FLT-1), VEGFR-2 (FLK-1/KDR), and VEGFR-3 (FLT-4)], and 2 co-receptors [neuropilin (NRP)-1 and NRP-2]. VEGF-A binding to VEGF receptor (VEGFR)-2 is believed to be the key signaling pathway mediating angiogenesis[10]; by the increase of endothelial proliferation and survival it promotes endothelial cell migration, increases vascular permeability, and alters gene expression. Tyrosine kinases are receptors for VEGF ligands and they are found primarily on vascular endothelial cells[11].
Tumor angiogenesis has peculiarities relating to the same process in healthy tissue. The endothelial layer is irregular and contains spaces that contribute to the extravasation of particles as large as 2 μm from tumor vessels[12], proliferating tumor cells cause compression of vessels[13], and interstitial pressure is increased. This causes an inadequate delivery of therapeutic agents such as monoclonal antibodies[14]. The ability of tumor vessels to supply oxygen and remove waste products is thus compromised, resulting in hypoxia and acidosis in the tumor microenvironment. This further lowers the effectiveness of anti-tumor treatments such as radiation therapy and chemotherapy[15].
Relative to the standard chemotherapy for advanced or mCRC; the guidelines advocate the use of the following schemes in initial therapy: fluorouracil, leucovorin, and oxaliplatin-based chemotherapy (FOLFOX), fluorouracil, leucovorin, and irinotecan-based chemotherapy (FOLFIRI)[16], capecitabine plus oxaliplatin (CapeOx or XELOX)[17] and fluorouracil, leucovorin, oxaliplatin and irinotecan-based chemotherapy (FOLFOXIRI)[18]. These drugs have been used since the 90’s for providing an improvement in the overall survival (OS) for metastatic patients[19].
In chemotherapy for advanced and mCRC, the addition of a biologic agent is also advocated. The biologics agents for CRC include an anti-VEGF (bevacizumab)[20] or an anti-EGFR (cetuximab or panitumumab) for KRAS wild-type (WT) gene patients. They are recommended or listed as an option in combination with some of the regimens above. The OS was not found to be associated with the order in which these drugs were received. After the first progression of the disease, the therapeutic regimen used should be changed, but some studies have demonstrated the benefit of continuing angiogenic suppression after first-progression following bevacizumab-containing cytotoxic regimen[21] though no benefit was observed with the use of bevacizumab in the adjuvant setting.
In mCRC, the anti-EGFR therapy that can be used is cetuximab and panitumumab. These are monoclonal antibodies that can be administered as monotherapy for patients with the KRAS wild type, or in association with standard chemotherapy[22]. Cetuximab was approved with 5-FU, leucovorin and irinotecan (FOLFIRI) for first-line therapy[23], on the other hand panitumumab was approved for use as monotherapy.
In the class of Anti-VEGF therapy, bevacizumab and aflibercept are currently interesting options. Bevacizumab is a recombinant humanized monoclonal IgG1 antibody that binds to and inhibits the biologic activity of VEGF by preventing its binding to VEGFR-1 and VEGFR-2 on the surface of endothelial cells. The result is inhibition of endothelial cell proliferation and new blood vessel formation[24-26]. Aflibercept (VEGF Trap, known in the United States as ziv-aflibercept), is a recombinant fusion protein with receptor components of VEGFR-1 and VEGFR-2 that binds multiple ligands in the angiogenesis network (VEGF-A, VEGF-B, and PlGF). It was demonstrated that this targeted therapy improved the survival of mCRC patients as second-line treatment in combination with the FOLFIRI regimen in patients who had disease progression during first-line oxaliplatin-based chemotherapy[27,28]. Despite the demonstrated effectiveness in progression-free-survival (PFS) and OS with the use of bevacizumab in combination with common cytotoxic chemotherapies[7], there are some mechanisms of resistance to VEGF targeting[29]. In this framework, some hypotheses have been raised, such as the VEGF axis related resistance, the role of non-VEGF modulators of angiogenesis in resistance, and the significance of the stroma in the response to angiogenesis targeting[30].
