Published online Jul 28, 2014. doi: 10.3748/wjg.v20.i28.9229
Revised: March 7, 2014
Accepted: April 30, 2014
Published online: July 28, 2014
Hepatocellular carcinoma (HCC) and pancreatic cancer remain difficult to treat, and despite the ongoing development of new treatments, the overall survival rate has only modestly improved over the past decade. Liver and pancreatic progenitors commonly develop from endoderm cells in the embryonic foregut. A previous study showed that HCC and pancreatic cancer cell lines variably express androgen receptor (AR), and these cancers and the surrounding tissues also express AR. AR is a ligand-dependent transcription factor that belongs to the nuclear receptor superfamily. Androgen response element is present in regulatory elements on the AR-responsive target genes, such as transforming growth factor beta-1 (TGF beta-1) and vascular endothelial growth factor (VEGF). It is well known that the activation of AR is associated with human carcinogenesis in prostate cancer as well as HCC and pancreatic cancer and that GRP78, TGF beta, and VEGF all play important roles in carcinogenesis and cancer development in these cancers. HCC is a male-dominant cancer irrespective of its etiology. Previous work has reported that vertebrae forkhead box A 1/2 are involved in estrogen receptors and/or AR signaling pathways, which may contribute to the gender differences observed with HCC. Our recent work also showed that AR has a critical role in pancreatic cancer development, despite pancreatic cancer not being a male dominant cancer. Aryl hydrocarbon (or dioxin) receptor is also involved in both HCC and pancreatic cancer through the formation of complex with AR. It is possible that AR might be involved in their carcinogenesis through major histocompatibility complex class I chain-related gene A/B. This review article describes AR and its role in HCC and pancreatic cancer and suggests that more specific AR signaling-inhibitors may be useful in the treatment of these “difficult to treat” cancers.
Core tip: Recent studies have shown that androgen receptor (AR) could play an important role in carcinogenesis and cancer development in hepatocellular carcinoma (HCC) and pancreatic cancer. HCC is a male-dominant cancer. Although pancreatic cancer is not male-dominant, because liver and pancreatic progenitors develop commonly from endoderm cells in the embryonic foregut, AR might play an important role in these cancers.
- Citation: Kanda T, Jiang X, Yokosuka O. Androgen receptor signaling in hepatocellular carcinoma and pancreatic cancers. World J Gastroenterol 2014; 20(28): 9229-9236
- URL: https://www.wjgnet.com/1007-9327/full/v20/i28/9229.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i28.9229
The liver and pancreas progenitors develop from endoderm cells in the embryonic foregut[1]. The liver arises from lateral domains of endoderm in the developing ventral foregut[1,2], and from a small group of endodermal cells tracking down the ventral midline[1-3]. The pancreas is also induced in lateral endoderm domains, adjacent and caudal to the lateral liver domains, and in cells near the dorsal midline of the foregut[4,5]. Under specific experimental conditions, pancreatic progenitor(s) and pancreatic oval cells may differentiate into hepatocytes[1]. Thus, given that the liver and pancreas share differentiation patterns[6], it is possible that carcinogenesis and cancer development in the liver and pancreas may resemble each other.
The occurrence of hepatocellular carcinoma (HCC) has increased in Japan[7] and is increasing in the United States[8]. Cirrhosis due to chronic hepatitis B virus (HBV) and hepatitis C virus (HCV) infections is the leading risk factor for HCC[9,10]. HCC is a male-dominant cancer[11]. Advanced HCC, defined as metastatic or locally advanced disease not responsive to locoregional therapies such as surgery[12], local ablation[13], or transcatheter arterial chemoembolization[14], is still associated with poor prognosis even if anti-angiogenesis therapies, especially treatment with sorafenib, were used for treatment[15].
The global annual incidence rate of pancreatic cancer is reported to be approximately 8/100000 persons and pancreatic cancer has an exceptionally high mortality rate[16,17]. Pancreatectomy seems to offer the only chance for long-term survival[18], although recent advancement in chemotherapies brought the improvement of survival of patients with invasive cancer in Japan[19]. These facts indicate the urgent need for the development of novel and more effective adjuvant therapies for HCC and pancreatic cancer.
Androgen and androgen receptor (AR) signaling has an important role in the initiation and progression of many hormone-related cancers including prostate and breast cancer[20]. AR consists of the N terminus harboring transcriptional activation domain(s), a central DNA-binding domain and a C-terminal ligand-binding domain. AR is a testosterone/5-alpha-dihydrotestosterone (DHT)-dependent transcription factor belonging to the nuclear receptor superfamily[21,22]. AR is primarily responsible for mediating the physiological effects of androgens by binding to specific DNA sequences, androgen responsive element (ARE), which is present in regulatory elements on the AR-responsive target genes. Androgen binding changes the protein conformation in AR and leads to its translocation from the cytoplasm into the nucleus. In the nucleus, AR forms a homodimer and is recruited to the ARE. ARE is present in regulatory elements on the AR-responsive target genes, such as transforming growth factor beta-1 (TGF beta-1)[23] and vascular endothelial growth factor (VEGF)[24]. Glucose-regulated protein 78 (GRP78/BiP), one of the endoplasmic reticulum chaperones, is also an AR-interacting protein[25]. Thus, the transcriptional activation function of AR is associated with gender differences as well as human carcinogenesis.
