Published online Aug 14, 2014. doi: 10.3748/wjg.v20.i30.10405
Revised: January 16, 2014
Accepted: April 30, 2014
Published online: August 14, 2014
Processing time: 304 Days and 17.9 Hours
Pancreatic adenocarcinoma is one of the most aggressive cancers, and the decline in mortality observed in most other cancer diseases, has so far not taken place in pancreatic cancer. Complete tumor resection is a requirement for potential cure, and the reorganization of care in the direction of high patient-volume centers, offering multimodal treatment, has improved survival and Quality of Life. Also the rates and severity grade of complications are improving in high-volume pancreatic centers. One of the major problems worldwide is underutilization of surgery in resectable pancreatic cancer. Suboptimal investigation, follow up and oncological treatment outside specialized centers are additional key problems. New chemotherapeutic regimens like FOLFIRINOX have improved survival in patients with metastatic disease, and different adjuvant treatment options result in well documented survival benefit. Neoadjuvant treatment is highly relevant, but needs further evaluation. Also adjuvant immunotherapy, in the form of vaccination with synthetic K-Ras-peptides, has been shown to produce long term immunological memory in cytotoxic T-cells in long term survivors. Improvement in clinical outcome is already achievable and further progress is expected in the near future for patients treated with curative as well as palliative intention.
Core tip: Curative treatment outcome for patients with pancreatic cancer is achievable if early surgical treatment is combined with adjuvant chemotherapy. Nevertheless, most patients end up in a palliative situation, earlier or later. Also palliative therapeutic interventions are improving, but a multidisciplinary team with advanced expertise is a prerequisite for optimal care.
- Citation: Buanes TA. Pancreatic cancer-improved care achievable. World J Gastroenterol 2014; 20(30): 10405-10418
- URL: https://www.wjgnet.com/1007-9327/full/v20/i30/10405.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i30.10405
Pancreatic adenocarcinoma is one of the most aggressive cancers. Despite all advances in cancer treatment it is still the fourth most-frequent tumor-related cause of death in the Western world[1]. The reasons for this are challenges associated with the diagnosis, which tend to be late and precarious, but more importantly limited therapeutic options. Therefore, even though cancer mortality in Europe has declined by approximately 10% during recent years, this is not the case for pancreatic cancer[2]. The development of new and potent treatment options is therefore strongly needed. In recent years there have been important advances in the organization of care for pancreatic cancer patients, also resulting in more focused studies on preoperative investigation, surgical, oncological and immunological treatment. This review summarizes available evidence, focusing best clinical practice based on the latest translational research.
Search in PubMed was performed with the key words: Pancreatic cancer, combined with pathogenesis, prevention, diagnosis and treatment. Reports were selected, based on publication date (preferring recent studies) and conceived internal validity in each single paper. A balanced mix of original papers, preferring randomized trials initiated after 2003, and Cochrane reviews, meta-analyses and review articles, relevant to the scope of this review, were prioritized.
The cause of pancreatic cancer remains unknown. Several environmental factors have been implicated, but a causal role has been shown only for tobacco. The risk of pancreatic cancer in smokers is 2.5 to 3.6 times that in non-smokers, increasing with greater tobacco use and longer duration of exposure[3]. Worldwide the proportion of early onset pancreatic cancer is strongly correlated with lung cancer mortality[4] (r2 = 0.53), suggesting that approximately half of the variation in the proportion of early onset pancreatic cancer can be explained by smoking. The possible roles of moderate intake of alcohol, coffee, and use of aspirin as contributing factors are supported by very limited data. Increased risk of pancreatic cancer among patients with blood type A, B or AB as compared with blood type O has been observed in recent reports[5,6]. Pancreatic cancer also occurs with increased frequency among persons with long-standing diabetes[7,8], but this does not necessarily imply that diabetes is a pathogenetic factor, as it may be a consequence of the cancer. The latter concept is supported by the recent observation that adrenomedullin is upregulated in patients with pancreatic cancer and causes insulin resistance in β cells[9]. A recent meta-analysis also favors the association between hepatitis B/C infection and pancreatic cancer[10].
There may be a causal relationship between chronic pancreatitis and pancreatic cancer, but the population attributable fraction was estimated to only 1.34% (95%CI: 0.612-2.07) in a recent study[11], suggesting that a relatively small proportion of pancreatic cancers might be avoided if pancreatitis could be prevented. Pancreatitis appearing shortly before the diagnosis of pancreatic cancer is probably the result of tumor-related ductal obstruction. But patients with hereditary pancreatitis, which is a rare subgroup of chronic pancreatitis, have a marked relative and absolute increased risk of pancreatic cancer[12] as compared to the general population, especially in smokers. This has been documented in two comprehensive international studies[13,14]. Whitcomb[15] identified in 1996 the first genetic defect in patients with hereditary pancreatitis on the cationic trysinogen gene (PRSS1).
Pancreatic cancer has been shown to result from a successive accumulation of gene mutations[16] in the ductal epithelium, evolving from premalignant lesions to fully invasive cancer. Pancreatic intraepithelial neoplasia is a precursor of pancreatic cancer[17], progressing from minimally dysplastic epithelium to invasive carcinoma. During carcinogenesis accumulation of mutations take place, initially activation of the KRAS2 oncogene, then inactivation of the tumor suppressor gene CDKN2A and inactivation of the tumor suppressor gene TP53 and finally deletion of the SMAD family member 4 gene[18,19].
At least one of four genetic defects are present in almost all patients with fully established pancreatic cancer[20]. Activated mutations in the KRAS2 oncogene is very frequent in pancreatic cancer cells, making this mutation an appropriate target for immunological attack from vaccine-activated cytotoxic T-cells[21]. The abnormal Ras protein, generated from transcription of the mutant KRAS gene, results in permanent activation of proliferative and survival signaling pathways in the cancer cells.
Comprehensive genetic analysis of 24 pancreatic cancers showed that the genetic basis of the tumor is extremely complex and heterogeneous[22]. An average of 63 genetic abnormalities per tumor was found, mainly point mutations, classified as likely to be carcinogenetically relevant. These abnormalities can be organized in 12 functional pathways. A model of this carcinogenetic process is presented graphically as the “Components of Pancreatic Cancer” in a clarifying review article by Hidalgo[19].
Genomic sequencing, evaluating the clonal relationships among primary and metastatic pancreatic cancer cells, has recently been performed. Based on differential accumulation of mutations, the authors estimated that pancreatic tumors cells are present for 6 to 12 years before development of metastatic disease, suggesting a broad time window for early detection of the primary tumor[23].
Universal primary screening for pancreatic cancer is currently not recommended, given the tools available and their performance[24,25], even though the time interval when pancreatic cancer cells are present in advance of their dissemination, is probably long[23,26]. Hence, the beneficial potential of a biomarker panel with the required accuracy, is huge. No imaging modality fills this requirement. Sensitivity as well as specificity of endoscopic ultrasound (EUS) examination has been improving during recent years, and enables fine needle aspiration during the same procedure. But even screening of high-risk groups by EUS combined with computed tomography (CT), should only be performed in the context of prospective trials[25,27].
The number of patients with incidentally diagnosed cystic pancreatic lesions is rising, most likely due to the increased use of high-resolution imaging[28]. The variable degree of malignancy potential in different cystic pancreatic lesions can be clarified by EUS guided aspiration/analysis of cystic fluid, as low levels of carcinoembryonic antigen (CEA) in cyst fluid from serous cystadenoma has been documented[29]. Oppositely, cyst fluid from mucinous lesions tends to have high CEA values[30]. Intraductal papillary mucinous neoplasms (IPMN) in the main duct develop invasive carcinoma more often than IPMN lesions in side branches[31], both supposed to be more indolent than sporadic pancreatic adenocarcinoma. But in patients with lymph node metastasis, long term survival curves are almost identical[32]. IPMN-lesions usually have a premalignant time interval of several years duration. Surgical resection of mucinous cystic lesions before they become invasive carcinoma, apparently represents one of the best opportunities to prevent pancreatic cancer. Also when incidentally recognized malignant lesions undergo surgical resection, survival is significantly improved[33]. Five year survival above 30% is reported after resection of incidentaloma, even in distal pancreatic carcinoma[34]. Prevention of death from pancreatic cancer is therefore increasingly affordable, even though screening programs have not become the way to do it up till now.
