Published online Jul 28, 2014. doi: 10.3748/wjg.v20.i28.9549
Revised: March 1, 2014
Accepted: April 5, 2014
Published online: July 28, 2014
Processing time: 208 Days and 9.1 Hours
AIM: To diagnose the clinical and histologic features that may be associated with or predictive of the need for dilation and dilation related complications; examine the safety of dilation in patients with eosinophilic esophagitis (EoE).
METHODS: The medical records of all patients diagnosed with EoE between January 2002 and July 2010 were retrospectively reviewed. Esophageal biopsies were reexamined by an experienced pathologist to confirm the diagnosis (≥ 15 eos/hpf per current guidelines). Patients were divided into 2 groups: patients who did not receive dilation therapy and those who did. Demographics, clinical history, the use of pharmacologic therapy, endoscopic and pathology findings, and the number of biopsies and dilations carried out, if any, and their locations were recorded for each patient. The dilation group was further examined based on the interval between diagnosis and dilation, and whether or not a complication occurred.
RESULTS: Sixty-one patients were identified with EoE and 22 (36%) of them underwent esophageal dilations for stricture/narrowing. The peak eos/hpf was significantly higher in patients who received a dilation (P = 0.04). Four (18% of pts.) minor complications occurred: deep mucosal tear 1, and small mucosal tears 3. There were no cases of esophageal perforations. Higher peak eos/hpf counts were not associated with increased risk of complications.
CONCLUSION: Esophageal dilation appears to be a safe procedure in EoE patients, carrying a low complication rate. No correlation was found between the peak of eosinophil count and complication rate. Complications can occur independently of the histologic features. The long-term outcome of EoE treatment, with or without dilation, needs to be determined.
Core tip: The field of gastroenterology does not currently have standardized treatment guidelines for eosinophilic esophagitis. Current data on the safety of dilations in patients with eosinophilic esophagitis (EoE) are conflicting and lack information on factors that influence whether or not a patient will require dilation. This study revealed that higher peak eos/hpf counts appear to influence whether or not an EoE patient will require dilation during the course of their treatment. However, complications appear to occur independently of the histologic features. Esophageal dilation appears to be a safe procedure in EoE patients, carrying a low complication rate.
- Citation: Ukleja A, Shiroky J, Agarwal A, Allende D. Esophageal dilations in eosinophilic esophagitis: A single center experience. World J Gastroenterol 2014; 20(28): 9549-9555
- URL: https://www.wjgnet.com/1007-9327/full/v20/i28/9549.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i28.9549
Eosinophilic esophagitis (EoE) is a recognized cause of dysphagia, food impaction, and heartburn that is unresponsive to anti-reflux measures. The disease is characterized by mucosal eosinophilic infiltration, a count of 15 or more eosinophils per high power field (eos/hpf) that is isolated to the esophagus[1-3]. Clinicians must rule out other esophageal disorders that cause similar physiological and histological presentations in order to diagnose an individual with EoE. This is because esophageal eosinophilia can be associated with gastroesophageal reflux disease (GERD), Crohn’s disease, and various types of gastritis and esophagitis[3,4]. An EoE diagnosis is typically differentiated from GERD if the individual does not respond to anti-reflux measures and proton pump inhibitors, and histologic criteria for EoE are met[5-7].
Chronic inflammatory eosinophilic infiltrates often lead to tissue remodeling and fibrosis, which causes luminal narrowing and loss of elasticity[8-10]. These morphological changes contribute to symptoms associated with EoE, such as chest pain, heartburn, acid reflux, feeding intolerance, and especially dysphagia and food impaction[11-12]. The methods of management can consist of dietary, pharmacological, and endoscopic interventions.
Persistent eosinophilia causes strictures to form over time such as confined and longitudinal narrowing, transient or fixed rings, feline esophagus, Schatzki rings, and small caliber esophagus[13-15]. Dysphagia and food impaction, attributable to these mucosal abnormalities, are currently treated with topical steroids and/or mechanical dilation[1,3,16]. Long-term use of steroids is discouraged due to adverse side effects and risks of toxicity. The disease process often rebounds after the individual stops using steroids[1,16].
Dilation is an important therapy for individuals who need immediate relief from dysphagia and prevention of food impaction. It also provides relief for patients whose symptoms have not responded to therapy with steroids, and those who do not want to be on long term medications or significantly restrict their diets[1,3,17].