It has been reported that AR is expressed in HCC and the surrounding liver tissues[26,27]. An immunohistochemical study showed that 67.7% of HCC were positive for AR; 51.6% for estrogen receptor (ER); 83.9% for progesterone receptor (PgR) and 38.7% for apolipoprotein D receptor and that chronic HCV infection correlated positively with AR and PgR status[28]. Some HCC specimens have elevated AR, ER and PgR expression, indicating that these hormone receptors could be involved in hepatocarcinogenesis and cancer development. The recurrence rate of HCC was significantly higher in the AR-positive group than in the AR-negative group, and the survival rate of HCC was better in AR-negative patients than in AR-positive patients[29]. Boix et al[30] reported that higher tumor recurrence was observed in AR-positive surrounding tissues than in AR-negative tissues. There were also contrary reports[30-32]. Only two-thirds of HCC contained AR, and there seemed no clear association between AR expression and liver fibrosis[30]; thus, further investigation might be needed.
More than 2 billion people have been exposed to HBV and 350 million are chronically infected with HBV globally. HBV causes acute and chronic hepatitis, cirrhosis, and HCC[33,34]. We compared the gene expression profile for nuclear receptors and related genes between HepG2.2.15, which secretes complete HBV virion, and HepG2 by real-time RT-PCR[35]. Among AR-related molecules, nuclear receptor subfamily 1, group I, member 3 (NR1I3), mediator complex subunit 16 (THRAP5), mediator complex subunit 4 (MED4), mediator complex subunit 17 (CRSP6), mediator complex subunit 24 (THRAP4), mediator complex subunit 13 (THRAP1) and mediator complex subunit 1 (PPARBP) all were upregulated significantly higher in HepG2.2.15 cells compared to HepG2 cells[35]. These data support the association between HBV and AR[35,36]. It has been reported in Taiwan[37-40] that the association between the trinucleotide (CAG) repeats in the AR gene and higher testosterone levels increase in HCC risk. Higher levels of androgen signaling, reflected by higher testosterone levels and 20 or fewer AR-CAG repeats, which is the association of CAG repeat in the AR gene, might be associated with an increased risk of HBV-related HCC in men[37]. The CAG polymorphism in exon 1 of the AR gene has been associated with the development of HCC with shorter AR alleles conferring a higher risk in men[37,39,40]. In contrast, women harboring 2 AR alleles with more than 23 CAG repeats had an increased risk of HCC compared to women with only short alleles or a single long allele[38]. In women, hepatocytes expressing the longer AR allele seem to confer a higher risk for HCC[38,39]. Polymorphisms of the AR-regulating genes and cytokine genes might be related to HCC[41].
HBx protein is about 17 kDa in size, and associated with hepatocarcinogenesis[42,43]. HBx likely augmented AR activity by increasing the phosphorylation of AR through HBx-mediated activation of the c-Src kinase signaling pathway[44]. HBx can physically bind to the AR and increase the gene transactivation activity of AR[45,46]. HBx increased the N-terminal transactivation domain (NTD) activation of AR through c-Src kinase and also enhanced AR dimerization by inhibiting glycogen synthase kinase-3β activity, which acts as a negative regulator of the conformational changes of AR[46].
Further, AR promotes transcription of HBV, resulting in a higher HBV titer in male HBV carriers and an increased risk of HCC[47-49]. It was reported that higher HBsAg and HBV titers were found in male HBV transgenic mice, compared with control mice, and that HBV enhancer I contained a DNA element responsive to transcriptional regulation by ligand-stimulated AR[47]. Similar observations in HBV transgenic mice lacking AR in liver hepatocytes, were also reported by another group[48]. The absence of HBx did not affect the effects of gender, androgen, and AR on HBV replication[49]. The androgen pathway could increase the transcription of HBV through direct binding to the androgen-responsive element sites in HBV enhancer I[47]. The direct relationship between HBV and AR was supported by our HepG2 experiments[35].
HCV infection affects approximately 4 million Americans and is the leading cause of cirrhosis and HCC in the United States[50]. HCV infection is a leading cause of HCC in Japan[7,11,51]. The HCV genome encodes structural proteins (core, E1, E2 and p7) and non-structural proteins (NS2, NS3, NS4A, NS4B, NS5A and NS5B). The HCV core protein is approximately 21 kDa in size and is associated with hepatocarcinogenesis[52-57]. HCV augments AR-mediated signaling through the HCV core protein[58]. HCV infection and HCV core protein enhance AR activation in the presence of the AR-ligand DHT.
AR is activated by the MAPK, phosphatidylinositol 3-kinase/AKT and JAK/signal transducer and activator of transcription 3 (STAT3) pathways[59]. STAT3 is also involved in HCV-induced AR activation, and HCV augments the phosphorylation status of STAT3. HCV core increases STAT3 phosphorylation at both Ser-727 and Tyr-705, which in turn activates AR. AR expression increases HCV-mediated VEGF mRNA expression and angiogenesis[58]. The status of angiogenesis in HCC has been correlated with disease progression and prognosis. It has also been reported that higher serum testosterone is associated with increased risk of advanced hepatitis C-related liver diseases in men[60]. In immortalized human hepatocytes, HCV increased AR mRNA expression[58]. However, further studies are needed to determine whether AR increases HCV replication.