High quality imaging plays a crucial role in the diagnosis of pancreatic tumors. One cross-sectional imaging modality is sufficient for adequate evaluation of tumor diagnosis and resectability in most patients. Multidetector CT angiography, performed by using a dedicated dual-phase pancreatic protocol[35] is the preference of most centers[36]. Adoption of a standardized template for radiology reporting in pancreatic neoplasms is strongly recommended in a consensus statement, authored by radiologists, gastroenterologists and hepatopancreaticobiliary surgeons under the sponsorship of the Society of Abdominal Radiologists and the American Pancreatic Association[35]. Magnetic resonance imaging (MRI), including magnetic resonance cholangiography, may help to differentiate cystic lesions, but does not add information about resectability. EUS guided fine-needle aspiration is essential for analysis of cystic fluid, and is the best method for obtaining a tissue diagnosis when needed, i.e., before neoadjuvant or palliative chemotherapy. Routine use of endoscopic retrograde cholangiopancreatography (ERCP) or 18F-fluorodeoxy-glycose (18F-FDG) PET cannot be recommended[36,37]. ERCP should only be used for therapeutic purposes, because of the high frequency of severe complications. Procedure-related mortality rate of 1.4% have recently been published[38]. Routine preoperative biopsy of resectable pancreatic tumors is not advisable, because malignant disease cannot be ruled out reliably[39]. Seeding of cancer cells along the path of the needle[40] after percutaneous biopsy is another reason for avoiding preoperative biopsy in patients with resectable tumors.
Numerous biomarkers for cancer have been developed[41], but the clinical benefit in pancreatic cancer patients has so far been limited, and the persistent search for a biomarker panel with improved sensitivity/specificity is important. New markers based on analysis of gene expression[42], proteomic analysis[43], radiolabeling with anti-Claudine 4[44] and membrane bound molecules[41] are developing, but a panel also including microRNA (miRNA) as a biomarker, seems presently most likely to obtain clinical significance[45]. The beneficial role of a secure biomarker panel is obvious for primary diagnosis, as well as monitoring of treatment outcome. Carbohydrate antigen 19-9 (CA 19-9) is in widespread clinical use, even though sensitivity and specificity are low[46]. Its clinical usefulness in early detection of recurrent disease and therapeutic monitoring is well documented[47].
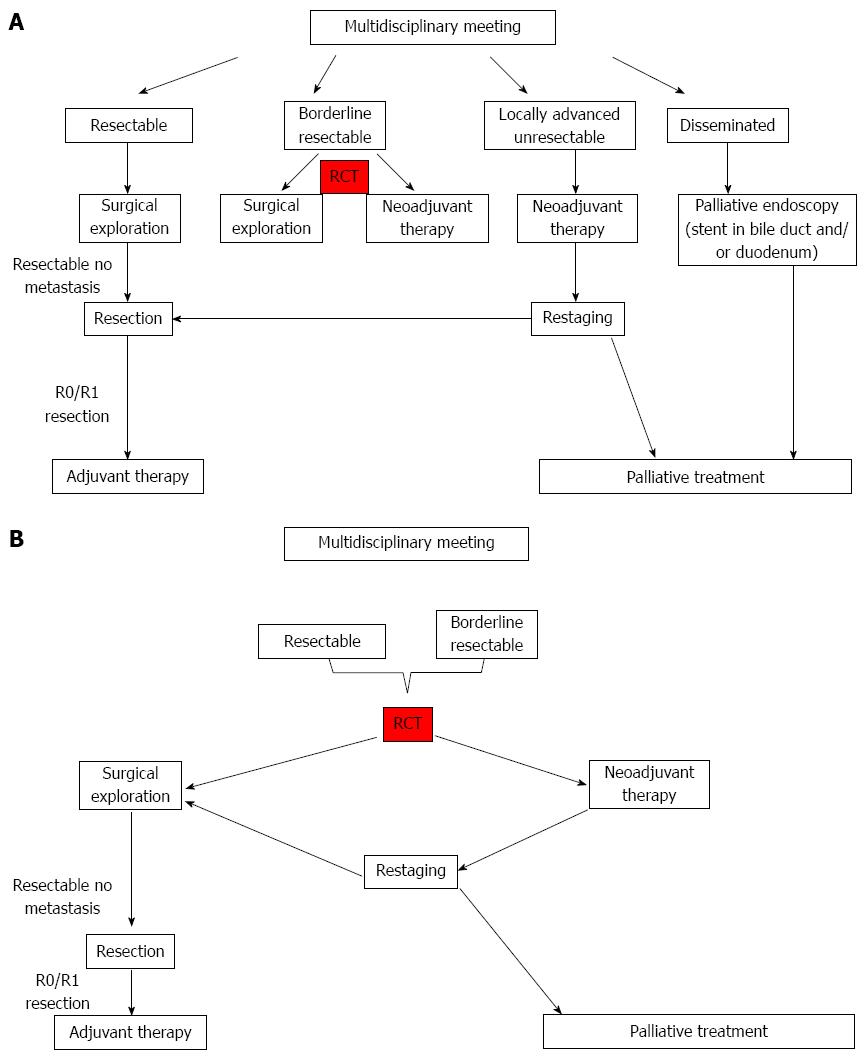
Pancreatic cancer is staged according to the most recent edition of the American Joint Committee on Cancer (AJCC) tumor-node-metastasis (TNM) classification[48]. Treatment of different stages have been changing during recent years, and the outcome, recorded with survival and Quality of life (QoL) as clinical endpoints, changes with the development of revised guidelines. Bilimoria et al[49] reported in 2007 survival data resulting from treatment according to staging by the 6th edition of AJCC Pancreatic Cancer Staging System, when T1, T2 and T3 tumors are considered potentially resectable, even though locally advanced T3 tumors involve the superior mesenteric veins (SMV), portal vein (PV), or splenic vein (SV). Median survival 24.1 mo was reported in stage 1A, decreasing to 4.5 mo in stage IV. Details of stage characteristics are given in Bilimoria et al[49]’s report from the National Cancer (NCD)-database and in Hidalgos et al[19]’s review. However, the practical consequences of staging, related to assessment of resectability and timing of an operation, is changing after the introduction of the concept of borderline resectable tumors. As indicated in Table 1, this question depends on the local handling program of each pancreatic center.
Long term survival is not achieved in pancreatic cancer patients without surgical resection of the tumor. This is true even when chemoradiation is used in early stage disease[50,51]. The superiority of surgery over chemoradiotherapy has even been documented in a randomized controlled trial (one year survival 62% vs 32%, P < 0.05)[52]. In the Surveillance Epidemiology and End Result (SEER) database, curative intent surgery was found to be the strongest predictor of prolonged survival[53]. In high-volume centers, resection of mesenteric vessels and even multivisceral resection are performed in patients with locally advanced disease[54-58] to enable long term survival.
A multidisciplinary approach is mandatory, already at the time of primary diagnosis of pancreatic cancer. The impact was evaluated in 203 consecutive patients at the Johns Hopkins pancreatic multidisciplinary clinic in 2006/2007[59], and a comprehensive and coordinated evaluation led to changes in therapeutic recommendations in almost one-quarter of patients. Patient logistics and care was organized around a nurse navigator in an “All-in-One Resort”[60,61], and high degree of patient satisfaction was reported. The continuous ongoing development of oncological and surgical treatment algorithms in patients with borderline resectable and locally advanced disease, further underlines the importance of close cooperation within the multidisciplinary team in order to maximize short- and long-term oncological outcomes[62].
Improved quality of preoperative staging has enabled radiological classification of pancreatic tumors as resectable, borderline resectable (a concept which has been defined by a consensus panel[63,64]) and locally advanced unresectable tumors. Borderline resectable tumors may be treated by neoadjuvant chemoradiation, which has been shown to result in high rates of R0 resections, and 5 years survival in the same range as primary resectable tumors[63]. But the downside of the neoadjuvant protocol was that significant numbers of included patients with potentially resectable tumors at inclusion (approximately ¼), progressed during neoadjuvant treatment and could never be resected[63,65]. These patients have the disadvantage of median survival 8 mo[66]. Future development of care for patients with pancreatic cancer is obviously emerging towards more advanced surgery for new patient groups combined with oncological efforts after surgery, probably also preoperatively. Further prospective clinical studies, focusing clinical outcome, are mandatory. But the underutilization of surgery in patients with localized pancreatic cancer is a major ethical problem: Numerous patients without any contraindication against surgery never receive surgical treatment of their serious disease[50,67].