At the current time, there are 8 new anti-angiogenic agents in trials and a new molecule approved by Food and Drug Administration (FDA) and European Medicine Agency (EMA)[31]. We will summarize below some important aspects regarding the main drugs in this field (Table 1).
| Drugs | Ref. | Trial | Summary of the study | Results (PFS; OS) | Toxicity (grade 3 or 4 adverse events) |
| Aflibercept | [33] | VELOUR trial | aflibercept with irinotecan/5-FU/LV as second line chemotherapy for mCRC | Median PFS for the aflibercept plus FOLFIRI arm was 6.90 mo vs 4.67 mo for the placebo-plus-FOLFIRI arm | Grade 3 or 4 adverse events were more common with the addition of aflibercept (n = 614) or placebo (n = 612), nearly 1/3 of these patients had previously been treated with bevacizumab |
| Median OS for the aflibercept-plus-FOLFIRI arm was 13.50 mo vs 12.06 mo for the placebo plus FOLFIRI arm | |||||
| Sunitinib | [36] | Carrat et al[36] | Double-blinded, phase III study participants were randomly assigned to sunitinib plus FOLFIRI or placebo plus FOLFIRI | Median PFS for the sunitinib arm was 7.8 mo (95%CI: 7.1-8.4 mo) vs 8.4 mo (95%CI: 7.6-9.2 mo) for the placebo arm | Diarrhea, stomatitis/oral syndromes, fatigue, hand-foot syndrome, neutropenia, thrombocytopenia, anemia, and febrile neutropenia) |
| Vatalanib | [38-40] | CONFIRM-1/CONFIRM-2 | These are multinational, randomized, double-blinded, phase III studies. Patients were randomly assigned to receive vatalanib plus FOLFOX4 or placebo plus FOLFOX4 in 1ª or 2º line respectively for CONFIRM-1 or CONFIRM-2 | Vatalanib and placebo, respectively, presented median OS was 13.1 and 11.9 mo (HR = 1.00; 95%CI: 0.87-1.16; P = 0.957). Median PFS was with placebo (5.6 and 4.2 mo, respectively; HR = 0.83; 95%CI: 0.71-0.96; P = 0.013). An exploratory, post hoc analysis demonstrated improved PFS in patients with high LDH, regardless of WHO PS (HR = 0.63; 95%CI: 0.48-0.83; P < 0.001) | The incidence of grade 3/4 neutropenia was similar for both groups; however, a higher percentage of patients in the vatalanib group experienced grade 3/4 hypertension, diarrhea, fatigue, nausea, vomiting, dizziness, confused state, deep vein thrombosis, and pulmonary embolism |
| Semaxanib | [42] | Study conducted with the objective of determining the maximum tolerated dose and dose-limiting toxicities in combination with weekly irinotecan in patients with advanced CRC who had failed at least one prior treatment | NA | There were no drug-related Grade 4 toxicities. There was one episode of Grade 3 headache and one episode of Grade 3 vomiting | |
| Regorafenib | [44] | CORRECT trial | Patients with mCRC and progression during or within 3 mo after the last standard therapy were randomised to receive best supportive care plus oral regorafenib 160 mg or placebo once daily, for the first 3 wk of each 4 wk cycle | Median OS was 6.4 mo in the regorafenib group vs 5.0 mo in the placebo group (HR = 0.77; 95%CI: 0.64-0.94; one-sided P = 0.0052) | The grade 3 or higher of adverse effects related to regorafenib were hand-foot skin reaction (83 patients, 17%), fatigue (48, 10%), diarrhoea (36, 7%), hypertension (36, 7%), and rash or desquamation (29, 6%) |
| Brivanib | [47] | Siu et al[47] | Patients previously treated were assigned 1:1 to receive cetuximab 400 mg/m intravenous loading dose followed by weekly maintenance of 250 mg/m plus either brivanib 800 mg orally daily (arm A) or placebo (arm B) | Median OS in the intent-to-treat population was 8.8 mo in arm A and 8.1 mo in arm B (HR = 0.88; 95%CI: 0.74-1.03; P = 0.12). Median PFS was 5.0 mo in arm A and 3.4 mo in arm B (HR = 0.72; 95%CI: 0.62-0.84; P < 0.001) | Grade ≥ 3 adverse events was 78% in arm A and 53% in arm B |
| Cediranib | [48] | HORIZON III | Patients randomly assigned 1:1:1 received mFOLFOX6 with cediranib or bevacizumab | There are not significant differences in PFS (HR = 1.10; 95%CI: 0.97-1.25; P = 0.119), OS (HR = 0.95; 95%CI: 0.82-1.10; P = 0.541) and overall response rate (46.3% vs 47.3%) | Cediranib arm adverse events included diarrhea, neutropenia, and hypertension |