The addition of functional AR in human HCC cells leads to the promotion of cell growth. AR may promote hepatocarcinogenesis via increased cellular oxidative stress and DNA damage as well as suppression of the p53-mediated DNA repairing system[61]. AR is also associated with HCC migration and invasion[62,63]. It has been reported that vertebrae forkhead box A 1/2 (Foxa1/2) is involved in estrogen receptor and/or AR signaling pathways, contributing to the observed gender differences of HCC[64]. Despite its cause, HCC is a male-dominant cancer; thus, AR could play a critical role in hepatocarcinogenesis and development. RNA expression profiling of LNCaP prostate cancer cells has described about 500 transcripts with altered expression[65,66]. Primary androgen-responsive genes (ARGs), that is, the subset regulated directly by AR-occupied androgen responsive elements (AREs), may in turn produce effects on secondary target genes[24]. Representative primary ARGs and ARGs lacking genomic AREs[24] that have been reported in HCC or pancreatic cancer are shown in Table 1. These genes have often been reported to be associated with hepatocarcinogenesis and/or pancreatocarcinogenesis.
| ARGs[24] | Signal pathways1 | Reported diseases |
| Primary ARGs | ||
| CYR61 | Wnt/β-catenin | HCC, pancreatic cancer |
| SNAI2 | Epithelial-mesenchymal transition (EMT) | HCC, pancreatic cancer |
| FN1 | Cell surface and extracellular matrix | HCC |
| ATP1A1 | Na,K-ATPase pump | Pancreatic cancer |
| FKBP5 | AKT | Pancreatic cancer |
| VEGFA | Angiogenesis | HCC, pancreatic cancer |
| SGK | Serine/threonine kinase | HCC, pancreatic cancer |
| SLC22A3 | Organic cation transporter | HCC, pancreatic cancer |
| NDRG1 | P53/N-myc | HCC, pancreatic cancer |
| NFKBIA | NF-κB inhibitory family | HCC |
| PYGB | Glycogen catabolism | HCC, pancreatic cancer |
| ARGs lacking AREs | ||
| ADAMTS1 | Inflammatory process | HCC, pancreatic cancer |
| CXCR7 | Orphan G-protein coupled receptor | HCC, pancreatic cancer |
| GATA2 | Transcription factor | Pancreatic cancer |
| MYC | Oncogene | HCC, pancreatic cancer |
| KLF4 | Cell proliferation | HCC, pancreatic cancer |
Human normal pancreas tissue and human pancreatic adenocarcinoma tissue also express AR[67]. Pancreatic cancer also over-expresses interleukin-6 (IL6), and serum IL6 levels are correlated with a poor prognosis of pancreatic cancer[68]. IL6 phospholylates MAPK and activates the AR NTD through STAT3[69,70]. MAPK and STAT3 increase the transactivation of AR[69,71]. We have demonstrated that IL6 increases the phosphorylation of STAT3 and MAPK, which in turn increases the activation of AR in pancreatic cancer cells[72]. IL6 also promotes pancreatic cancer cell migration in the presence of AR[72]. AR might play an important role in pancreatic carcinogenesis and the development of pancreatic cancer, making AR a candidate of therapeutic targets for new pancreatic cancer treatments[72,73].
Both aryl hydrocarbon (or dioxin) receptor (AHR) and AHR nuclear translocator (ARNT) are known to interact with AR in a testosterone-dependent manner[74,75]. AHR, but not ARNT, increased the AR-transcriptional activity independent of exogenous AHR ligand treatment [2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD)][74]. It has also been shown that normal expression levels of ERK1/2 are required for testosterone to induce the interaction between AR and AHR and the expression of liver receptor homolog 1 in ovarian granulosa cells[75]. In humans, a wide variety of health effects and cancer development are associated with the exposure to dioxins[76]. Once the ligand is bound, AHR translocates into the nucleus, and the AHR-ARNT-dioxin complex then binds to dioxin response elements acting as transcriptional enhancers and induces the transcription of CYP1A1[77]. Previous work[78] has revealed that the liver and pancreas from AHR-deficient mice had no significant 2,3,7,8-TCDD-induced lesions. It has also been shown that AHR is involved in many physiological functions, including circadian rhythm[79], which is involved in both HCC[80-82] and pancreatic cancer[83,84]. AR might influence the progression of pancreatic cancer and HCC by affecting circadian rhythm, the disruption of which is associated with cancer development[84].
AHR also downregulates natural killer (NK) cell inflammatory cytokine production[85]. Several matrix metalloproteases (MMPs), such as a disintegrin and metalloprotease (ADAM)10 and MMP-9, are targets of AHR pathways[86]. ADAM10 and MMP-9 cleave NKG2D ligands, leading to the release of soluble major histocompatibility complex class I chain-related gene A (MICA) and MICB, which then act to inhibit NK cells, γδTCR bearing cells and some other T lymphocyte subsets, contributing to immune-suppression[79]. Interestingly, recent work has suggested that MICA is involved in human hepatocarcinogenesis[87,88] and that MICA/B is expressed in human HCC and hepatoma cell lines[89], despite their expression being significantly decreased in HCV-infected Huh7.5 cells[90].
The migration of tumor cells play a role in tumor progression and this process requires acquisition of an invasive phenotype involving MMPs production and cell motility[91]. Targeting the AR domain involved in AR/Src association impairs EGF signaling, which stimulates transmigration and MMP-9, in human fibrosarcoma HT1080 cells[92], suggesting AR might be new potential targets in the therapeutic approach to human pancreatic cancer. Further studies are needed.