The best outcome of surgical treatment is histologically free resection margin (R0) and it has been unclear whether an R1 resection confers any survival benefit at all over no surgical removal of locally advanced tumors. Even the predictive value of an R0 resection has been queried: In 360 consecutive patients, undergoing pancreaticoduodenectomy, R0 was found in 300 (83.3%), but R0 status did not come out as survival predictor in multivariate analyses[68]. Patients who underwent an R1 resection had a median overall survival of 21.5 mo compared with 27.8 mo after R0 resection, which was found not significantly different. This might in part be explained by the fact that up till 2006, pathological examination of pancreaticoduodenectomy specimens were not standardized between different pancreatic centers[69,70]. Verbeke et al[71] published a systematic, detailed technique for handling and evaluation of resected specimens with colouring of the resectional margins, redefining R1 resection as tumor cells within 1 mm of the resection margin. The Heidelberg group documented that R0 resection came out as predictor of long term survival in multivariate analyses after the introduction of this standardized handling of resected specimens[54]. Accordingly, refinement of surgical technique, aiming at increased rates of R0 resection, defined by new standards, is mandatory. This may require increased rates of resection of the SMV/PV[72], altered dissection strategy[73] or it might be advantageous to alter the whole treatment algorithm, introducing neoadjuvant chemoradiotherapy in patients with borderline resectable tumours, as described in the United States National Comprehensive Cancer Network guidelines[36,74]. European guidelines are different, as neoadjuvant chemoradiotherapy is not recommended in patients with resectable pancreatic cancer[75]. The intention behind the neoadjuvant treatment algorithm is to avoid surgery in patients with rapidly progressive disease, and to achieve better local tumour control for the residual group, potentially even to down-size unresectable locally advanced tumours to allow secondary resection. Chemoradiotherapy before any surgical resection selects patients with more stable disease for surgery and putative micrometastasis may be treated at an earlier stage. On the other hand significant numbers of primary resectable patients become unresectable during neoadjuvant treatment and the outcome of primary resection followed by adjuvant chemotherapy is lost in many of these cases. A median survival of 23 mo[76] to 28 mo[77] has been documented in two recent randomized controlled trials. This life expectancy is replaced by the prospects of an unresectable tumor, being less than a year, ie. significantly shorter[78].
Table 2 puts together core data from four studies illustrating principal difficulties, arising when outcome of neoadjuvant treatment is compared with upfront surgery followed by adjuvant chemotherapy. Katz et al[63] published 2008 median postoperative survival 40 mo and 94% R0 resections, which is the best outcome for resected patients. But most patients included in the study could never be respected, and it is an open question what the clinical outcome of an earlier operation would have been in these cases. On the other hand, Nordby et al[72] published in 2013, that almost 90% of patients scheduled for upfront surgery were actually resected, but the rate of R0 resection was low, and it is underlined that alteration of surgical technique might be an opportunity of improvement: Increased frequency of resection of the PV/SMV and/or artery first dissection strategy. Finally, ESPAC centers resected PV/SMV in 17 % of operated patients, and obtained similar oncological outcome in the whole group of included patients, when surgery was performed first. Also the Heidelberg group (Schmidt 2012) has reported equivalent survival after upfront surgery. The different outcome in these series is probably explained by diverse patient selection, differences in preoperative and intraoperative criteria for resectability and variable surgical technique. These parameters illustrate important confounding factors when outcome is compared between neoadjuvant and upfront surgical treatment algorithms. Further efforts are therefore needed to standardize and clarify critical determining factors of outcome in advance of future randomized clinical trials. According to the current available evidence, neoadjuvant therapy is usually not recommended for patients with curatively resectable pancreatic cancer[36,75], but the prospective evaluation in well-designed controlled trials is mandatory. A synthesis of the considerations above is summarized in Figure 1A, suggesting upfront surgery for all patients with resectable tumor, followed by adjuvant chemotherapy. However, the inclusion of resectable as well as borderline resectable tumors in a neoadjuvant protocol is the preference of MD Anderson Cancer Center[79] as shown in Figure 1B. Further details on tumor biology, enabling personalized medical treatment plans would significantly improve outcome in both arms of these trials, and probably reduce health care costs[80,81].
| Ref. | Patients included | Inclusion periode | Treatment algorithm | Proportion not resected | R0 status | Frequency of vascular resection | Median survival in months |
| Katz et al[63] 2008 | 160 | 1999-2006 | Neo-adjuvant | 59% | 94% | 27% | 40 |
| Nordby et al[84] 2013 | 135 | 2008-2010 | Upfront surgery+ adjuvance | 11% | 42% | 6% | Na1 |
| Neop-tolemos et al[76] 2010 | 1088 | 2000-2007 | Upfront surgery+ adjuvance | Only resected patients included | 65% | 17% | 23 |
| Schmidt et al[77] 2012 | 132 | 2004-2007 | Upfront surgery+ adjuvance | Only resected patients included | 61% | Na2 | 28 |
The purpose of surgical resection of pancreatic tumors is radicality, but final R1 status occurs in all centers. Already in 1996 Lillemoe published data, suggesting a survival benefit of R1 resection over locally advanced unresectable tumours[82]. Two recent publications have verified increased survival after R1 resection. Konstantinidis et al[83] found median survival 14 mo in 157 R1 resected patients vs 11 mo in 286 locally advanced, unresectable cases. Nordby et al[84] found median 18 mo survival after R1 resection vs 8.1 mo in the locally advanced unresectable group and also QoL, recorded longitudinally, was found improved in the resected group. Collective evidence supports the concept that there is a significant clinical benefit of removing the pancreatic tumor, even if the resectional status is R1.
Pancreatic adenocarcinoma tend to be chemoresistant and for a long period little progress has been obtained by traditional anti-tumor treatment, illustrated by the fact that gemcitabine has been standard of care since 1997[85]. But Conroy et al[86] published 2011 a randomized controlled trial including 342 metastatic patients, comparing FOLFIRINOX (oxaliplatin, irinotecan, leucoverin and fluouracil) with gemcitabine, and found significantly increased survival (11.1 mo vs 6.8 mo). The objective response rate was 31.6% in the FOLFIRINOX group vs 9.4% in the gemcitabine group (P < 0.001). During ASCO 2012 the FOLFIRINOX regimen was characterized as a paradigm shift in oncological treatment of pancreatic cancer, and this regimen is now under evaluation in potentially curative subgroups, as those with borderline resectable tumours[87]. The results are positive, but the toxicity of FOLFIRINOX generates significant limitations, as only patients with relatively good health can be included.
Evaluation of adjuvant chemotherapy, combined with radiotherapy or alone, was first analysed in well-designed randomized trials in the ESPAC 1 study which showed no survival benefit for adjuvant chemoradiotherapy but a significant prolonged survival in patients treated by fluouracil and folic acid[88,89]. However, a meta-analysis suggests that further studies with chemoradiation is warranted in patients with positive resection margins, as chemotherapy appears relatively ineffective in this subgroup[90]. Another important observation in the ESPAC 1 study was that also QoL, recorded prospectively, was not negatively affected by adjuvant chemotherapy compared to surgery alone[91]. Also adjuvant gemcitabine was found to delay recurrence after complete resection of pancreatic cancer[92]. Finally adjuvant gemcitabine was compared with fluouracil/folic acid in the ESPAC 3 trial, which did not find any difference[76].
Neoadjuvant chemoradiotherapy seems to have an obvious place in patients with locally advanced unresectable tumors, who may become resectable after downstaging[93]. However, downstaging filling the RECIST (Response Evaluation Criteria in Solid Tumors) criteria, was found very rare in a recent review[79]. In patients with resectable or borderline resectable tumors, the role of gemcitabine is controversial[94] due to objective response rates below 10%. But potent new regimens like FOLFIRINOX may expand this window of opportunity.
After decades of disappointment, the recent success of immunotherapy in metastatic melanoma, including proof-of concept trials[95], have renewed the interest in this form of therapy also against pancreatic cancer. In fact, the concept of immune attach against pancreatic tumor cells by CD4+/CD8+ T-lymphocytes was published already in 1997[96]. A second treatment protocol was initiated simultaneously, utilizing adjuvant vaccination with synthetic ras peptides encompassing residues 5-21 of p21 ras in patients operated for pancreatic cancer[21]. This phase I/II trial included 23 resected patients, receiving adjuvant vaccination, subsequently followed till death or for more than ten years. Three patients mounted a memory response immunologically up to nine years after vaccination. Recurrence was found in a fourth patient six years after the Whipple procedure, and her T-cells had then lost their reactivity. After baseline vaccination (1998), she mounted a strong immune response. The evaluation of K-ras peptides in phase II/III trials are ongoing, so far in the Targovax-study, but numerous other basic and translational efforts are in progress[97].
Also the catalytic subunit of telomerase, hTERT, expressed in 85%-90% of human cancer tissue[98], is an attractive “universal” tumour antigen. A synthetic peptide, GV1001, has been tested in unresectable pancreatic cancer patients, with promising outcome: Vaccination initiated CD4+/CD8+ immune response[99] via multiple MHC class II alleles. The intermediate dose of GV1001 resulted in immune response in 3/4 of included patients, with significantly increased survival (median 7.2 mo vs 2.9 mo) in responding patients. This resulted in a following phase III trial, the Primovax Study, evaluating GV1001 as monotherapy in one arm, compared with standard gemcitabine in the other arm. The intention was to randomize 520 patients to each arm. But the study was closed after inclusion of 360 patients when preliminary data on the deaths of 174 patients showed no survival benefit in the GV1001 group[100]. Another randomized trial with three arms, comparing survival in metastatic pancreatic cancer after gemcitabine plus capecitabine, vs gemcitabine plus capecitabine followed by GV 1001 in the second arm and concurrent gemcitabine/capecitabine in the third arm, could neither improve outcome by adding the vaccine[101].