Early descriptions of routine endoscopies and therapeutic interventions, such as dilation, in EoE patients indicated that the fragile and inelastic mucosa associated with the disease increased risks of deep tissue tears, lacerations, and perforations[4,11,12,14,18]. However, more recent studies with larger patient samples have indicated esophageal dilation as a safe therapy in EoE patients[16,19-21]. Schoepfer et al[16] reported in a multi-center study a series of 207 patients with EoE who received mechanical dilation. No major complications defined as severe esophageal injury were observed. Jung et al[19] reviewed 293 dilations performed in 161 EoE patients and reported complications such as deep mucosal tears in 9.2%, major bleeding in 0.3%, and immediate perforation in 1.0% of cases. In a systematic literature review by Jacobs et al[20], including 468 patients and combined total of 671 dilations, a single perforation was found, which confirmed that earlier heightened fear of perforation had indeed been exaggerated. Researchers also looked at whether or not a correlation exists between dilation-related complications and eos/hpf count.
This study aimed to examine the differences in clinical and pathological presentations between EoE patients who did and did not require a dilation throughout their clinical history. The relations between dilation, rate of complications and histologic features in EoE patients who received a dilation were evaluated as well. We were especially interested if higher peak eos/hpf was associated with or predictive of the need for dilation and higher rates of complication. The study also further assessed the safety and outcomes of esophageal dilations in EoE patients.
A retrospective systematic chart review of patients diagnosed with EoE between January 2004 and July 2010 was conducted at the Cleveland Clinic Florida. Medical records, endoscopic reports, and biopsy reports and slides were reviewed. Age, gender, clinical history of allergic or chronic sinusitis or rhinitis, endoscopic presentation, pharmacological therapy for EoE number of endoscopies (EGDs), number of biopsies and esophageal dilations and their locations, outcomes of dilations, histopathology (eos/hpf), and length of follow up were collected. All dilations were done using the rule of 3.
Patients were divided into 2 groups: patients who did not receive dilation (ND) and patients who did (YD). Those who received dilation were further divided in to groups based on if they were diagnosed with EoE prior to their first dilation, or at the time of the dilation (AT), and if there was a complication (YC) or not (NC).
Complications were defined as bleeding, perforation, deep mucosal tears, chest pain, and hospitalizations that were related to the esophageal dilation procedure. Rents were not considered a complication.
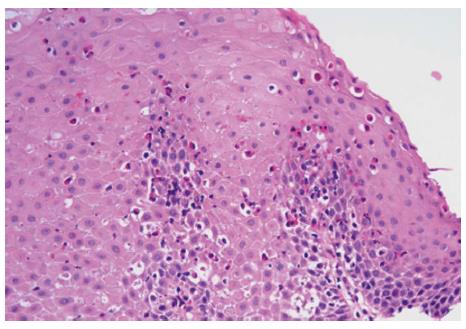
Histological slides were reexamined and confirmed to have a peak eosinophil count greater than or equal to 15 eos/hpf by an expert GI pathologist (D.A.) (Figure 1). The peak values of eos/hpf at time of diagnosis and at time of dilation were reported in every case, as well as the number of slides reviewed. Affected sites were classified as proximal, mid, or distal esophagus or the gastroesophageal junction (GEJ).
Peak eos/hpf count at diagnosis, number of EGDs, and sites affected of ND and YD groups were compared using t-tests for independent samples. The peak eos/hpf and size of dilation in YD groups of YC and NC, PR and AT were compared using t-tests for independent samples. A P value of 0.05 or less was considered statistically significant. An ad hoc one-way ANOVA weighted means analysis with a Tukey’s HSD was done to compare peak eos/hpf count at diagnosis between ND, PR, and AT groups.
Sixty-one patients were diagnosed with EoE during the study’s review period. Esophageal dilations were performed in 22 (36%) patients. Eighteen (29%) patients were lost to follow up. All remaining patients were treated with topical steroids. The treatment consisted of 2 puffs of 110-220 mcg of fluticasone to swallow twice a day in 40 patients, 160 mcg ciclesonide for 2, and 1 mg of budesonide in liquid form for 1.