First, aflibercept, a fully humanized recombinant fusion protein that is composed of domain 2 of VEGFR-1 and domain 3 of VEGFR-2 with the Fc fragment of IgG1[32] as mentioned above. The phase III VELOUR trial determined that patients receiving aflibercept with irinotecan/5-FU as second line chemotherapy for mCRC experienced increased time of outcomes: median PFS for the aflibercept plus FOLFIRI arm was 6.90 mo vs 4.67 mo for the placebo-plus-FOLFIRI arm; and median OS for the aflibercept-plus-FOLFIRI arm was 13.50 mo vs 12.06 mo for the placebo plus FOLFIRI arm. In this study, 1226 patients were randomized to receive FOLFIRI plus placebo or aflibercept. However, toxicity (grade 3 or 4 of adverse events) were more common with the addition of aflibercept (n = 614) than in placebo (n = 612); also, nearly 1/3 of these patients had previously been treated with bevacizumab[33]. The most common adverse events were: fatigue (63.8%), nausea (36.2%) and vomiting (27.7%), while the most common toxicities included dysphonia (46.8%), hypertension (38.3%) and proteinuria (10.6%)[34].
Sunitinib is a small-molecule inhibitor of the VEGF pathway. It is a multi-targeted receptor tyrosine kinase inhibitor (TKI), with both direct anti-proliferative effects and anti-angiogenic properties, targeting the VEGFR-2 as well as PDGFR-b, c-Kit and Ret[35]. However a recent study showed that sunitinib plus FOLFIRI was not superior to FOLFIRI alone and has a poorer safety profile in treatment of mCRC patients[36].
Another biological agent is vatalanib, a TKI that blocks the activity of VEGFR-1, VEGFR-2 and VEGFR-3. It also inhibits other class III kinases, such as the platelet-derived growth factor (PDGF) receptor beta tyrosine kinase, c-Kit, and c-Fms, but at higher concentrations[37]. Phase I/II studies have shown good results, but the data from a phase III study, CONFIRM-1, showed that the addition of vatalanib to FOLFOX-4 did not improve PFS. This was compared with FOLFOX-4 alone in patients with mCRC in first line treatment[38]. Nevertheless, the CONFIRM-2[39], which evaluated the same therapy as second line, showed that in patients with high levels of lactate dehydrogenase, vatalanib could be useful[40].
Semaxanib is an inhibitor of VEGFR-1 and 2 tyrosine kinases that was shown to inhibit VEGF-dependent mitogenesis of human endothelial cells without inhibiting the growth of a variety of tumor cells in vitro[41]. However, the results in phase III trials did not show any improvement in clinical outcome with semaxanib in combination with irinotecan vs fluorouracil irinotecan alone, as therapy for advanced CRC patients that had failed at least one prior treatment[42]. As well as this, severe toxicity was observed.
Regorafenib is a TKI against various pro-angiogenic and pro-proliferation targets[43]. It is the first anti-angiogenic molecule with survival benefits in mCRC which has progressed after all standard therapies[44]. In the CORRECT phase III trial, it was randomized for 753 patients to initiate treatment (regorafenib n = 500; placebo n = 253; population for safety analyses). Median OS, the primary end-point, was 6.4 mo in the regorafenib group vs 5.0 mo in the placebo group (HR = 0.77; 95%CI: 0.64-0.94; one-sided P = 0.0052). Treatment-related adverse events occurred in 465 (93%) patients assigned regorafenib and in 154 (61%) of those assigned placebo. The most common adverse events of grade three or higher related to regorafenib were hand-foot skin reaction (83 patients, 17%), fatigue (48.10%), diarrhea (36.7%), hypertension (36.7%), and rash or desquamation (29.6%). These results were interesting, particularly, for the patients with KRAS mutant mCRC.
Another molecule being studied is brivanib, a TKI that specifically inhibits the VEGR-2 and FGFR-1 signaling[45]. Some studies have been showed that brivanib can be used to restore the antiangiogenic activity in bevacizumab-resistant patients[46]. A recent phase III trial that compared the use of brivanib and cetuximab with or without chemotherapy, demonstrated that despite positive effects on PFS and objective response, cetuximab plus brivanib increased toxicity and did not significantly improve OS in patients with metastatic, chemotherapy-refractory, wild-type K-RAS colorectal cancer[47]. Taking this in account, more data are needed to clarify this issue.