The majority of patients with pancreatic cancer are not resectable at the time of presentation, with life expectancy less than one year for approximately 80%-90%. Palliative interventions for these patients intend to solve problems associated with biliary occlusion and/or duodenal obstruction. The advantage of surgical palliation with double bypass has been to obtain lifelong palliation with one single procedure[102]. But improved radiological staging enables secure prediction of resectability in most cases, and the advantage of avoiding surgical exploration of unresectable patients favors endoscopic stenting, also of patients with duodenal obstruction[103]. The development of defined quality indicators for the different aspects of the handling of pancreatic cancer patients[104] enables better focus on clinical outcome in future treatment guidelines. The symptom profile of advanced pancreatic cancer is dominated by fatigue and pain[105] and appropriate treatment of nausea and vomiting is important[106]. The palliative functions of the multidisciplinary team have to be closely integrated to offer well-timed help when treatment aspirations change from curative to palliative ambitions[107]: Endoscopic and radiological interventions, together with nutritional support may significantly improve clinical outcome[108].
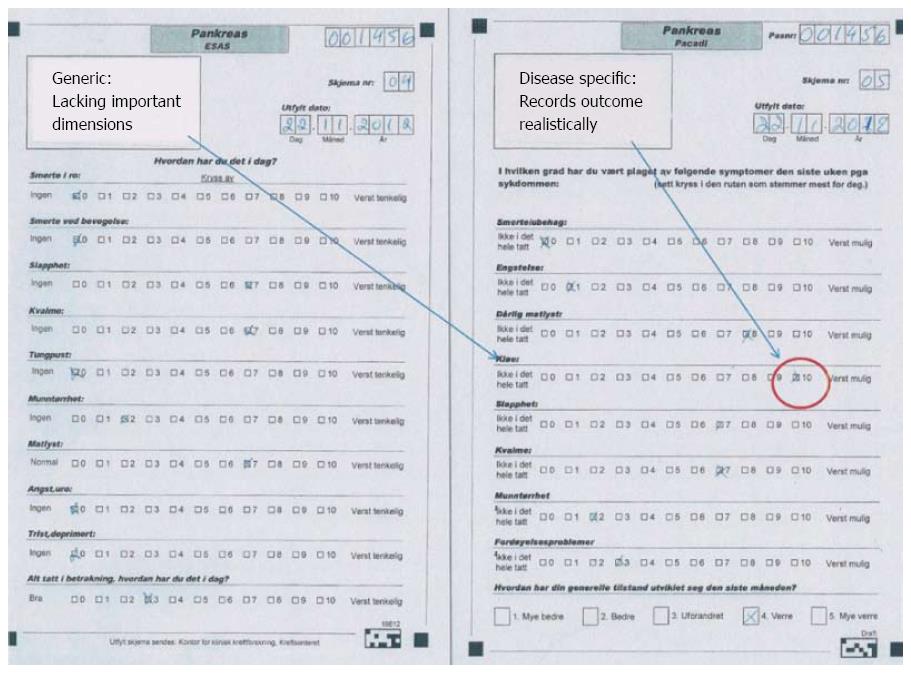
The short survival in most patients with pancreatic cancer makes clinical research difficult due to limited follow-up before transition into a general palliative stage. The symptom profile adds to this problem, because fatigue is a major problem for the majority of patients already at the time of primary diagnosis[105], and several patients are unable to fill comprehensive report forms. Available knowledge about health-related QoL in pancreatic cancer patients is constrained - for these and several other reasons. The lack of disease-specific tools for QoL-registration in patients with pancreatic cancer is one of the main reasons for shortage of information about clinical outcome. Several self-reported measures have been used in research, but only the European Organisation for Research and Treatment in Cancer (EORTC) has developed a disease-specific instrument for pancreatic cancer[109]. The QoL module for pancreatic cancer (EORTC QLQ-PANC26) has 26 questions and must be used in conjunction with the generic instrument EORTC Quality of Life Questioinnaire-C30 (EORTC C-30). Ultimately, altogether 56 questions have to be completed, strongly restricting the feasibility of the instrument both in research and clinical practice. This applies particularly for patients with severe, disabling disease[105]. The Edmonton Symptom System (ESAS) form is short and hence feasible, but generic. A recent new instrument is now developed, which is short and disease-specific, the pancreatic cancer disease impact (PACADI) score[110]. The methodology behind the PACADI score utilized experience from rheumatology, where the Rheumatoid Arthritis Impact of Disease (RAID) score, based on patients’ selection of dimensions where the disease has the most important impact on their QoL, has been developed and validated[111,112]. The RAID score is proven to be feasible and is now widely used in research. Similarly, the PACADI score asked for the patients’ priorities. The three dimensions with most severe negative impact on pancreatic cancer patients QoL, was pain/discomfort, fatigue and problems with bowel/digestion. But patients with severe icterus reported itching as their most important problem. In order to characterize clinical outcome of therapeutic interventions in a cohort with the short life expectancy of pancreatic cancer patients, it is of utmost importance to obtain valid data on patient reported outcome (PRO). Figure 2 illustrates the difference between a generic (ESAS) and disease-specific (PACADI) instrument in this regard.
Pancreatic surgery has now been accepted as one of the most recognized high-risk, low-volume surgical procedures, but this has not taken place without widespread reluctance in the medical community. One early comprehensive analysis of the relationship between a hospitals patient volume and outcome, was published in 2002 by Birkmeyer et al[113], focused on selected cardiovascular and cancer procedures. Absolute differences in adjusted postoperative mortality rates after pancreatic resections ranged from 16.3% (low volume) and 3.8% (high volume). Several subsequent reports supported the concept that outcome is best in high-volume hospitals, first because complications are recognized earlier and handled better, second because better oncological surgery and chemotherapy is offered[114-116]. The statement that postoperative mortality rates as well as long-term survival are improved with high patient volume, is now clearly evidence-based[117]. The aggressiveness of the tumor combined with the rates and severity grade of complications associated with pancreatic surgery, resulted in an almost nihilistic therapeutic attitude for several years[118]. The fact that most patients with pancreatic cancer die shortly after diagnosis was for years a “self-verifying prophecy”, uphold by negative expectations in most of the medical world. This was a real observation - nevertheless, evidence-based medicine is something very different.
The room for improvement is huge in diagnostic as well as therapeutic aspects of pancreatic cancer. The development of a panel of biomarkers enabling early detection of small and localized cancerous lesions is still only a dream, but progress is speeding up, particularly the stability of free miRNA in serum[119] has fostered optimism. Even the recurrence risk after surgery and the probable response to anti-tumor therapy may be predicted and become a key to individualized treatment plans in the near future. Novel chemotherapy regimens with documented improved survival are now available[86], and even chemo- and radiotherapy resistance may be reversed through utilization of the regulatory effect of miRNA on essential molecular pathways[120].
Surgical performance has improved significantly in large volume centers and the laparoscopic technique is well established for distal resections[121,122]. Skepticism remains for laparoscopic resection of adenocarcinoma but the rate of R0 resection was 91% and five year survival 30% in a recent report[34]. Accordingly, oncological results are equal or may even be better after laparoscopic than open resection. Also pancreaticoduodenectomy (Whipple-procedures) may be performed laparoscopically, but available data on outcome are scarce[123]. Robotic surgery might generate security advantages in this field[124], and it seems reasonable to assume that the immunosuppressive effect of surgery can be significantly reduced when an open Whipple-procedure is replaced by a laparoscopic operation. This might represent a greater window of opportunity for adjuvant immunotherapy, becoming more effective when inhibitory immunoregulation is downgraded or even eliminated.
The need of well-designed prospective trials clarifying the role of neoadjuvant chemotherapy is underlined also by other authors[125,126]. Important standardization of staging and treatment is incorporated in the Intergroup trial (Alliance A021101)[79], which is conducted as a single arm pilot study, intended to serve as paradigm for future randomized comparative trials.
Curative treatment outcome for patients with pancreatic cancer is achievable if early surgical treatment is combined with adjuvant chemotherapy. Nevertheless, most patients end up in a palliative situation, earlier or later. Also palliative therapeutic interventions are improving, but a multidisciplinary team with advanced expertise is a prerequisite for optimal care. Translational research is the key to personalized treatment plans, which is strongly needed in patients with pancreatic cancer[127].