Twenty-seven (69%) of the 39 patients were males. The mean age of patients at time of diagnosis was 38 years (range 18-61). Fifteen (38%) patients presented with dysphagia, 9 (23%) with food impaction, 9 (23%) with reflux, and 6 (15%) were asymptomatic (EoE was an incidental finding). All patients who presented with food impaction were treated and diagnosed based on an EGD done during a visit to the emergency room (ER) (Table 1).
| Characteristic | No Dilation | Dilation |
| (n= 39) | (n= 22) | |
| Age (yr) | 38 (18-61) | 49 (23-61) |
| Asthma/allergic history | 12 (31) | 10 (45) |
| Presenting symptoms | 0 | 0 |
| Dysphagia | 15 (38) | 22 (100) |
| Food impaction | 9 (23) | 10 (45)2 |
| Reflux | 9 (23) | 0 |
| No symptoms | 6 (15) | 0 |
| Symptomatic | 33 (85) | 22 (100) |
| Peak eos/hpf | 47.9 eos/hpf | 52.6 eos/hpf |
| Length of follow up (yr) | 4.8 ± 1.51 yr | 5.2 ± 1.4 yr |
| Number of EGDs | 1.4 ± 0.7 | 2.6 ± 1.5 |
Endoscopic appearance: In 24 (62%) patients the whole esophagus was observed as abnormal, in 8 (20%) only one segment of esophagus appeared abnormal, and in 7 (18%) no clear cut abnormalities were seen. Twenty (51%) patients had 1 abnormality reported, 11 (28%) patients had 2, and 1 (3%) had 3 (Table 2).
| Type of findings | No dilation | Dilation |
| Erosions | 6 (15) | 2 (9) |
| Inflammation | 1 (3) | 2 (9) |
| Linear furrowing | 13 (33) | 4 (18) |
| Ridges and cobblestone | 1 (3) | 0 |
| Ringed | 14 (35) | 7 (32) |
| Schatzki ring | 2 (5) | 6 (37) |
| Stenosis | 0 | 4 (18) |
| Benign strictures | 0 | 9 (41) |
| Tortuous | 1 (3) | 0 |
| White plaques | 4 (10) | 2 (9) |
| No abnormalities | 8 (21) | 0 |
Histological findings: An average of 2.1 ± 0.8 biopsies were taken (ranging from 1 to 4). The mean peak count of eos/hpf was 47.9 (range 15-99). The number of areas involved varied from 1 to 4. Nine (23%) patients had a single biopsy from the esophagus, 20 (51%) patients were biopsied at 2 different locations, 9 (23%) at 3 different locations, and 1 (3%) at 4 locations. Eighteen (46%) patients had one site involved, 16 (41%) had 2 sites involved, 4 (10%) had 3, and 1 (3%) had 4. Fifteen (38%) had documented upper and lower esophagus involvement by EoE (Table 3).
| Location | No dilation | Dilation |
| Proximal | 7 | 1 |
| Mid | 31 | 19 |
| Distal | 22 | 8 |
| GEJ | 4 | 3 |
| Not specified | 3 | 2 |
Seventeen (77%) of the 22 patients who required dilations were males. The mean age of patients at time of dilation was 49 years (range 23-67). All patients presented with dysphagia. Ten (45%) of them were treated for food impactions.
Endoscopic appearance: Endoscopic abnormalities were observed in all patients who received dilations. In 13 (59%) patients the whole esophagus was abnormal and in 9 (41%) only one site appeared abnormal. Thirteen (59%) patients had 1 abnormality reported, 6 (27%) had 2, and 2 had 3 (14%) (Table 2).
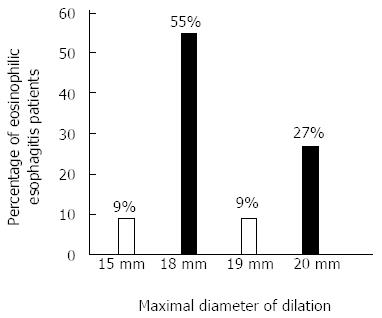
A total of 28 esophageal dilations were performed, 24 of them were done using TTS balloon and 4 with over the guidewire Savary dilator. Eighteen (82%) patients had a single dilation, 2 (9%) received 2, and 2 (9%) had 3 procedures. The mean dilation size was 18.4 mm. Six patients received a maximum dilation size of 20 mm, 2 received a 19 mm, 12 received an 18 mm, and 2 received a 15 mm (Figure 2). Three (11%) dilations were performed in the mid-esophagus, 11 (39%) in the distal part of the esophagus, 12 (43%) at the GEJ, 1 (3%) involved the entire esophagus, and 1 (3%) was not specified. Over the course of their treatments, group YD had significantly more EGDs than group ND.