Cediranib, a VEGFR tyrosine kinase inhibitor (VEGFR TKI), was tested in the HORIZON III trial[48] in which cediranib plus FOLFOX6 was compared with bevacizumab plus FOLFOX6 in patients with untreated mCRC. The results did not show significant differences in the use of cediranib or bevacizumab with respect to OS and PFS. Thus, this drug is not currently used in our clinical practice.
Nowadays, the new era of molecular cancer profiling has emerged in order to help clinicians in their decision making. Taking this into account, many targeted drugs are objects of extensive interest for the scientific community. In colorectal cancer, only bevacizumab, cetuximab, regorafenib and panitumumab have acquired sufficient evidence to be used in combination with standard chemotherapy to improve patient outcomes. However, the results are not strong enough for the national public systems to support their complete use in all countries. Pharmaco-economics studies are needed prior to approve those biological drugs towards the cancer care institutions. However, it is important for oncologists to be aware of patients cost and benefit in order to provide the best care for mCRC patients.
P- Reviewer: Cullen JJ, Perini MV, Sipos F S- Editor: Qi Y L- Editor: A E- Editor: Liu XM
| 1. | Siegel R, DeSantis C, Virgo K, Stein K, Mariotto A, Smith T, Cooper D, Gansler T, Lerro C, Fedewa S. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin. 2012;62:220-241. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1973] [Cited by in F6Publishing: 2026] [Article Influence: 168.8] [Reference Citation Analysis (1)] |
| 2. | Van Cutsem E, Nordlinger B, Adam R, Köhne CH, Pozzo C, Poston G, Ychou M, Rougier P. Towards a pan-European consensus on the treatment of patients with colorectal liver metastases. Eur J Cancer. 2006;42:2212-2221. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 362] [Cited by in F6Publishing: 405] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 3. | Muratore A, Zorzi D, Bouzari H, Amisano M, Massucco P, Sperti E, Capussotti L. Asymptomatic colorectal cancer with un-resectable liver metastases: immediate colorectal resection or up-front systemic chemotherapy? Ann Surg Oncol. 2007;14:766-770. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 104] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 4. | Midgley R, Kerr D. Conventional cytotoxic and novel therapeutic concepts in colorectal cancer. Expert Opin Investig Drugs. 2001;10:1011-1019. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Cercek A, Saltz L. Evolving treatment of advanced colorectal cancer. Curr Oncol Rep. 2010;12:153-159. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A, Bets D, Mueser M, Harstrick A, Verslype C. Cetuximab Monotherapy and Cetuximab plus Irinotecan in Irinotecan-Refractory Metastatic Colorectal Cancer. N Engl J Med. 2004;351:337-345. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3767] [Cited by in F6Publishing: 3625] [Article Influence: 181.3] [Reference Citation Analysis (0)] |
| 7. | Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335-2342. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7832] [Cited by in F6Publishing: 7527] [Article Influence: 376.4] [Reference Citation Analysis (1)] |
| 8. | Laurent-Puig P, Cayre A, Manceau G, Buc E, Bachet JB, Lecomte T, Rougier P, Lievre A, Landi B, Boige V. Analysis of PTEN, BRAF, and EGFR status in determining benefit from cetuximab therapy in wild-type KRAS metastatic colon cancer. J Clin Oncol. 2009;27:5924-5930. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 516] [Cited by in F6Publishing: 562] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 9. | Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307:58-62. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3925] [Cited by in F6Publishing: 3784] [Article Influence: 199.2] [Reference Citation Analysis (0)] |
| 10. | Sun W. Angiogenesis in metastatic colorectal cancer and the benefits of targeted therapy. J Hematol Oncol. 2012;5:63. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 81] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 11. | Dvorak HF. Vascular permeability factor/vascular endothelial growth factor: a critical cytokine in tumor angiogenesis and a potential target for diagnosis and therapy. J Clin Oncol. 2002;20:4368-4380. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1170] [Cited by in F6Publishing: 1115] [Article Influence: 50.7] [Reference Citation Analysis (0)] |