P- Reviewer: Lee KT, Talukdar R S- Editor: Qi Y L- Editor: A E- Editor: Liu XM
| 1. | Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8406] [Cited by in RCA: 8971] [Article Influence: 690.1] [Reference Citation Analysis (0)] |
| 2. | Bosetti C, Bertuccio P, Malvezzi M, Levi F, Chatenoud L, Negri E, La Vecchia C. Cancer mortality in Europe, 2005-2009, and an overview of trends since 1980. Ann Oncol. 2013;24:2657-2671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 218] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 3. | Hassan MM, Bondy ML, Wolff RA, Abbruzzese JL, Vauthey JN, Pisters PW, Evans DB, Khan R, Chou TH, Lenzi R. Risk factors for pancreatic cancer: case-control study. Am J Gastroenterol. 2007;102:2696-2707. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 246] [Cited by in RCA: 229] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 4. | Raimondi S, Maisonneuve P, Löhr JM, Lowenfels AB. Early onset pancreatic cancer: evidence of a major role for smoking and genetic factors. Cancer Epidemiol Biomarkers Prev. 2007;16:1894-1897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 98] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 5. | Matsuyama M, Ishii H, Kuraoka K, Yukisawa S, Kasuga A, Ozaka M, Suzuki S, Takano K, Sugiyama Y, Itoi T. Ultrasound-guided vs endoscopic ultrasound-guided fine-needle aspiration for pancreatic cancer diagnosis. World J Gastroenterol. 2013;19:2368-2373. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Wolpin BM, Chan AT, Hartge P, Chanock SJ, Kraft P, Hunter DJ, Giovannucci EL, Fuchs CS. ABO blood group and the risk of pancreatic cancer. J Natl Cancer Inst. 2009;101:424-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 281] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 7. | Everhart J, Wright D. Diabetes mellitus as a risk factor for pancreatic cancer. A meta-analysis. JAMA. 1995;273:1605-1609. [PubMed] |
| 8. | Pezzilli R, Pagano N. Is diabetes mellitus a risk factor for pancreatic cancer? World J Gastroenterol. 2013;19:4861-4866. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 9. | Aggarwal G, Ramachandran V, Javeed N, Arumugam T, Dutta S, Klee GG, Klee EW, Smyrk TC, Bamlet W, Han JJ. Adrenomedullin is up-regulated in patients with pancreatic cancer and causes insulin resistance in β cells and mice. Gastroenterology. 2012;143:1510-1517.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 138] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 10. | Xu JH, Fu JJ, Wang XL, Zhu JY, Ye XH, Chen SD. Hepatitis B or C viral infection and risk of pancreatic cancer: a meta-analysis of observational studies. World J Gastroenterol. 2013;19:4234-4241. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 54] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 11. | Duell EJ, Lucenteforte E, Olson SH, Bracci PM, Li D, Risch HA, Silverman DT, Ji BT, Gallinger S, Holly EA. Pancreatitis and pancreatic cancer risk: a pooled analysis in the International Pancreatic Cancer Case-Control Consortium (PanC4). Ann Oncol. 2012;23:2964-2970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 170] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 12. | Rebours V, Boutron-Ruault MC, Schnee M, Férec C, Maire F, Hammel P, Ruszniewski P, Lévy P. Risk of pancreatic adenocarcinoma in patients with hereditary pancreatitis: a national exhaustive series. Am J Gastroenterol. 2008;103:111-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 166] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 13. | Lowenfels AB, Maisonneuve P, DiMagno EP, Elitsur Y, Gates LK, Perrault J, Whitcomb DC. Hereditary pancreatitis and the risk of pancreatic cancer. International Hereditary Pancreatitis Study Group. J Natl Cancer Inst. 1997;89:442-446. [PubMed] |
| 14. | Howes N, Lerch MM, Greenhalf W, Stocken DD, Ellis I, Simon P, Truninger K, Ammann R, Cavallini G, Charnley RM. Clinical and genetic characteristics of hereditary pancreatitis in Europe. Clin Gastroenterol Hepatol. 2004;2:252-261. [PubMed] |
| 15. | Whitcomb DC, Gorry MC, Preston RA, Furey W, Sossenheimer MJ, Ulrich CD, Martin SP, Gates LK, Amann ST, Toskes PP. Hereditary pancreatitis is caused by a mutation in the cationic trypsinogen gene. Nat Genet. 1996;14:141-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1123] [Cited by in RCA: 1028] [Article Influence: 35.4] [Reference Citation Analysis (0)] |
| 16. | Vogelstein B, Kinzler KW. Cancer genes and the pathways they control. Nat Med. 2004;10:789-799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2905] [Cited by in RCA: 2774] [Article Influence: 132.1] [Reference Citation Analysis (0)] |
| 17. | Hruban RH, Maitra A, Goggins M. Update on pancreatic intraepithelial neoplasia. Int J Clin Exp Pathol. 2008;1:306-316. [PubMed] |
| 18. | Feldmann G, Beaty R, Hruban RH, Maitra A. Molecular genetics of pancreatic intraepithelial neoplasia. J Hepatobiliary Pancreat Surg. 2007;14:224-232. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 201] [Cited by in RCA: 180] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 19. | Hidalgo M. Pancreatic cancer. N Engl J Med. 2010;362:1605-1617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2078] [Cited by in RCA: 2206] [Article Influence: 147.1] [Reference Citation Analysis (2)] |
| 20. | Maitra A, Hruban RH. Pancreatic cancer. Annu Rev Pathol. 2008;3:157-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 604] [Cited by in RCA: 589] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 21. | Wedén S, Klemp M, Gladhaug IP, Møller M, Eriksen JA, Gaudernack G, Buanes T. Long-term follow-up of patients with resected pancreatic cancer following vaccination against mutant K-ras. Int J Cancer. 2011;128:1120-1128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 140] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 22. | Jones S, Zhang X, Parsons DW, Lin JC, Leary RJ, Angenendt P, Mankoo P, Carter H, Kamiyama H, Jimeno A. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science. 2008;321:1801-1806. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3216] [Cited by in RCA: 3027] [Article Influence: 178.1] [Reference Citation Analysis (0)] |
| 23. | Yachida S, Jones S, Bozic I, Antal T, Leary R, Fu B, Kamiyama M, Hruban RH, Eshleman JR, Nowak MA. Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature. 2010;467:1114-1117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2041] [Cited by in RCA: 1946] [Article Influence: 129.7] [Reference Citation Analysis (0)] |
| 24. | Greenhalf W, Grocock C, Harcus M, Neoptolemos J. Screening of high-risk families for pancreatic cancer. Pancreatology. 2009;9:215-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 25. | Poruk KE, Firpo MA, Adler DG, Mulvihill SJ. Screening for pancreatic cancer: why, how, and who? Ann Surg. 2013;257:17-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 185] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 26. | Campbell PJ, Yachida S, Mudie LJ, Stephens PJ, Pleasance ED, Stebbings LA, Morsberger LA, Latimer C, McLaren S, Lin ML. The patterns and dynamics of genomic instability in metastatic pancreatic cancer. Nature. 2010;467:1109-1113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1118] [Cited by in RCA: 1027] [Article Influence: 68.5] [Reference Citation Analysis (0)] |
| 27. | Larghi A, Verna EC, Lecca PG, Costamagna G. Screening for pancreatic cancer in high-risk individuals: a call for endoscopic ultrasound. Clin Cancer Res. 2009;15:1907-1914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 28. | Klibansky DA, Reid-Lombardo KM, Gordon SR, Gardner TB. The clinical relevance of the increasing incidence of intraductal papillary mucinous neoplasm. Clin Gastroenterol Hepatol. 2012;10:555-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 126] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 29. | Tatsuta M, Iishi H, Ichii M, Noguchi S, Yamamoto R, Yamamura H, Okuda S. Values of carcinoembryonic antigen, elastase 1, and carbohydrate antigen determinant in aspirated pancreatic cystic fluid in the diagnosis of cysts of the pancreas. Cancer. 1986;57:1836-1839. [PubMed] |
| 30. | van der Waaij LA, van Dullemen HM, Porte RJ. Cyst fluid analysis in the differential diagnosis of pancreatic cystic lesions: a pooled analysis. Gastrointest Endosc. 2005;62:383-389. [PubMed] |
| 31. | Tanaka M, Fernández-del Castillo C, Adsay V, Chari S, Falconi M, Jang JY, Kimura W, Levy P, Pitman MB, Schmidt CM. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology. 2012;12:183-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1714] [Cited by in RCA: 1614] [Article Influence: 124.2] [Reference Citation Analysis (0)] |