Histological findings: An average of 1.5 ± 0.7 biopsies were taken at time of diagnosis (ranging from 1 to 3). The mean peak count of eos/hpf at diagnosis was 52.6 (range 15-145). The number of slides involved varied from 1 to 3. Ten (45%) patients had a single biopsy from the esophagus, 10 (45%) patients had biopsies from 2 different locations, and 2 (10%) cases were biopsied at 3 different locations. Ten (45%) patients had one site involved, 10 (45%) had 2 sites involved, and 2 (10%) had 3. Ten patients had one site involved, 10 had 2 sites involved, and 2 had 3. All sites biopsied were positive for eosinophilia with ≥ 15 eos/hpf. The peak eos/hpf counts were significantly higher in patients who received a dilation (P < 0.05).
Time of diagnosis: The diagnosis of EoE was established for 6 (27%) patients based on biopsies from EGDs performed prior to their first dilation. The average time interval between diagnosis and need for dilation was 10.5 mo (range of 1-18 mo). Sixteen (73%) patients were diagnosed with EoE based on biopsies obtained at the time of the initial EGD with dilation. Therefore, those patients had no established diagnosis of EoE at the time of dilation. Three (14%) patients were lost to follow up (Table 4).
| Characteristics | PR Group (Diagnosis before 1st dilation)(n= 6) | AT Group(Diagnosis at 1st dilation)(n= 16) | ||
| Mean | Range | Mean | Range | |
| Peak eos/hpf at 1st diagnosis | 31.6 ± 18.4 | 15-92 | ||
| Peak eos/hpf at 1st dilation | 45.7 ± 30.8 | 73.6 ± 42.4 | 15-145 | |
| Number of biopsies at diagnosis | 1.7 ± 0.8 | 1-3 | 1.7 ± 0.6 | 1-3 |
| Number of EGDs | 3.7 ± 2.0 | 2-7 | 2.2 ± 1.2 | 1-5 |
| Number of dilations | 1 | 1 | 1.4 ± 0.7 | 1-3 |
| Length of follow up (yr)1 | 5 ± 1.2 | 4-7 | 5.2 ± 1.4 | 4-9 |
The peak eos/hpf at time of diagnosis was significantly higher (P < 0.05) for AT group when compared to PR group. However, there was no significant difference in peak eos/hpf between both groups at the time of dilation. PR underwent significantly more EGDs during their treatment period. There were no significant differences in peak eos/hpf at time of diagnosis between ND, PR and AT groups.
Complications: Minor complications were reported following 4 (14%) dilations. Three (11%) dilations were associated with a small mucosal tear and 1 (4%) case of very deep mucosal tear. All of them occurred in AT patients. Two of the small tears occurred at the GEJ and one in the distal esophagus. Two of these patients reported severe chest pain after the procedure and had X-ray negative for perforation. The deep mucosal tear occurred in the mid esophagus. No perforations occurred. No patients were hospitalized for procedure related complications.
There was no significant difference in peak eos/hpf count between YC and NC groups (Table 5). There was also no significant difference in size of dilation.
| Complications | No complications | |
| Age (yr) | 35 (20-51) | 41 (20-54) |
| Asthma/allergy history | 2 (50) | 8 (44) |
| Peak eos/hpf at time of dilation | 78 ± 51 | 63 ± 37 |
The prevalence of EoE has increased over the last decade in both children and adults in Western countries. EoE has been documented in most racial groups, although many studies have reported predominance in Caucasian males[22]. The prevalence of EoE in an outpatient population undergoing upper endoscopy was reported 0.4%-0.5%[23], but EoE was found in 12% of patients presenting with dysphagia[24]. Based on recent studies, the prevalence of EoE is lower in Asia with reported rate 0.34% in China[25] and 0.02% of the routine endoscopies in Japan[26].
In adults with EoE, solid food dysphagia is the most common presenting symptom, followed by food impaction. Other common symptoms in adults include heartburn, chest pain and upper abdominal pain. Therefore any of the above symptoms and reflux not responding to proton pump inhibitor therapy should raise a high index of suspicion about possibility of EoE while evaluating those cases.
The need for esophageal dilatation in EoE patients is relatively high and reported in 27%-30.3% of cases[16,27]. In our series, 36% of EoE patients underwent esophageal dilations.