| 12. | Choong HL, Pwee HS, Woo KT, Lim CH. Maintenance haemodialysis in Singapore. Singapore Med J. 1991;32:133-138. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1179] [Cited by in F6Publishing: 1109] [Article Influence: 46.2] [Reference Citation Analysis (0)] |
| 13. | Padera TP, Stoll BR, Tooredman JB, Capen D, di Tomaso E, Jain RK. Pathology: cancer cells compress intratumour vessels. Nature. 2004;427:695. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 585] [Cited by in F6Publishing: 544] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 14. | Boucher Y, Baxter LT, Jain RK. Interstitial pressure gradients in tissue-isolated and subcutaneous tumors: implications for therapy. Cancer Res. 1990;50:4478-4484. [PubMed] [Cited in This Article: ] |
| 15. | Fukumura D, Duda DG, Munn LL, Jain RK. Tumor microvasculature and microenvironment: novel insights through intravital imaging in pre-clinical models. Microcirculation. 2010;17:206-225. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 290] [Cited by in F6Publishing: 290] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 16. | Douillard JY, Cunningham D, Roth AD, Navarro M, James RD, Karasek P, Jandik P, Iveson T, Carmichael J, Alakl M. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet. 2000;355:1041-1047. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2407] [Cited by in F6Publishing: 2336] [Article Influence: 97.3] [Reference Citation Analysis (1)] |
| 17. | Cassidy J, Clarke S, Díaz-Rubio E, Scheithauer W, Figer A, Wong R, Koski S, Lichinitser M, Yang TS, Rivera F. Randomized phase III study of capecitabine plus oxaliplatin compared with fluorouracil/folinic acid plus oxaliplatin as first-line therapy for metastatic colorectal cancer. J Clin Oncol. 2008;26:2006-2012. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 592] [Cited by in F6Publishing: 607] [Article Influence: 37.9] [Reference Citation Analysis (0)] |
| 18. | Falcone A, Ricci S, Brunetti I, Pfanner E, Allegrini G, Barbara C, Crinò L, Benedetti G, Evangelista W, Fanchini L. Phase III trial of infusional fluorouracil, leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) compared with infusional fluorouracil, leucovorin, and irinotecan (FOLFIRI) as first-line treatment for metastatic colorectal cancer: the Gruppo Oncologico Nord Ovest. J Clin Oncol. 2007;25:1670-1676. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 830] [Cited by in F6Publishing: 838] [Article Influence: 49.3] [Reference Citation Analysis (0)] |
| 19. | Grothey A, Sargent D, Goldberg RM, Schmoll HJ. Survival of patients with advanced colorectal cancer improves with the availability of fluorouracil-leucovorin, irinotecan, and oxaliplatin in the course of treatment. J Clin Oncol. 2004;22:1209-1214. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 800] [Cited by in F6Publishing: 858] [Article Influence: 42.9] [Reference Citation Analysis (1)] |
| 20. | Saif MW. Is there a benefit from addiction to anti-VEGF therapy in patients with colorectal cancer? Anticancer Res. 2013;33:2377-2380. [PubMed] [Cited in This Article: ] |
| 21. | Bennouna J, Sastre J, Arnold D, Österlund P, Greil R, Van Cutsem E, von Moos R, Viéitez JM, Bouché O, Borg C. Continuation of bevacizumab after first progression in metastatic colorectal cancer (ML18147): a randomised phase 3 trial. Lancet Oncol. 2013;14:29-37. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 760] [Cited by in F6Publishing: 827] [Article Influence: 68.9] [Reference Citation Analysis (0)] |
| 22. | Haraldsdottir S, Bekaii-Saab T. Integrating anti-EGFR therapies in metastatic colorectal cancer. J Gastrointest Oncol. 2013;4:285-298. [PubMed] [Cited in This Article: ] |
| 23. | Van Cutsem E, Köhne CH, Hitre E, Zaluski J, Chang Chien CR, Makhson A, D’Haens G, Pintér T, Lim R, Bodoky G. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360:1408-1417. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2901] [Cited by in F6Publishing: 3021] [Article Influence: 201.4] [Reference Citation Analysis (1)] |