| 32. | Wasif N, Bentrem DJ, Farrell JJ, Ko CY, Hines OJ, Reber HA, Tomlinson JS. Invasive intraductal papillary mucinous neoplasm versus sporadic pancreatic adenocarcinoma: a stage-matched comparison of outcomes. Cancer. 2010;116:3369-3377. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 81] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 33. | Winter JM, Cameron JL, Lillemoe KD, Campbell KA, Chang D, Riall TS, Coleman J, Sauter PK, Canto M, Hruban RH. Periampullary and pancreatic incidentaloma: a single institution’s experience with an increasingly common diagnosis. Ann Surg. 2006;243:673-680; discussion 680-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 123] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 34. | Marangos IP, Buanes T, Røsok BI, Kazaryan AM, Rosseland AR, Grzyb K, Villanger O, Mathisen Ø, Gladhaug IP, Edwin B. Laparoscopic resection of exocrine carcinoma in central and distal pancreas results in a high rate of radical resections and long postoperative survival. Surgery. 2012;151:717-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 35. | Al-Hawary MM, Francis IR, Chari ST, Fishman EK, Hough DM, Lu DS, Macari M, Megibow AJ, Miller FH, Mortele KJ. Pancreatic ductal adenocarcinoma radiology reporting template: consensus statement of the society of abdominal radiology and the american pancreatic association. Gastroenterology. 2014;146:291-304.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 192] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 36. | Tempero MA, Arnoletti JP, Behrman SW, Ben-Josef E, Benson AB, Casper ES, Cohen SJ, Czito B, Ellenhorn JD, Hawkins WG. Pancreatic Adenocarcinoma, version 2.2012: featured updates to the NCCN Guidelines. J Natl Compr Canc Netw. 2012;10:703-713. [PubMed] |
| 37. | Callery MP, Chang KJ, Fishman EK, Talamonti MS, William Traverso L, Linehan DC. Pretreatment assessment of resectable and borderline resectable pancreatic cancer: expert consensus statement. Ann Surg Oncol. 2009;16:1727-1733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 717] [Cited by in RCA: 618] [Article Influence: 38.6] [Reference Citation Analysis (0)] |
| 38. | Glomsaker T, Hoff G, Kvaløy JT, Søreide K, Aabakken L, Søreide JA. Patterns and predictive factors of complications after endoscopic retrograde cholangiopancreatography. Br J Surg. 2013;100:373-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 101] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 39. | Hartwig W, Schneider L, Diener MK, Bergmann F, Büchler MW, Werner J. Preoperative tissue diagnosis for tumours of the pancreas. Br J Surg. 2009;96:5-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 126] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 40. | Torreggiani WC, Lyburn I, Harris AA, Zwirewich CV. Seeding of pancreatic cancer along the path of a surgical drain: case report and literature review. Can Assoc Radiol J. 2000;51:241-243. [PubMed] |
| 41. | Harsha HC, Kandasamy K, Ranganathan P, Rani S, Ramabadran S, Gollapudi S, Balakrishnan L, Dwivedi SB, Telikicherla D, Selvan LD. A compendium of potential biomarkers of pancreatic cancer. PLoS Med. 2009;6:e1000046. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 231] [Cited by in RCA: 239] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 42. | Argani P, Rosty C, Reiter RE, Wilentz RE, Murugesan SR, Leach SD, Ryu B, Skinner HG, Goggins M, Jaffee EM. Discovery of new markers of cancer through serial analysis of gene expression: prostate stem cell antigen is overexpressed in pancreatic adenocarcinoma. Cancer Res. 2001;61:4320-4324. [PubMed] |
| 43. | Faca VM, Song KS, Wang H, Zhang Q, Krasnoselsky AL, Newcomb LF, Plentz RR, Gurumurthy S, Redston MS, Pitteri SJ. A mouse to human search for plasma proteome changes associated with pancreatic tumor development. PLoS Med. 2008;5:e123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 205] [Cited by in RCA: 199] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 44. | Foss CA, Fox JJ, Feldmann G, Maitra A, Iacobuzio-Donohue C, Kern SE, Hruban R, Pomper MG. Radiolabeled anti-claudin 4 and anti-prostate stem cell antigen: initial imaging in experimental models of pancreatic cancer. Mol Imaging. 2007;6:131-139. [PubMed] |
| 45. | Ma MZ, Kong X, Weng MZ, Cheng K, Gong W, Quan ZW, Peng CH. Candidate microRNA biomarkers of pancreatic ductal adenocarcinoma: meta-analysis, experimental validation and clinical significance. J Exp Clin Cancer Res. 2013;32:71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 76] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 46. | Hawes RH, Xiong Q, Waxman I, Chang KJ, Evans DB, Abbruzzese JL. A multispecialty approach to the diagnosis and management of pancreatic cancer. Am J Gastroenterol. 2000;95:17-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 120] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 47. | Hess V, Glimelius B, Grawe P, Dietrich D, Bodoky G, Ruhstaller T, Bajetta E, Saletti P, Figer A, Scheithauer W. CA 19-9 tumour-marker response to chemotherapy in patients with advanced pancreatic cancer enrolled in a randomised controlled trial. Lancet Oncol. 2008;9:132-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 178] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 48. | Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471-1474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5537] [Cited by in RCA: 6465] [Article Influence: 431.0] [Reference Citation Analysis (0)] |
| 49. | Bilimoria KY, Bentrem DJ, Ko CY, Ritchey J, Stewart AK, Winchester DP, Talamonti MS. Validation of the 6th edition AJCC Pancreatic Cancer Staging System: report from the National Cancer Database. Cancer. 2007;110:738-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 347] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 50. | Bilimoria KY, Bentrem DJ, Ko CY, Stewart AK, Winchester DP, Talamonti MS. National failure to operate on early stage pancreatic cancer. Ann Surg. 2007;246:173-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 428] [Cited by in RCA: 442] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 51. | Bilimoria KY, Bentrem DJ, Ko CY, Tomlinson JS, Stewart AK, Winchester DP, Talamonti MS. Multimodality therapy for pancreatic cancer in the U.S. : utilization, outcomes, and the effect of hospital volume. Cancer. 2007;110:1227-1234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 182] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 52. | Imamura M, Doi R, Imaizumi T, Funakoshi A, Wakasugi H, Sunamura M, Ogata Y, Hishinuma S, Asano T, Aikou T. A randomized multicenter trial comparing resection and radiochemotherapy for resectable locally invasive pancreatic cancer. Surgery. 2004;136:1003-1011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 81] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 53. | Shaib Y, Davila J, Naumann C, El-Serag H. The impact of curative intent surgery on the survival of pancreatic cancer patients: a U.S. Population-based study. Am J Gastroenterol. 2007;102:1377-1382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 99] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 54. | Hartwig W, Hackert T, Hinz U, Gluth A, Bergmann F, Strobel O, Büchler MW, Werner J. Pancreatic cancer surgery in the new millennium: better prediction of outcome. Ann Surg. 2011;254:311-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 328] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 55. | Hartwig W, Hackert T, Hinz U, Hassenpflug M, Strobel O, Büchler MW, Werner J. Multivisceral resection for pancreatic malignancies: risk-analysis and long-term outcome. Ann Surg. 2009;250:81-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 136] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 56. | Mollberg N, Rahbari NN, Koch M, Hartwig W, Hoeger Y, Büchler MW, Weitz J. Arterial resection during pancreatectomy for pancreatic cancer: a systematic review and meta-analysis. Ann Surg. 2011;254:882-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 383] [Cited by in RCA: 334] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 57. | Burdelski CM, Reeh M, Bogoevski D, Gebauer F, Tachezy M, Vashist YK, Cataldegirmen G, Yekebas E, Izbicki JR, Bockhorn M. Multivisceral resections in pancreatic cancer: identification of risk factors. World J Surg. 2011;35:2756-2763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 58. | Heinemann V, Haas M, Boeck S. Neoadjuvant treatment of borderline resectable and non-resectable pancreatic cancer. Ann Oncol. 2013;24:2484-2492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 83] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 59. | Pawlik TM, Laheru D, Hruban RH, Coleman J, Wolfgang CL, Campbell K, Ali S, Fishman EK, Schulick RD, Herman JM. Evaluating the impact of a single-day multidisciplinary clinic on the management of pancreatic cancer. Ann Surg Oncol. 2008;15:2081-2088. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 207] [Cited by in RCA: 205] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 60. | Gantos-O'Brien E. A Patient’s Perspective on the Multidisciplinary Liver/Pancreas Tumor Clinic: An All-in-One Resort. J Oncol Pract. 2010;6:292-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 61. | Gardner TB, Barth RJ, Zaki BI, Boulay BR, McGowan MM, Sutton JE, Ripple GH, Colacchio TA, Smith KD, Byock IR. Effect of initiating a multidisciplinary care clinic on access and time to treatment in patients with pancreatic adenocarcinoma. J Oncol Pract. 2010;6:288-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 62. | Cooper AB, Tzeng CW, Katz MH. Treatment of borderline resectable pancreatic cancer. Curr Treat Options Oncol. 2013;14:293-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 63. | Katz MH, Pisters PW, Evans DB, Sun CC, Lee JE, Fleming JB, Vauthey JN, Abdalla EK, Crane CH, Wolff RA. Borderline resectable pancreatic cancer: the importance of this emerging stage of disease. J Am Coll Surg. 2008;206:833-846; discussion 846-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 652] [Cited by in RCA: 605] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 64. | Evans DB, Erickson BA, Ritch P. Borderline resectable pancreatic cancer: definitions and the importance of multimodality therapy. Ann Surg Oncol. 2010;17:2803-2805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 65. | Evans DB, Varadhachary GR, Crane CH, Sun CC, Lee JE, Pisters PW, Vauthey JN, Wang H, Cleary KR, Staerkel GA. Preoperative gemcitabine-based chemoradiation for patients with resectable adenocarcinoma of the pancreatic head. J Clin Oncol. 2008;26:3496-3502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 590] [Cited by in RCA: 547] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 66. | Gillen S, Schuster T, Meyer Zum Büschenfelde C, Friess H, Kleeff J. Preoperative/neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of response and resection percentages. PLoS Med. 2010;7:e1000267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1243] [Cited by in RCA: 1170] [Article Influence: 78.0] [Reference Citation Analysis (1)] |