The findings of our study contribute to preceding research on the use and safety of dilation in EoE patients in order to help develop a standard of care for EoE patients. Who needs dilation therapy?
Based on our findings, peak eos/hpf at time of diagnosis can be linked to severity of findings and presence of a significant clinical stricture, and may influence whether or not an EoE patient requires a dilation. The severity of eosinophilic infiltration should be taken into account when deciding to dilate. However often at the time of dilation this information is not available. Endoscopists should also consider the severity of the patients’ symptoms of dysphagia and recurring food impaction, the presence of benign strictures, and endoscopic appearance when choosing to dilate based on the endoscopic appearance.
All sites biopsied in YD patients were positive for eosinophilic infiltration with ≥ 15 eos/hpf. Whereas not all esophageal biopsies taken from ND patients presented with eosinophil count that qualify as EoE. Another interesting observation is that even though the whole esophagus appeared abnormal in many cases, the biopsies did not always reflect eosinophilic infiltration of the entire esophagus. This may be because most endoscopists avoided additional biopsies of the proximal esophagus since there was no developed protocol regarding esophagus biopsies in EoE. It may also suggest that localized increases in eosinophils may affect the mucosal integrity of surrounding areas or the whole esophagus.
Unfortunately due to its retrospective design, this study did not have standardized guidelines for the number and location of biopsies taken to diagnose or rule out EoE. Overall more biopsies were taken at time of diagnosis in patients that did not receive dilations. One reason may be that patients who did receive dilations exhibited symptoms and abnormalities that were more obviously associated with EoE. Therefore endoscopists might have felt fewer biopsies would suffice for diagnosis. The AT group also may have had less biopsies taken because dilations were done during the same procedure. Endoscopists may have been wary to cause increased trauma or may not have been able to biopsy at the site of dilation.
Performing dilation overall increases the number of EGDs, EoE patients will require over the course of their treatment. This may be because those who received dilations are watched more closely or have more complicated cases of the disease. PR group had significantly more EGDs than AT group. This is most likely to be attributable to the fact that they received 2 EGDs for diagnosis and dilation, while the other 2 groups only received 1. Therefore it may be more economical to dilate patients who endoscopists think might be likely to later require dilations at the time of their first endoscopy. ND patients did have the lowest average of EGDs, 1.4 ± 0.7, which may suggest less severe disease and/or symptoms.
When do dilations happen: Higher peak eos/hpf counts appear to correlate with the need for dilation. Although the peak eos/hpf at time of diagnosis for PR was significantly lower than AT, there was no difference in peak count at the time of dilation. Therefore peak eos/hpf in PR patients had significantly increased during the time between diagnosis and dilation. Similar to the ND group, the PR patients were treated with topical steroids after diagnosis. However, unlike the ND group, their symptoms recurred with such severity that they later required dilation. The topical steroids did not provide lasting relief. Based on this, peak eos/hpf count at time of diagnosis does not appear to help predict whether or not a patient will later require dilation. Future research should examine factors that may bring differences between ND and PR patients to light.
Is it safe to dilate: Based on our study’s results, dilation therapy appears to be a safe and effective measure to alleviate dysphagia in EoE patients by enlarging the diameter of the esophagus. Only a minority of patients had recurrent symptoms requiring repeat dilations. Higher peak eos/hpf counts were associated with increased need for dilation. No association was found between peak eos/hpf and rate of complication. Higher eos/hpf counts and the length of affected segment do not appear to be associated with increased risks of complication.
The safety of dilation is important because dysphagia is the most uniform symptom and common complaint in adults with EoE[12,13,20,27]. Dilation allows for immediate resolution of dysphagia while steroids take more time. This is especially important in patients suffering from food impactions. Dilations also benefit patients with recurring dysphagia who refuse to take steroids, do not respond well to them, or have cannot take them for long periods of time. Schoepfer et al[16] suggested that the benefits of dilation were long-term with or without topical steroids. Their study found long-term resolution of dysphagia and food impaction symptoms, with recurrence after 23 ± 22 mo in patients who did not receive steroid treatment afterward vs 20 ± 14 mo in patients who did.
In our study we did not report rents and superficial mucosal tears as major complications because they are expected to occur during dilation in EoE patients as previously described[20,28]. Vasilopoulos et al[29] suggested that rents after an upper endoscopy can be considered markers for identifying patients with EoE. However patients should be advised prior to the procedure of the likelihood of rents because they can be associated with acute post-procedural chest pain[20,28,29].
No perforations occurred in our study. This finding, alongside other recent studies, indicates that the risk of perforation is not nearly as high as the earliest studies suggested. However, decisions concerning dilation therapy should be individualized to the EoE patient. Studies often suggested potential factors that may increase risks of complications such as: proximal location, smaller diameter of strictures, younger age, steroid use, and the number of dilations[4,19,27,28]. However conclusive evidence that any of these factors significantly increase risk of dilation has not been presented in prospective fashion. Therefore, endoscopists should have a comprehensive understanding of conservative dilation methods and patient history when considering dilation.
Cohen et al[4] encountered more complications with patients who had an eos/hpf of 40 or more, the difference was not statistically significant. However, both Cohen’s study and ours were limited by small sample group. Larger studies would be needed to justify whether or not endoscopists should be concerned about eos/hpf count in relation to risk of dilation-related complications. Especially because the repercussions of this knowledge might require EoE patients to undergo two endoscopies; one to biopsy the mucosa for histologic reading and the second one to perform dilation. This could also raise cost of care of EoE patients. Currently, no publications conclusively suggest a positive correlation between eos/hpf and complication rate.
EoE has increasingly come to the attention of endoscopists over the past two decades. Despite progress in the field, current publications are still limited in their abilities to provide a comprehensive understanding of the causes of the chronic disease and long-term outcomes. This study is limited by its retrospective design and consequently the absence of a standardized method of recording EGD reports, as well as the loss of 29% patients to follow up. The body of research also lacks successful long-term treatment options for alleviating symptoms and diminishing the inflammatory response, as well as preventative and curative measures. Recommendations for treatment of EoE have primarily been based on retrospective reviews of small patient samples by expert gastroenterologists and endoscopists[1-3,13].
The field of research on EoE would greatly benefit from prospective studies. Future research must work to establish standardized methods of recording patient symptoms, histories, and EGD reports, deciding when and how to perform dilation, and reporting endoscopic findings. They should also follow standardized guidelines for both balloon and Savary dilations. Long-term follow up of patients would provide longitudinal data on the effectiveness of disease interventions and the course of the disease. Unfortunately, no cure for EoE is currently known. Longitudinal, prospective studies will be a step in the right direction towards developing curative therapies for EoE patients. Until then, our study supports the use of dilation therapy to help relieve acute dysphagia when mucosal abnormalities are present in EoE patients.
Symptoms of eosinophilic esophagitis (EoE) are managed with pharmacologic, endoscopic, and dietary interventions. Endoscopic esophageal dilations help relieve symptoms dysphagia and food impaction in individuals who have developed esophageal strictures as a result of food impaction.
The field of gastroenterology does not currently have standardized treatment guidelines for eosinophilic esophagitis. Current data on the safety of dilations in patients with EoE is conflicting. Research on EoE also lacks information on factors that may influence whether or not a patient will require dilation and when.
Initial studies on the safety of dilation in patients with eosinophilic esophagitis classified the procedure as high risk of tearing and perforation. However, larger follow up studies suggested that the initial cautionary reports exaggerated the risks. This study reports that higher peak eos/hpf counts do not appear to be associated with increased risk of complication. Higher peak eos/hpf counts do appear to influence whether or not an EoE patient will require a dilation during the course of their treatment.
By understanding the clinical and histologic characteristics that influence whether or not a patient will develop a stricture, need dilation, or be at risk for complications from dilation will help clinicians and endoscopists make more informed decisions when caring for patients with EoE.
EoE is characterized by mucosal eosinophilic infiltration, a count of 15 or more eosinophils per high power field. The mucosal eosinophilic infiltration leads to chronic inflammation within esophageal tissue. Clinicians must rule out other esophageal disorders that cause similar physiological and histological presentations in order to diagnose an individual with EoE. Common symptoms include heartburn that is unresponsive to anti-reflux measures, dysphagia, and food impaction.
The authors investigated clinical and histologic features that may be associated with or predictive of the need for dilation and dilation related complications. The safety of dilation in patients with EoE was also assessed to contribute to existing understanding. The study revealed no correlation between the peak of eosinophil count and complication rate. Complications can occur independently of the histologic features. Esophageal dilation appears to be a safe procedure in EoE patients, carrying a low complication rate.