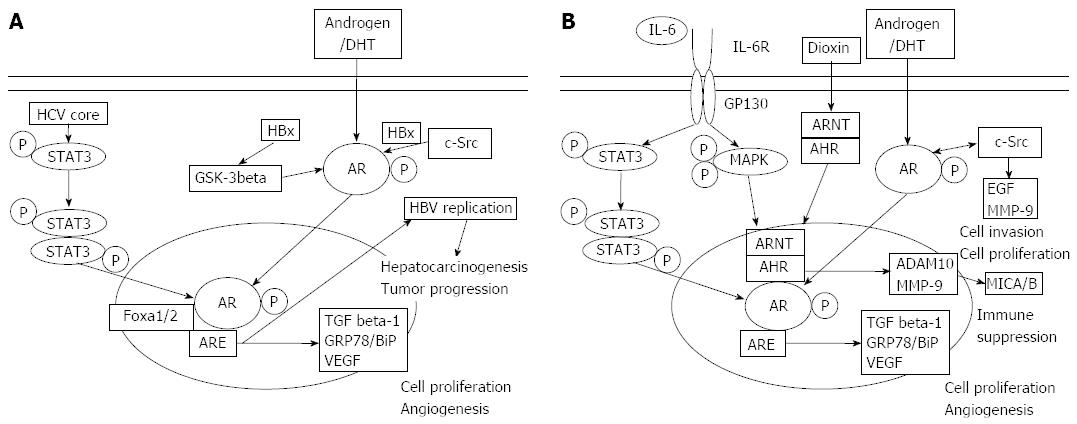
In summary, HCC, pancreatic cancers, and their surrounding tissues express AR at various levels (Figure 1). AR, but not androgen, might be involved in the carcinogenesis and cancer development of HCC or pancreatic cancer. In HBV-related hepatocarcinogenesis, the interaction between the CAG repeats in the AR gene as was reported from Taiwan[37-41]. AR also promotes the transcription of HBV, which leads to a higher HBV titer in male HBV carriers and an increased risk of HCC[47-49,93]. Currently, the effect of AR on HCV replication is unclear, however, HCV increases AR-mediated transcriptional activity especially in the presence of AR[58]. AR might play an important role in pancreatic carcinogenesis and the development of pancreatic cancer[72]. AHR is involved in both HCC[80-82] and pancreatic cancer[83,84]. AR could be in involved the carcinogenesis of HCC and pancreatic cancer through MICA/B[87-90]. Future directions in treatment development should specifically target AR in these cancers.
P- Reviewer: Andrada S, Michalopoulos GK, Mizuguchi T, Ross JA S- Editor: Ma YJ L- Editor: A E- Editor: Wang CH
| 1. | Zaret KS, Grompe M. Generation and regeneration of cells of the liver and pancreas. Science. 2008;322:1490-1494. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 436] [Cited by in F6Publishing: 462] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 2. | Chalmers AD, Slack JM. The Xenopus tadpole gut: fate maps and morphogenetic movements. Development. 2000;127:381-392. [PubMed] [Cited in This Article: ] |
| 3. | Tremblay KD, Zaret KS. Distinct populations of endoderm cells converge to generate the embryonic liver bud and ventral foregut tissues. Dev Biol. 2005;280:87-99. [PubMed] [Cited in This Article: ] |
| 4. | Field HA, Dong PD, Beis D, Stainier DY. Formation of the digestive system in zebrafish. II. Pancreas morphogenesis. Dev Biol. 2003;261:197-208. [PubMed] [Cited in This Article: ] |
| 5. | Slack JM. Developmental biology of the pancreas. Development. 1995;121:1569-1580. [PubMed] [Cited in This Article: ] |
| 6. | Shen CN, Slack JM, Tosh D. Molecular basis of transdifferentiation of pancreas to liver. Nat Cell Biol. 2000;2:879-887. [PubMed] [Cited in This Article: ] |
| 7. | Tanaka H, Imai Y, Hiramatsu N, Ito Y, Imanaka K, Oshita M, Hijioka T, Katayama K, Yabuuchi I, Yoshihara H. Declining incidence of hepatocellular carcinoma in Osaka, Japan, from 1990 to 2003. Ann Intern Med. 2008;148:820-826. [PubMed] [Cited in This Article: ] |
| 8. | Mittal S, El-Serag HB. Epidemiology of hepatocellular carcinoma: consider the population. J Clin Gastroenterol. 2013;47 Suppl:S2-S6. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 770] [Cited by in F6Publishing: 832] [Article Influence: 75.6] [Reference Citation Analysis (0)] |
| 9. | Beasley RP, Hwang LY, Lin CC, Chien CS. Hepatocellular carcinoma and hepatitis B virus. A prospective study of 22 707 men in Taiwan. Lancet. 1981;2:1129-1133. [PubMed] [Cited in This Article: ] |
| 10. | Hayashi PH, Di Bisceglie AM. The progression of hepatitis B- and C-infections to chronic liver disease and hepatocellular carcinoma: epidemiology and pathogenesis. Med Clin North Am. 2005;89:371-389. [PubMed] [Cited in This Article: ] |
| 11. | Akita T, Ohisa M, Kimura Y, Fujimoto M, Miyakawa Y, Tanaka J. Validation and limitation of age-period-cohort model in simulating mortality due to hepatocellular carcinoma from 1940 to 2010 in Japan. Hepatol Res. 2013;Epub ahead of print. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 12. | Ikai I, Arii S, Kojiro M, Ichida T, Makuuchi M, Matsuyama Y, Nakanuma Y, Okita K, Omata M, Takayasu K. Reevaluation of prognostic factors for survival after liver resection in patients with hepatocellular carcinoma in a Japanese nationwide survey. Cancer. 2004;101:796-802. [PubMed] [Cited in This Article: ] |
| 13. | Shiina S, Tateishi R, Arano T, Uchino K, Enooku K, Nakagawa H, Asaoka Y, Sato T, Masuzaki R, Kondo Y. Radiofrequency ablation for hepatocellular carcinoma: 10-year outcome and prognostic factors. Am J Gastroenterol. 2012;107:569-77; quiz 578. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 484] [Cited by in F6Publishing: 537] [Article Influence: 44.8] [Reference Citation Analysis (0)] |
| 14. | Yoshikawa M, Ono N, Yodono H, Ichida T, Nakamura H. Phase II study of hepatic arterial infusion of a fine-powder formulation of cisplatin for advanced hepatocellular carcinoma. Hepatol Res. 2008;38:474-483. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 92] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 15. | Shao YY, Hsu CH, Cheng AL. Predictive biomarkers of antiangiogenic therapy for advanced hepatocellular carcinoma: where are we? Liver Cancer. 2013;2:93-107. [PubMed] [Cited in This Article: ] |