| 67. | Riall TS, Lillemoe KD. Underutilization of surgical resection in patients with localized pancreatic cancer. Ann Surg. 2007;246:181-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 45] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 68. | Raut CP, Tseng JF, Sun CC, Wang H, Wolff RA, Crane CH, Hwang R, Vauthey JN, Abdalla EK, Lee JE. Impact of resection status on pattern of failure and survival after pancreaticoduodenectomy for pancreatic adenocarcinoma. Ann Surg. 2007;246:52-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 449] [Cited by in RCA: 446] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 69. | Esposito I, Kleeff J, Bergmann F, Reiser C, Herpel E, Friess H, Schirmacher P, Büchler MW. Most pancreatic cancer resections are R1 resections. Ann Surg Oncol. 2008;15:1651-1660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 454] [Cited by in RCA: 562] [Article Influence: 33.1] [Reference Citation Analysis (0)] |
| 70. | Gaedcke J, Gunawan B, Grade M, Szöke R, Liersch T, Becker H, Ghadimi BM. The mesopancreas is the primary site for R1 resection in pancreatic head cancer: relevance for clinical trials. Langenbecks Arch Surg. 2010;395:451-458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 209] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 71. | Verbeke CS, Leitch D, Menon KV, McMahon MJ, Guillou PJ, Anthoney A. Redefining the R1 resection in pancreatic cancer. Br J Surg. 2006;93:1232-1237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 419] [Cited by in RCA: 444] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 72. | Nordby T, Ikdahl T, Lothe IM, Ånonsen K, Hauge T, Edwin B, Line PD, Labori KJ, Buanes T. Opportunities of improvement in the management of pancreatic and periampullary tumors. Scand J Gastroenterol. 2013;48:617-625. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 73. | Weitz J, Rahbari N, Koch M, Büchler MW. The “artery first” approach for resection of pancreatic head cancer. J Am Coll Surg. 2010;210:e1-e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 180] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 74. | Tempero MA, Arnoletti JP, Behrman S, Ben-Josef E, Benson AB, Berlin JD, Cameron JL, Casper ES, Cohen SJ, Duff M. Pancreatic adenocarcinoma. J Natl Compr Canc Netw. 2010;8:972-1017. [PubMed] |
| 75. | Adler G, Seufferlein T, Bischoff SC, Brambs HJ, Feuerbach S, Grabenbauer G, Hahn S, Heinemann V, Hohenberger W, Langrehr JM. [Carcinoma of the pancreas: summary of guidelines 2007, issued jointly by 15 German specialist medical societies]. Dtsch Med Wochenschr. 2007;132:1696-1700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 76. | Neoptolemos JP, Stocken DD, Bassi C, Ghaneh P, Cunningham D, Goldstein D, Padbury R, Moore MJ, Gallinger S, Mariette C. Adjuvant chemotherapy with fluorouracil plus folinic acid vs gemcitabine following pancreatic cancer resection: a randomized controlled trial. JAMA. 2010;304:1073-1081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1144] [Cited by in RCA: 999] [Article Influence: 66.6] [Reference Citation Analysis (0)] |
| 77. | Schmidt J, Abel U, Debus J, Harig S, Hoffmann K, Herrmann T, Bartsch D, Klein J, Mansmann U, Jäger D. Open-label, multicenter, randomized phase III trial of adjuvant chemoradiation plus interferon Alfa-2b versus fluorouracil and folinic acid for patients with resected pancreatic adenocarcinoma. J Clin Oncol. 2012;30:4077-4083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 104] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 78. | Abbott DE, Tzeng CW, Merkow RP, Cantor SB, Chang GJ, Katz MH, Bentrem DJ, Bilimoria KY, Crane CH, Varadhachary GR. The cost-effectiveness of neoadjuvant chemoradiation is superior to a surgery-first approach in the treatment of pancreatic head adenocarcinoma. Ann Surg Oncol. 2013;20 Suppl 3:S500-S508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 79. | Katz MH, Marsh R, Herman JM, Shi Q, Collison E, Venook AP, Kindler HL, Alberts SR, Philip P, Lowy AM. Borderline resectable pancreatic cancer: need for standardization and methods for optimal clinical trial design. Ann Surg Oncol. 2013;20:2787-2795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 248] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 80. | Farrell JJ, Elsaleh H, Garcia M, Lai R, Ammar A, Regine WF, Abrams R, Benson AB, Macdonald J, Cass CE. Human equilibrative nucleoside transporter 1 levels predict response to gemcitabine in patients with pancreatic cancer. Gastroenterology. 2009;136:187-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 351] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 81. | Ko AH, Tempero MA. Personalized medicine for pancreatic cancer: a step in the right direction. Gastroenterology. 2009;136:43-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 82. | Lillemoe KD, Cameron JL, Yeo CJ, Sohn TA, Nakeeb A, Sauter PK, Hruban RH, Abrams RA, Pitt HA. Pancreaticoduodenectomy. Does it have a role in the palliation of pancreatic cancer? Ann Surg. 1996;223:718-725; discussion 725-728. [PubMed] |
| 83. | Konstantinidis IT, Warshaw AL, Allen JN, Blaszkowsky LS, Castillo CF, Deshpande V, Hong TS, Kwak EL, Lauwers GY, Ryan DP. Pancreatic ductal adenocarcinoma: is there a survival difference for R1 resections versus locally advanced unresectable tumors? What is a “true” R0 resection? Ann Surg. 2013;257:731-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 307] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 84. | Nordby T, Ikdahl T, Bowitz Lothe IM, Fagerland MW, Heiberg T, Hauge T, Labori KJ, Buanes T. Improved survival and quality of life in patients undergoing R1 pancreatic resection compared to patients with locally advanced unresectable pancreatic adenocarcinoma. Pancreatology. 2013;13:180-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 85. | Burris HA, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo AM, Tarassoff P. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403-2413. [PubMed] |
| 86. | Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de la Fouchardière C. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817-1825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4838] [Cited by in RCA: 5640] [Article Influence: 402.9] [Reference Citation Analysis (1)] |
| 87. | Boone BA, Steve J, Krasinskas AM, Zureikat AH, Lembersky BC, Gibson MK, Stoller RG, Zeh HJ, Bahary N. Outcomes with FOLFIRINOX for borderline resectable and locally unresectable pancreatic cancer. J Surg Oncol. 2013;108:236-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 146] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 88. | Neoptolemos JP, Dunn JA, Stocken DD, Almond J, Link K, Beger H, Bassi C, Falconi M, Pederzoli P, Dervenis C. Adjuvant chemoradiotherapy and chemotherapy in resectable pancreatic cancer: a randomised controlled trial. Lancet. 2001;358:1576-1585. [PubMed] |
| 89. | Neoptolemos JP, Stocken DD, Friess H, Bassi C, Dunn JA, Hickey H, Beger H, Fernandez-Cruz L, Dervenis C, Lacaine F. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004;350:1200-1210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1945] [Cited by in RCA: 1909] [Article Influence: 90.9] [Reference Citation Analysis (0)] |
| 90. | Stocken DD, Büchler MW, Dervenis C, Bassi C, Jeekel H, Klinkenbijl JH, Bakkevold KE, Takada T, Amano H, Neoptolemos JP. Meta-analysis of randomised adjuvant therapy trials for pancreatic cancer. Br J Cancer. 2005;92:1372-1381. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 268] [Cited by in RCA: 240] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 91. | Ghaneh P, Slavin J, Sutton R, Hartley M, Neoptolemos JP. Adjuvant therapy in pancreatic cancer. World J Gastroenterol. 2001;7:482-489. [PubMed] |
| 92. | Oettle H, Post S, Neuhaus P, Gellert K, Langrehr J, Ridwelski K, Schramm H, Fahlke J, Zuelke C, Burkart C. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA. 2007;297:267-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1779] [Cited by in RCA: 1764] [Article Influence: 98.0] [Reference Citation Analysis (0)] |