| 24. | Jain RK, Duda DG, Clark JW, Loeffler JS. Lessons from phase III clinical trials on anti-VEGF therapy for cancer. Nat Clin Pract Oncol. 2006;3:24-40. [PubMed] [Cited in This Article: ] |
| 25. | Ferrara N, Hillan KJ, Gerber HP, Novotny W. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat Rev Drug Discov. 2004;3:391-400. [PubMed] [Cited in This Article: ] |
| 26. | Cohen MH, Gootenberg J, Keegan P, Pazdur R. FDA drug approval summary: bevacizumab plus FOLFOX4 as second-line treatment of colorectal cancer. Oncologist. 2007;12:356-361. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 127] [Cited by in F6Publishing: 137] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 27. | Tabernero J, Van Cutsem E, Lakomý R, Prausová J, Ruff P, van Hazel GA, Moiseyenko VM, Ferry DR, McKendrick JJ, Soussan-Lazard K. Aflibercept versus placebo in combination with fluorouracil, leucovorin and irinotecan in the treatment of previously treated metastatic colorectal cancer: prespecified subgroup analyses from the VELOUR trial. Eur J Cancer. 2014;50:320-331. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 162] [Cited by in F6Publishing: 155] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 28. | Chung C, Pherwani N. Ziv-aflibercept: a novel angiogenesis inhibitor for the treatment of metastatic colorectal cancer. Am J Health Syst Pharm. 2013;70:1887-1896. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 29. | Menon RS, Rusinko MS, Allen PS. Proton relaxation studies of water compartmentalization in a model neurological system. Magn Reson Med. 1992;28:264-274. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 80] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 30. | Clarke JM, Hurwitz HI. Understanding and targeting resistance to anti-angiogenic therapies. J Gastroint Oncol. 2013;4:253-263. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 41] [Reference Citation Analysis (0)] |
| 31. | Ciombor KK, Berlin J, Chan E. Aflibercept. Clin Cancer Res. 2013;19:1920-1925. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 77] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 32. | Chu QS. Aflibercept (AVE0005): an alternative strategy for inhibiting tumour angiogenesis by vascular endothelial growth factors. Expert Opin Biol Ther. 2009;9:263-271. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 92] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 33. | Van Cutsem E, Tabernero J, Lakomy R, Prenen H, Prausová J, Macarulla T, Ruff P, van Hazel GA, Moiseyenko V, Ferry D. Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin-based regimen. J Clin Oncol. 2012;30:3499-3506. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 933] [Cited by in F6Publishing: 954] [Article Influence: 79.5] [Reference Citation Analysis (0)] |
| 34. | Lockhart AC, Rothenberg ML, Dupont J, Cooper W, Chevalier P, Sternas L, Buzenet G, Koehler E, Sosman JA, Schwartz LH. Phase I study of intravenous vascular endothelial growth factor trap, aflibercept, in patients with advanced solid tumors. J Clin Oncol. 2010;28:207-214. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 185] [Cited by in F6Publishing: 206] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 35. | O'Farrell AM, Abrams TJ, Yuen HA, Ngai TJ, Louie SG, Yee KW, Wong LM, Hong W, Lee LB, Town A. SU11248 is a novel FLT3 tyrosine kinase inhibitor with potent activity in vitro and in vivo. Blood. 2003;101:3597-3605. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 668] [Cited by in F6Publishing: 653] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 36. | Carrato A, Swieboda-Sadlej A, Staszewska-Skurczynska M, Lim R, Roman L, Shparyk Y, Bondarenko I, Jonker DJ, Sun Y, De la Cruz JA. Fluorouracil, leucovorin, and irinotecan plus either sunitinib or placebo in metastatic colorectal cancer: a randomized, phase III trial. J Clin Oncol. 2013;31:1341-1347. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 111] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 37. | Wood JM, Bold G, Buchdunger E, Cozens R, Ferrari S, Frei J, Hofmann F, Mestan J, Mett H, O’Reilly T. PTK787/ZK 222584, a novel and potent inhibitor of vascular endothelial growth factor receptor tyrosine kinases, impairs vascular endothelial growth factor-induced responses and tumor growth after oral administration. Cancer Res. 2000;60:2178-2189. [PubMed] [Cited in This Article: ] |
| 38. | Abstracts of the 41st Annual Meeting of the American Society of Clinical Oncology (ASCO). May 13-17, 2005. Orlando, Florida, USA. J Clin Oncol. 2005;23:1s-1087s. [PubMed] [Cited in This Article: ] |
| 39. | Van Cutsem E, Bajetta E, Valle J, Köhne CH, Hecht JR, Moore M, Germond C, Berg W, Chen BL, Jalava T. Randomized, placebo-controlled, phase III study of oxaliplatin, fluorouracil, and leucovorin with or without PTK787/ZK 222584 in patients with previously treated metastatic colorectal adenocarcinoma. J Clin Oncol. 2011;29:2004-2010. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 120] [Cited by in F6Publishing: 135] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 40. | Kohne C, Bajetta E, Lin E. Final results of CONFIRM 2: A multinational, randomized, double-blind, phase III study in 2nd line patients (pts) with metastatic colorectal cancer (mCRC) receiving FOLFOX4 and PTK787/ZK 222584 (PTK/ZK) or placebo. J Clin Oncol. 2007;25:4033. [Cited in This Article: ] |
| 41. | Fong TA, Shawver LK, Sun L, Tang C, App H, Powell TJ, Kim YH, Schreck R, Wang X, Risau W. SU5416 is a potent and selective inhibitor of the vascular endothelial growth factor receptor (Flk-1/KDR) that inhibits tyrosine kinase catalysis, tumor vascularization, and growth of multiple tumor types. Cancer Res. 1999;59:99-106. [PubMed] [Cited in This Article: ] |
| 42. | Hoff PM, Wolff RA, Bogaard K, Waldrum S, Abbruzzese JL. A Phase I study of escalating doses of the tyrosine kinase inhibitor semaxanib (SU5416) in combination with irinotecan in patients with advanced colorectal carcinoma. Jpn J Clin Oncol. 2006;36:100-103. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 30] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 43. | Kies M, Blumenschein GJ, Christensen O. Phase I study of regorafenib (BAY 73-4506), an inhibitor of oncogenic and angiogenic kinases, administered continuously in patients (pts) with advanced refractory non-small cell lung cancer (NSCLC). J Clin Oncol. 2010;28:7585. [Cited in This Article: ] |
| 44. | Grothey A, Van Cutsem E, Sobrero A, Siena S, Falcone A, Ychou M, Humblet Y, Bouché O, Mineur L, Barone C. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381:303-312. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1808] [Cited by in F6Publishing: 1899] [Article Influence: 172.6] [Reference Citation Analysis (0)] |
| 45. | Cai ZW, Zhang Y, Borzilleri RM, Qian L, Barbosa S, Wei D, Zheng X, Wu L, Fan J, Shi Z. Discovery of brivanib alaninate ((S)-((R)-1-(4-(4-fluoro-2-methyl-1H-indol-5-yloxy)-5-methylpyrrolo[2,1-f][1,2,4]triazin-6-yloxy)propan-2-yl)2-aminopropanoate), a novel prodrug of dual vascular endothelial growth factor receptor-2 and fibroblast growth factor receptor-1 kinase inhibitor (BMS-540215). J Med Chem. 2008;51:1976-1980. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 113] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 46. | Lieu C, Heymach J, Overman M, Tran H, Kopetz S. Beyond VEGF: inhibition of the fibroblast growth factor pathway and antiangiogenesis. Clin Cancer Res. 2011;17:6130-6139. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 212] [Cited by in F6Publishing: 222] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 47. | Siu LL, Shapiro JD, Jonker DJ, Karapetis CS, Zalcberg JR, Simes J, Couture F, Moore MJ, Price TJ, Siddiqui J. Phase III randomized, placebo-controlled study of cetuximab plus brivanib alaninate versus cetuximab plus placebo in patients with metastatic, chemotherapy-refractory, wild-type K-RAS colorectal carcinoma: the NCIC Clinical Trials Group and AGITG CO.20 Trial. J Clin Oncol. 2013;31:2477-2484. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 100] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 48. | Schmoll HJ, Cunningham D, Sobrero A, Karapetis CS, Rougier P, Koski SL, Kocakova I, Bondarenko I, Bodoky G, Mainwaring P. Cediranib with mFOLFOX6 versus bevacizumab with mFOLFOX6 as first-line treatment for patients with advanced colorectal cancer: a double-blind, randomized phase III study (HORIZON III). J Clin Oncol. 2012;30:3588-3595. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 161] [Cited by in F6Publishing: 166] [Article Influence: 13.8] [Reference Citation Analysis (0)] |