P- Reviewer: Kar P, Sporea I S- Editor: Qi Y L- Editor: A E- Editor: Liu XM
| 1. | Furuta GT, Liacouras CA, Collins MH, Gupta SK, Justinich C, Putnam PE, Bonis P, Hassall E, Straumann A, Rothenberg ME. Eosinophilic esophagitis in children and adults: a systematic review and consensus recommendations for diagnosis and treatment. Gastroenterology. 2007;133:1342-1363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1253] [Cited by in RCA: 1154] [Article Influence: 64.1] [Reference Citation Analysis (0)] |
| 2. | Liacouras CA, Bonis P, Putnam PE, Straumann A, Ruchelli E, Gupta SK, Lee JJ, Hogan SP, Wershil BK, Rothenberg ME. Summary of the First International Gastrointestinal Eosinophil Research Symposium. J Pediatr Gastroenterol Nutr. 2007;45:370-391. |
| 3. | Dellon ES, Gonsalves N, Hirano I, Furuta GT, Liacouras CA, Katzka DA; American College of Gastroenterology. ACG clinical guideline: Evidenced based approach to the diagnosis and management of esophageal eosinophilia and eosinophilic esophagitis (EoE). Am J Gastroenterol. 2013;108:679-692. [RCA] [DOI] [Full Text] [Cited by in Crossref: 784] [Cited by in RCA: 844] [Article Influence: 70.3] [Reference Citation Analysis (0)] |
| 4. | Cohen MS, Kaufman AB, Palazzo JP, Nevin D, Dimarino AJ, Cohen S. An audit of endoscopic complications in adult eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2007;5:1149-1153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 99] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 5. | Dellon ES, Speck O, Woodward K, Gebhart JH, Madanick RD, Levinson S, Fritchie KJ, Woosley JT, Shaheen NJ. Clinical and endoscopic characteristics do not reliably differentiate PPI-responsive esophageal eosinophilia and eosinophilic esophagitis in patients undergoing upper endoscopy: a prospective cohort study. Am J Gastroenterol. 2013;108:1854-1860. [RCA] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 203] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 6. | Rodrigo S, Abboud G, Oh D, DeMeester SR, Hagen J, Lipham J, DeMeester TR, Chandrasoma P. High intraepithelial eosinophil counts in esophageal squamous epithelium are not specific for eosinophilic esophagitis in adults. Am J Gastroenterol. 2008;103:435-442. [RCA] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 141] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 7. | Molina-Infante J, Ferrando-Lamana L, Fernandez-Bermejo M, Porcel-Carreño S. Eosinophilic esophagitis in GERD patients: a clinicopathological diagnosis using proton pump inhibitors. Am J Gastroenterol. 2009;104:2856-1857. [RCA] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 8. | Chehade M, Sampson HA, Morotti RA, Magid MS. Esophageal subepithelial fibrosis in children with eosinophilic esophagitis. J Pediatr Gastroenterol Nutr. 2007;45:319-328. [PubMed] |
| 9. | Aceves SS, Ackerman SJ. Relationships between eosinophilic inflammation, tissue remodeling, and fibrosis in eosinophilic esophagitis. Immunol Allergy Clin North Am. 2009;29:197-211, xiii-xiv. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 115] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 10. | Nicodème F, Hirano I, Chen J, Robinson K, Lin Z, Xiao Y, Gonsalves N, Kwasny MJ, Kahrilas PJ, Pandolfino JE. Esophageal distensibility as a measure of disease severity in patients with eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2013;11:1101-1107.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 230] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 11. | Kaplan M, Mutlu EA, Jakate S, Bruninga K, Losurdo J, Losurdo J, Keshavarzian A. Endoscopy in eosinophilic esophagitis: “feline” esophagus and perforation risk. Clin Gastroenterol Hepatol. 2003;1:433-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 114] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 12. | Straumann A, Rossi L, Simon HU, Heer P, Spichtin HP, Beglinger C. Fragility of the esophageal mucosa: a pathognomonic endoscopic sign of primary eosinophilic esophagitis? Gastrointest Endosc. 2003;57:407-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 111] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 13. | Croese J, Fairley SK, Masson JW, Chong AK, Whitaker DA, Kanowski PA, Walker NI. Clinical and endoscopic features of eosinophilic esophagitis in adults. Gastrointest Endosc. 2003;58:516-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 248] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 14. | Langdon DE. Corrugated ringed and too small esophagi. Am J Gastroenterol. 1999;94:542-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Schoepfer AM, Safroneeva E, Bussmann C, Kuchen T, Portmann S, Simon HU, Straumann A. Delay in diagnosis of eosinophilic esophagitis increases risk for stricture formation in a time-dependent manner. Gastroenterology. 2013;145:1230-6.e1-2. [RCA] [DOI] [Full Text] [Cited by in Crossref: 455] [Cited by in RCA: 572] [Article Influence: 47.7] [Reference Citation Analysis (1)] |
| 16. | Schoepfer AM, Gonsalves N, Bussmann C, Conus S, Simon HU, Straumann A, Hirano I. Esophageal dilation in eosinophilic esophagitis: effectiveness, safety, and impact on the underlying inflammation. Am J Gastroenterol. 2010;105:1062-1070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 233] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 17. | Alexander JA, Jung KW, Arora AS, Enders F, Katzka DA, Kephardt GM, Kita H, Kryzer LA, Romero Y, Smyrk TC. Swallowed fluticasone improves histologic but not symptomatic response of adults with eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2012;10:742-749.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 244] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 18. | Straumann A, Bussmann C, Zuber M, Vannini S, Simon HU, Schoepfer A. Eosinophilic esophagitis: analysis of food impaction and perforation in 251 adolescent and adult patients. Clin Gastroenterol Hepatol. 2008;6:598-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 164] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 19. | Jung KW, Gundersen N, Kopacova J, Arora AS, Romero Y, Katzka D, Francis D, Schreiber J, Dierkhising RA, Talley NJ. Occurrence of and risk factors for complications after endoscopic dilation in eosinophilic esophagitis. Gastrointest Endosc. 2011;73:15-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 74] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 20. | Jacobs JW, Spechler SJ. A systematic review of the risk of perforation during esophageal dilation for patients with eosinophilic esophagitis. Dig Dis Sci. 2010;55:1512-1515. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 78] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 21. | Bohm ME, Richter JE. Review article: oesophageal dilation in adults with eosinophilic oesophagitis. Aliment Pharmacol Ther. 2011;33:748-757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 22. | Ferreira CT, Goldani HA. Contribution of endoscopy in the management of eosinophilic esophagitis. World J Gastrointest Endosc. 2012;4:347-355. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 23. | Prasad GA, Talley NJ, Romero Y, Arora AS, Kryzer LA, Smyrk TC, Alexander JA. Prevalence and predictive factors of eosinophilic esophagitis in patients presenting with dysphagia: a prospective study. Am J Gastroenterol. 2007;102:2627-2632. [PubMed] |
| 24. | Mackenzie SH, Go M, Chadwick B, Thomas K, Fang J, Kuwada S, Lamphier S, Hilden K, Peterson K. Eosinophilic oesophagitis in patients presenting with dysphagia--a prospective analysis. Aliment Pharmacol Ther. 2008;28:1140-1146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 145] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 25. | Shi YN, Sun SJ, Xiong LS, Cao QH, Cui Y, Chen MH. Prevalence, clinical manifestations and endoscopic features of eosinophilic esophagitis: a pathological review in China. J Dig Dis. 2012;13:304-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 26. | Fujishiro H, Amano Y, Kushiyama Y, Ishihara S, Kinoshita Y. Eosinophilic esophagitis investigated by upper gastrointestinal endoscopy in Japanese patients. J Gastroenterol. 2011;46:1142-1144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 60] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 27. | Dellon ES, Gibbs WB, Rubinas TC, Fritchie KJ, Madanick RD, Woosley JT, Shaheen NJ. Esophageal dilation in eosinophilic esophagitis: safety and predictors of clinical response and complications. Gastrointest Endosc. 2010;71:706-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 110] [Article Influence: 7.3] [Reference Citation Analysis (1)] |
| 28. | Hirano I. Dilation in eosinophilic esophagitis: to do or not to do? Gastrointest Endosc. 2010;71:713-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 29. | Vasilopoulos S, Murphy P, Auerbach A, Massey BT, Shaker R, Stewart E, Komorowski RA, Hogan WJ. The small-caliber esophagus: an unappreciated cause of dysphagia for solids in patients with eosinophilic esophagitis. Gastrointest Endosc. 2002;55:99-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 123] [Article Influence: 5.3] [Reference Citation Analysis (0)] |