| 93. | Small W, Berlin J, Freedman GM, Lawrence T, Talamonti MS, Mulcahy MF, Chakravarthy AB, Konski AA, Zalupski MM, Philip PA. Full-dose gemcitabine with concurrent radiation therapy in patients with nonmetastatic pancreatic cancer: a multicenter phase II trial. J Clin Oncol. 2008;26:942-947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 121] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 94. | Kleeff J, Friess H. Nonmetastatic pancreatic cancer: many trials, little progress. J Clin Oncol. 2008;26:3100; author reply 3100-a-313101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 95. | Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature. 2011;480:480-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2348] [Cited by in RCA: 2908] [Article Influence: 207.7] [Reference Citation Analysis (0)] |
| 96. | Gjertsen MK, Bjorheim J, Saeterdal I, Myklebust J, Gaudernack G. Cytotoxic CD4+ and CD8+ T lymphocytes, generated by mutant p21-ras (12Val) peptide vaccination of a patient, recognize 12Val-dependent nested epitopes present within the vaccine peptide and kill autologous tumour cells carrying this mutation. Int J Cancer. 1997;72:784-790. [PubMed] |
| 97. | Uram JN, Le DT. Current advances in immunotherapy for pancreatic cancer. Curr Probl Cancer. 2013;37:273-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 98. | Vasef MA, Ross JS, Cohen MB. Telomerase activity in human solid tumors. Diagnostic utility and clinical applications. Am J Clin Pathol. 1999;112:S68-S75. [PubMed] |
| 99. | Bernhardt SL, Gjertsen MK, Trachsel S, Møller M, Eriksen JA, Meo M, Buanes T, Gaudernack G. Telomerase peptide vaccination of patients with non-resectable pancreatic cancer: A dose escalating phase I/II study. Br J Cancer. 2006;95:1474-1482. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 227] [Cited by in RCA: 221] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 100. | Buanes T, Maurel J, Liauw W, Hebbar M, Nemunaitis J. A randomized Phase III study of gemcitabine (G) versus GV1001 in sequential combination with G in patients with unresectable and metastatic pancreatic cancer (PC). J Clin Oncol. 2009;27:4601. |
| 101. | Middleton G, Valle J, Wadsley J, Propper D, Coxon F, Ross P. A phase III randomized trial of chemoimmunotherapy comprising gemcitabine and capecitabine with or without telomerase vaccine GV1001 in patients with locally advanced or metastatic pancreatic cancer. J Clin Oncol. 2013;31:abstr LBA4004. |
| 102. | Lillemoe KD, Pitt HA. Palliation. Surgical and otherwise. Cancer. 1996;78:605-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 103. | Larssen L, Medhus AW, Korner H, Glomsaker T, Soberg T, Gleditsch D, Hovde O, Tholfsen JK, Skreden K, Nesbakken A. Long-term outcome of palliative treatment with self-expanding metal stents for malignant obstructions of the GI tract. Scand J Gastroenterol. 2012;47:1505-1514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 104. | Bilimoria KY, Bentrem DJ, Lillemoe KD, Talamonti MS, Ko CY. Assessment of pancreatic cancer care in the United States based on formally developed quality indicators. J Natl Cancer Inst. 2009;101:848-859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 105] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 105. | Labori KJ, Hjermstad MJ, Wester T, Buanes T, Loge JH. Symptom profiles and palliative care in advanced pancreatic cancer: a prospective study. Support Care Cancer. 2006;14:1126-1133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 61] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 106. | Glare PA, Dunwoodie D, Clark K, Ward A, Yates P, Ryan S, Hardy JR. Treatment of nausea and vomiting in terminally ill cancer patients. Drugs. 2008;68:2575-2590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 107. | Currow DC, Wheeler JL, Glare PA, Kaasa S, Abernethy AP. A framework for generalizability in palliative care. J Pain Symptom Manage. 2009;37:373-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 56] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 108. | Glare PA, Sinclair CT. Palliative medicine review: prognostication. J Palliat Med. 2008;11:84-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 186] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 109. | Fitzsimmons D, Johnson CD, George S, Payne S, Sandberg AA, Bassi C, Beger HG, Birk D, Büchler MW, Dervenis C. Development of a disease specific quality of life (QoL) questionnaire module to supplement the EORTC core cancer QoL questionnaire, the QLQ-C30 in patients with pancreatic cancer. EORTC Study Group on Quality of Life. Eur J Cancer. 1999;35:939-941. [PubMed] |
| 110. | Heiberg T, Nordby T, Kvien TK, Buanes T. Development and preliminary validation of the pancreatic cancer disease impact score. Support Care Cancer. 2013;21:1677-1684. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 111. | Heiberg T, Kvien TK. Preferences for improved health examined in 1,024 patients with rheumatoid arthritis: pain has highest priority. Arthritis Rheum. 2002;47:391-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 193] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 112. | Gossec L, Paternotte S, Aanerud GJ, Balanescu A, Boumpas DT, Carmona L, de Wit M, Dijkmans BA, Dougados M, Englbrecht M. Finalisation and validation of the rheumatoid arthritis impact of disease score, a patient-derived composite measure of impact of rheumatoid arthritis: a EULAR initiative. Ann Rheum Dis. 2011;70:935-942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 223] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 113. | Birkmeyer JD, Siewers AE, Finlayson EV, Stukel TA, Lucas FL, Batista I, Welch HG, Wennberg DE. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002;346:1128-1137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3695] [Cited by in RCA: 3773] [Article Influence: 164.0] [Reference Citation Analysis (0)] |
| 114. | Birkmeyer JD, Sun Y, Wong SL, Stukel TA. Hospital volume and late survival after cancer surgery. Ann Surg. 2007;245:777-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 478] [Cited by in RCA: 512] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 115. | Ghaferi AA, Birkmeyer JD, Dimick JB. Variation in hospital mortality associated with inpatient surgery. N Engl J Med. 2009;361:1368-1375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1040] [Cited by in RCA: 1098] [Article Influence: 68.6] [Reference Citation Analysis (0)] |
| 116. | Finks JF, Osborne NH, Birkmeyer JD. Trends in hospital volume and operative mortality for high-risk surgery. N Engl J Med. 2011;364:2128-2137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1119] [Cited by in RCA: 1066] [Article Influence: 76.1] [Reference Citation Analysis (0)] |
| 117. | Gooiker GA, van Gijn W, Wouters MW, Post PN, van de Velde CJ, Tollenaar RA. Systematic review and meta-analysis of the volume-outcome relationship in pancreatic surgery. Br J Surg. 2011;98:485-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 273] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 118. | Buanes T. The prognosis for pancreatic cancer patients--better than feared. Tidsskr Nor Laegeforen. 2012;132:7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 119. | Zöller M. Pancreatic cancer diagnosis by free and exosomal miRNA. World J Gastrointest Pathophysiol. 2013;4:74-90. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 59] [Cited by in RCA: 64] [Article Influence: 5.3] [Reference Citation Analysis (2)] |
| 120. | Drakaki A, Iliopoulos D. MicroRNA-gene signaling pathways in pancreatic cancer. Biomed J. 2013;36:200-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 121. | Røsok BI, Marangos IP, Kazaryan AM, Rosseland AR, Buanes T, Mathisen O, Edwin B. Single-centre experience of laparoscopic pancreatic surgery. Br J Surg. 2010;97:902-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 53] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 122. | Iacobone M, Citton M, Nitti D. Laparoscopic distal pancreatectomy: up-to-date and literature review. World J Gastroenterol. 2012;18:5329-5337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 32] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 123. | Asbun HJ, Stauffer JA. Laparoscopic vs open pancreaticoduodenectomy: overall outcomes and severity of complications using the Accordion Severity Grading System. J Am Coll Surg. 2012;215:810-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 295] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 124. | Cirocchi R, Partelli S, Trastulli S, Coratti A, Parisi A, Falconi M. A systematic review on robotic pancreaticoduodenectomy. Surg Oncol. 2013;22:238-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 66] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 125. | Werner J, Combs SE, Springfeld C, Hartwig W, Hackert T, Büchler MW. Advanced-stage pancreatic cancer: therapy options. Nat Rev Clin Oncol. 2013;10:323-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 171] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 126. | Hartwig W, Werner J, Jäger D, Debus J, Büchler MW. Improvement of surgical results for pancreatic cancer. Lancet Oncol. 2013;14:e476-e485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 277] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 127. | Álvaro-Meca A, Akerkar R, Alvarez-Bartolome M, Gil-Prieto R, Rue H, de Miguel ÁG. Factors involved in health-related transitions after curative resection for pancreatic cancer. 10-years experience: a multi state model. Cancer Epidemiol. 2013;37:91-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |