Copyright
©2014 Baishideng Publishing Group Inc.
World J Gastroenterol. Aug 7, 2014; 20(29): 9882-9897
Published online Aug 7, 2014. doi: 10.3748/wjg.v20.i29.9882
Published online Aug 7, 2014. doi: 10.3748/wjg.v20.i29.9882
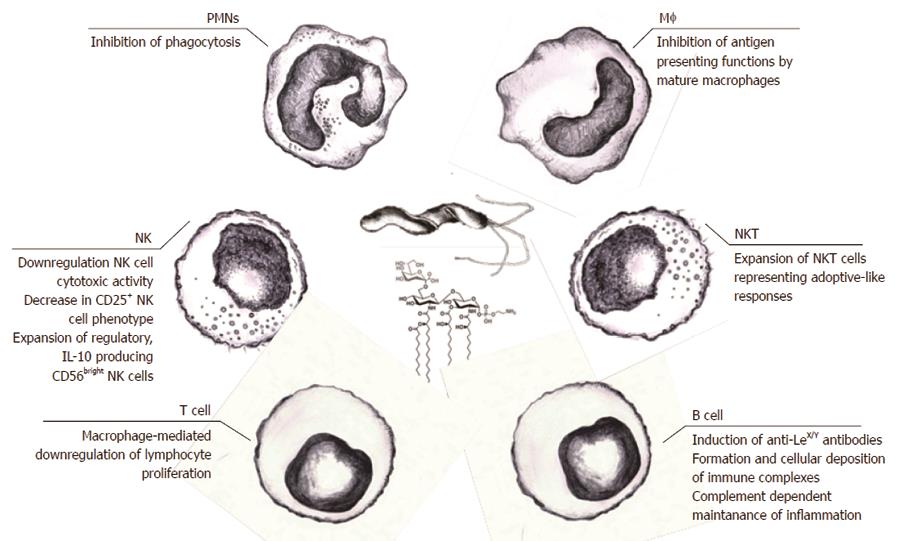
Figure 3 Local and systemic consequences of Helicobacter pylori-driven immunomodulation.
Helicobacter pylori (H. pylori) lipopolysaccharide (LPS) may facilitate colonization of the gastric mucosa through extracellular matrix proteins and induction of local inflammation. Increased permeability of the gastric epithelium due to the activity of H. pylori proteins such as urease and vacuolating cytotoxin, which contribute to the degradation of intercellular tight junctions, may result in broad interactions of soluble H. pylori LPS with host cells. This LPS can bind to various immune cells infiltrating the mucosal basal lamina or even enter the circulation, where the LPS may contribute to the inflammatory response via a direct influence on immune cells or through the formation of complexes with low density lipoprotein (LDL)s, and possibly oxidized LDL. Released H. pylori LPS can also move along the gastrointestinal tract and, together with the intestinal microbiota, may induce complex immunoregulatory mechanisms. These interactions can result in an increased expression of adhesins, pathogen recognition receptors (PRRs), and proinflammatory cytokines. Different PRRs can mediate the interactions of H. pylori LPSs with the immune cells, including Toll-like receptor 4 or 2, dendritic cell-specific intracellular adhesion molecule-3-grabbing non-integrin, and triggering receptor expressed on myeloid cells-like receptors. The signaling pathways triggered by these receptors may cause cytokine secretion. NKT: Natural killer T lymphocytes; PMNs: Polymorphonuclear cells.
-
Citation: Chmiela M, Miszczyk E, Rudnicka K. Structural modifications of
Helicobacter pylori lipopolysaccharide: An idea for how to live in peace. World J Gastroenterol 2014; 20(29): 9882-9897 - URL: https://www.wjgnet.com/1007-9327/full/v20/i29/9882.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i29.9882