| 16. | Raimondi S, Maisonneuve P, Lowenfels AB. Epidemiology of pancreatic cancer: an overview. Nat Rev Gastroenterol Hepatol. 2009;6:699-708. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 430] [Cited by in F6Publishing: 432] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 17. | Yadav D, Lowenfels AB. The epidemiology of pancreatitis and pancreatic cancer. Gastroenterology. 2013;144:1252-1261. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1151] [Cited by in F6Publishing: 1218] [Article Influence: 110.7] [Reference Citation Analysis (0)] |
| 18. | Conlon KC, Klimstra DS, Brennan MF. Long-term survival after curative resection for pancreatic ductal adenocarcinoma. Clinicopathologic analysis of 5-year survivors. Ann Surg. 1996;223:273-279. [PubMed] [Cited in This Article: ] |
| 19. | Egawa S, Toma H, Ohigashi H, Okusaka T, Nakao A, Hatori T, Maguchi H, Yanagisawa A, Tanaka M. Japan Pancreatic Cancer Registry; 30th year anniversary: Japan Pancreas Society. Pancreas. 2012;41:985-992. [PubMed] [Cited in This Article: ] |
| 20. | Chang C, Lee SO, Yeh S, Chang TM. Androgen receptor (AR) differential roles in hormone-related tumors including prostate, bladder, kidney, lung, breast and liver. Oncogene. 2014;33:3225-3234. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 135] [Cited by in F6Publishing: 149] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 21. | Gelmann EP. Molecular biology of the androgen receptor. J Clin Oncol. 2002;20:3001-3015. [PubMed] [Cited in This Article: ] |
| 22. | Heinlein CA, Chang C. Androgen receptor in prostate cancer. Endocr Rev. 2004;25:276-308. [PubMed] [Cited in This Article: ] |
| 23. | Yoon G, Kim JY, Choi YK, Won YS, Lim IK. Direct activation of TGF-beta1 transcription by androgen and androgen receptor complex in Huh7 human hepatoma cells and its tumor in nude mice. J Cell Biochem. 2006;97:393-411. [PubMed] [Cited in This Article: ] |
| 24. | Bolton EC, So AY, Chaivorapol C, Haqq CM, Li H, Yamamoto KR. Cell- and gene-specific regulation of primary target genes by the androgen receptor. Genes Dev. 2007;21:2005-2017. [PubMed] [Cited in This Article: ] |
| 25. | Jiang X, Kanda T, Nakamoto S, Miyamura T, Wu S, Yokosuka O. Involvement of androgen receptor and glucose-regulated protein 78 kDa in human hepatocarcinogenesis. Exp Cell Res. 2014;323:326-336. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 26. | Nagasue N, Ito A, Yukaya H, Ogawa Y. Androgen receptors in hepatocellular carcinoma and surrounding parenchyma. Gastroenterology. 1985;89:643-647. [PubMed] [Cited in This Article: ] |
| 27. | Negro F, Papotti M, Pacchioni D, Galimi F, Bonino F, Bussolati G. Detection of human androgen receptor mRNA in hepatocellular carcinoma by in situ hybridisation. Liver. 1994;14:213-219. [PubMed] [Cited in This Article: ] |
| 28. | Vizoso FJ, Rodriguez M, Altadill A, González-Diéguez ML, Linares A, González LO, Junquera S, Fresno-Forcelledo F, Corte MD, Rodrigo L. Liver expression of steroid hormones and Apolipoprotein D receptors in hepatocellular carcinoma. World J Gastroenterol. 2007;13:3221-3227. [PubMed] [Cited in This Article: ] |
| 29. | Nagasue N, Yu L, Yukaya H, Kohno H, Nakamura T. Androgen and oestrogen receptors in hepatocellular carcinoma and surrounding liver parenchyma: impact on intrahepatic recurrence after hepatic resection. Br J Surg. 1995;82:542-547. [PubMed] [Cited in This Article: ] |
| 30. | Boix L, Castells A, Bruix J, Solé M, Brú C, Fuster J, Rivera F, Rodés J. Androgen receptors in hepatocellular carcinoma and surrounding liver: relationship with tumor size and recurrence rate after surgical resection. J Hepatol. 1995;22:616-622. [PubMed] [Cited in This Article: ] |
| 31. | Zhang X, He L, Lu Y, Liu M, Huang X. Androgen receptor in primary hepatocellular carcinoma and its clinical significance. Chin Med J (Engl). 1998;111:1083-1086. [PubMed] [Cited in This Article: ] |
| 32. | Kalra M, Mayes J, Assefa S, Kaul AK, Kaul R. Role of sex steroid receptors in pathobiology of hepatocellular carcinoma. World J Gastroenterol. 2008;14:5945-5961. [PubMed] [Cited in This Article: ] |
| 33. | Lok AS, McMahon BJ. Chronic hepatitis B. Hepatology. 2007;45:507-539. [PubMed] [Cited in This Article: ] |
| 34. | Di Bisceglie AM. Hepatitis B and hepatocellular carcinoma. Hepatology. 2009;49:S56-S60. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 215] [Cited by in F6Publishing: 229] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 35. | Wu S, Kanda T, Imazeki F, Nakamoto S, Shirasawa H, Yokosuka O. Nuclear receptor mRNA expression by HBV in human hepatoblastoma cell lines. Cancer Lett. 2011;312:33-42. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 36. | Yu MW, Yang YC, Yang SY, Cheng SW, Liaw YF, Lin SM, Chen CJ. Hormonal markers and hepatitis B virus-related hepatocellular carcinoma risk: a nested case-control study among men. J Natl Cancer Inst. 2001;93:1644-1651. [PubMed] [Cited in This Article: ] |
| 37. | Yu MW, Cheng SW, Lin MW, Yang SY, Liaw YF, Chang HC, Hsiao TJ, Lin SM, Lee SD, Chen PJ. Androgen-receptor gene CAG repeats, plasma testosterone levels, and risk of hepatitis B-related hepatocellular carcinoma. J Natl Cancer Inst. 2000;92:2023-2028. [PubMed] [Cited in This Article: ] |
| 38. | Yu MW, Yang YC, Yang SY, Chang HC, Liaw YF, Lin SM, Liu CJ, Lee SD, Lin CL, Chen PJ. Androgen receptor exon 1 CAG repeat length and risk of hepatocellular carcinoma in women. Hepatology. 2002;36:156-163. [PubMed] [Cited in This Article: ] |
| 39. | Yeh SH, Chang CF, Shau WY, Chen YW, Hsu HC, Lee PH, Chen DS, Chen PJ. Dominance of functional androgen receptor allele with longer CAG repeat in hepatitis B virus-related female hepatocarcinogenesis. Cancer Res. 2002;62:4346-4351. [PubMed] [Cited in This Article: ] |
| 40. | Yeh SH, Chiu CM, Chen CL, Lu SF, Hsu HC, Chen DS, Chen PJ. Somatic mutations at the trinucleotide repeats of androgen receptor gene in male hepatocellular carcinoma. Int J Cancer. 2007;120:1610-1617. [PubMed] [Cited in This Article: ] |
| 41. | Chan HL, Sung JJ. Hepatocellular carcinoma and hepatitis B virus. Semin Liver Dis. 2006;26:153-161. [PubMed] [Cited in This Article: ] |
| 42. | Kim CM, Koike K, Saito I, Miyamura T, Jay G. HBx gene of hepatitis B virus induces liver cancer in transgenic mice. Nature. 1991;351:317-320. [PubMed] [Cited in This Article: ] |
| 43. | Kanda T, Yokosuka O, Imazeki F, Yamada Y, Imamura T, Fukai K, Nagao K, Saisho H. Hepatitis B virus X protein (HBx)-induced apoptosis in HuH-7 cells: influence of HBV genotype and basal core promoter mutations. Scand J Gastroenterol. 2004;39:478-485. [PubMed] [Cited in This Article: ] |
| 44. | Chiu CM, Yeh SH, Chen PJ, Kuo TJ, Chang CJ, Chen PJ, Yang WJ, Chen DS. Hepatitis B virus X protein enhances androgen receptor-responsive gene expression depending on androgen level. Proc Natl Acad Sci USA. 2007;104:2571-2578. [PubMed] [Cited in This Article: ] |
| 45. | Zheng Y, Chen WL, Ma WL, Chang C, Ou JH. Enhancement of gene transactivation activity of androgen receptor by hepatitis B virus X protein. Virology. 2007;363:454-461. [PubMed] [Cited in This Article: ] |
| 46. | Yang WJ, Chang CJ, Yeh SH, Lin WH, Wang SH, Tsai TF, Chen DS, Chen PJ. Hepatitis B virus X protein enhances the transcriptional activity of the androgen receptor through c-Src and glycogen synthase kinase-3beta kinase pathways. Hepatology. 2009;49:1515-1524. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 87] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 47. | Wang SH, Yeh SH, Lin WH, Wang HY, Chen DS, Chen PJ. Identification of androgen response elements in the enhancer I of hepatitis B virus: a mechanism for sex disparity in chronic hepatitis B. Hepatology. 2009;50:1392-1402. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 130] [Cited by in F6Publishing: 136] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 48. | Wu MH, Ma WL, Hsu CL, Chen YL, Ou JH, Ryan CK, Hung YC, Yeh S, Chang C. Androgen receptor promotes hepatitis B virus-induced hepatocarcinogenesis through modulation of hepatitis B virus RNA transcription. Sci Transl Med. 2010;2:32ra35. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 112] [Cited by in F6Publishing: 153] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 49. | Tian Y, Kuo CF, Chen WL, Ou JH. Enhancement of hepatitis B virus replication by androgen and its receptor in mice. J Virol. 2012;86:1904-1910. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 50. | Lee WM, Dienstag JL, Lindsay KL, Lok AS, Bonkovsky HL, Shiffman ML, Everson GT, Di Bisceglie AM, Morgan TR, Ghany MG. Evolution of the HALT-C Trial: pegylated interferon as maintenance therapy for chronic hepatitis C in previous interferon nonresponders. Control Clin Trials. 2004;25:472-492. [PubMed] [Cited in This Article: ] |
| 51. | Saito I, Miyamura T, Ohbayashi A, Harada H, Katayama T, Kikuchi S, Watanabe Y, Koi S, Onji M, Ohta Y. Hepatitis C virus infection is associated with the development of hepatocellular carcinoma. Proc Natl Acad Sci USA. 1990;87:6547-6549. [PubMed] [Cited in This Article: ] |
| 52. | Ray RB, Lagging LM, Meyer K, Steele R, Ray R. Transcriptional regulation of cellular and viral promoters by the hepatitis C virus core protein. Virus Res. 1995;37:209-220. [PubMed] [Cited in This Article: ] |
| 53. | Ray RB, Lagging LM, Meyer K, Ray R. Hepatitis C virus core protein cooperates with ras and transforms primary rat embryo fibroblasts to tumorigenic phenotype. J Virol. 1996;70:4438-4443. [PubMed] [Cited in This Article: ] |
| 54. | Moriya K, Fujie H, Shintani Y, Yotsuyanagi H, Tsutsumi T, Ishibashi K, Matsuura Y, Kimura S, Miyamura T, Koike K. The core protein of hepatitis C virus induces hepatocellular carcinoma in transgenic mice. Nat Med. 1998;4:1065-1067. [PubMed] [Cited in This Article: ] |
| 55. | Ray RB, Meyer K, Ray R. Hepatitis C virus core protein promotes immortalization of primary human hepatocytes. Virology. 2000;271:197-204. [PubMed] [Cited in This Article: ] |
| 56. | Basu A, Meyer K, Lai KK, Saito K, Di Bisceglie AM, Grosso LE, Ray RB, Ray R. Microarray analyses and molecular profiling of Stat3 signaling pathway induced by hepatitis C virus core protein in human hepatocytes. Virology. 2006;349:347-358. [PubMed] [Cited in This Article: ] |
| 57. | Saito K, Meyer K, Warner R, Basu A, Ray RB, Ray R. Hepatitis C virus core protein inhibits tumor necrosis factor alpha-mediated apoptosis by a protective effect involving cellular FLICE inhibitory protein. J Virol. 2006;80:4372-4379. [PubMed] [Cited in This Article: ] |
| 58. | Kanda T, Steele R, Ray R, Ray RB. Hepatitis C virus core protein augments androgen receptor-mediated signaling. J Virol. 2008;82:11066-11072. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 68] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 59. | Ueda T, Mawji NR, Bruchovsky N, Sadar MD. Ligand-independent activation of the androgen receptor by interleukin-6 and the role of steroid receptor coactivator-1 in prostate cancer cells. J Biol Chem. 2002;277:38087-38094. [PubMed] [Cited in This Article: ] |
| 60. | White DL, Tavakoli-Tabasi S, Kuzniarek J, Pascua R, Ramsey DJ, El-Serag HB. Higher serum testosterone is associated with increased risk of advanced hepatitis C-related liver disease in males. Hepatology. 2012;55:759-768. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 61. | Ma WL, Hsu CL, Wu MH, Wu CT, Wu CC, Lai JJ, Jou YS, Chen CW, Yeh S, Chang C. Androgen receptor is a new potential therapeutic target for the treatment of hepatocellular carcinoma. Gastroenterology. 2008;135:947-55, 955.e1-5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 173] [Cited by in F6Publishing: 183] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 62. | Ma WL, Hsu CL, Yeh CC, Wu MH, Huang CK, Jeng LB, Hung YC, Lin TY, Yeh S, Chang C. Hepatic androgen receptor suppresses hepatocellular carcinoma metastasis through modulation of cell migration and anoikis. Hepatology. 2012;56:176-185. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 109] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 63. | Ao J, Meng J, Zhu L, Nie H, Yang C, Li J, Gu J, Lin Q, Long W, Dong X. Activation of androgen receptor induces ID1 and promotes hepatocellular carcinoma cell migration and invasion. Mol Oncol. 2012;6:507-515. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 64. | Li Z, Tuteja G, Schug J, Kaestner KH. Foxa1 and Foxa2 are essential for sexual dimorphism in liver cancer. Cell. 2012;148:72-83. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 256] [Cited by in F6Publishing: 281] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 65. | DePrimo SE, Diehn M, Nelson JB, Reiter RE, Matese J, Fero M, Tibshirani R, Brown PO, Brooks JD. Transcriptional programs activated by exposure of human prostate cancer cells to androgen. Genome Biol. 2002;3:RESEARCH0032. [PubMed] [Cited in This Article: ] |
| 66. | Nelson PS, Clegg N, Arnold H, Ferguson C, Bonham M, White J, Hood L, Lin B. The program of androgen-responsive genes in neoplastic prostate epithelium. Proc Natl Acad Sci USA. 2002;99:11890-11895. [PubMed] [Cited in This Article: ] |
| 67. | Corbishley TP, Iqbal MJ, Wilkinson ML, Williams R. Androgen receptor in human normal and malignant pancreatic tissue and cell lines. Cancer. 1986;57:1992-1995. [PubMed] [Cited in This Article: ] |
| 68. | Noh KW, Pungpapong S, Wallace MB, Woodward TA, Raimondo M. Do cytokine concentrations in pancreatic juice predict the presence of pancreatic diseases? Clin Gastroenterol Hepatol. 2006;4:782-789. [PubMed] [Cited in This Article: ] |
| 69. | Ueda T, Bruchovsky N, Sadar MD. Activation of the androgen receptor N-terminal domain by interleukin-6 via MAPK and STAT3 signal transduction pathways. J Biol Chem. 2002;277:7076-7085. [PubMed] [Cited in This Article: ] |
| 70. | Furukawa T. Molecular targeting therapy for pancreatic cancer: current knowledge and perspectives from bench to bedside. J Gastroenterol. 2008;43:905-911. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 71. | Yang L, Wang L, Lin HK, Kan PY, Xie S, Tsai MY, Wang PH, Chen YT, Chang C. Interleukin-6 differentially regulates androgen receptor transactivation via PI3K-Akt, STAT3, and MAPK, three distinct signal pathways in prostate cancer cells. Biochem Biophys Res Commun. 2003;305:462-469. [PubMed] [Cited in This Article: ] |
| 72. | Okitsu K, Kanda T, Imazeki F, Yonemitsu Y, Ray RB, Chang C, Yokosuka O. Involvement of interleukin-6 and androgen receptor signaling in pancreatic cancer. Genes Cancer. 2010;1:859-867. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 73. | Konduri S, Schwarz MA, Cafasso D, Schwarz RE. Androgen receptor blockade in experimental combination therapy of pancreatic cancer. J Surg Res. 2007;142:378-386. [PubMed] [Cited in This Article: ] |
| 74. | Kollara A, Brown TJ. Four and a half LIM domain 2 alters the impact of aryl hydrocarbon receptor on androgen receptor transcriptional activity. J Steroid Biochem Mol Biol. 2010;118:51-58. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 75. | Wu Y, Baumgarten SC, Zhou P, Stocco C. Testosterone-dependent interaction between androgen receptor and aryl hydrocarbon receptor induces liver receptor homolog 1 expression in rat granulosa cells. Mol Cell Biol. 2013;33:2817-2828. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 76. | Gradin K, McGuire J, Wenger RH, Kvietikova I, fhitelaw ML, Toftgård R, Tora L, Gassmann M, Poellinger L. Functional interference between hypoxia and dioxin signal transduction pathways: competition for recruitment of the Arnt transcription factor. Mol Cell Biol. 1996;16:5221-5231. [PubMed] [Cited in This Article: ] |
| 77. | Rowlands JC, McEwan IJ, Gustafsson JA. Trans-activation by the human aryl hydrocarbon receptor and aryl hydrocarbon receptor nuclear translocator proteins: direct interactions with basal transcription factors. Mol Pharmacol. 1996;50:538-548. [PubMed] [Cited in This Article: ] |
| 78. | Fernandez-Salguero PM, Hilbert DM, Rudikoff S, Ward JM, Gonzalez FJ. Aryl-hydrocarbon receptor-deficient mice are resistant to 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced toxicity. Toxicol Appl Pharmacol. 1996;140:173-179. [PubMed] [Cited in This Article: ] |
| 79. | Anderson G, Beischlag TV, Vinciguerra M, Mazzoccoli G. The circadian clock circuitry and the AHR signaling pathway in physiology and pathology. Biochem Pharmacol. 2013;85:1405-1416. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 80. | Buzzelli G, Dattolo P, Pinzani M, Brocchi A, Romano S, Gentilini P. Circulating growth hormone and insulin-like growth factor-I in nonalcoholic liver cirrhosis with or without superimposed hepatocarcinoma: evidence of an altered circadian rhythm. Am J Gastroenterol. 1993;88:1744-1748. [PubMed] [Cited in This Article: ] |
| 81. | Zhao B, Lu J, Yin J, Liu H, Guo X, Yang Y, Ge N, Zhu Y, Zhang H, Xing J. A functional polymorphism in PER3 gene is associated with prognosis in hepatocellular carcinoma. Liver Int. 2012;32:1451-1459. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 82. | Naik A, Košir R, Rozman D. Genomic aspects of NAFLD pathogenesis. Genomics. 2013;102:84-95. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 83. | Sato F, Nagata C, Liu Y, Suzuki T, Kondo J, Morohashi S, Imaizumi T, Kato Y, Kijima H. PERIOD1 is an anti-apoptotic factor in human pancreatic and hepatic cancer cells. J Biochem. 2009;146:833-838. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 84. | Relles D, Sendecki J, Chipitsyna G, Hyslop T, Yeo CJ, Arafat HA. Circadian gene expression and clinicopathologic correlates in pancreatic cancer. J Gastrointest Surg. 2013;17:443-450. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 78] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 85. | Monteleone I, MacDonald TT, Pallone F, Monteleone G. The aryl hydrocarbon receptor in inflammatory bowel disease: linking the environment to disease pathogenesis. Curr Opin Gastroenterol. 2012;28:310-313. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 71] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 86. | Kung T, Murphy KA, White LA. The aryl hydrocarbon receptor (AhR) pathway as a regulatory pathway for cell adhesion and matrix metabolism. Biochem Pharmacol. 2009;77:536-546. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 70] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 87. | Kumar V, Kato N, Urabe Y, Takahashi A, Muroyama R, Hosono N, Otsuka M, Tateishi R, Omata M, Nakagawa H. Genome-wide association study identifies a susceptibility locus for HCV-induced hepatocellular carcinoma. Nat Genet. 2011;43:455-458. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 271] [Cited by in F6Publishing: 267] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 88. | Kumar V, Yi Lo PH, Sawai H, Kato N, Takahashi A, Deng Z, Urabe Y, Mbarek H, Tokunaga K, Tanaka Y. Soluble MICA and a MICA variation as possible prognostic biomarkers for HBV-induced hepatocellular carcinoma. PLoS One. 2012;7:e44743. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 70] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 89. | Jinushi M, Takehara T, Tatsumi T, Kanto T, Groh V, Spies T, Kimura R, Miyagi T, Mochizuki K, Sasaki Y. Expression and role of MICA and MICB in human hepatocellular carcinomas and their regulation by retinoic acid. Int J Cancer. 2003;104:354-361. [PubMed] [Cited in This Article: ] |
| 90. | Kim H, Bose SK, Meyer K, Ray R. Hepatitis C virus impairs natural killer cell-mediated augmentation of complement synthesis. J Virol. 2014;88:2564-2571. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 91. | Friedl P, Sahai E, Weiss S, Yamada KM. New dimensions in cell migration. Nat Rev Mol Cell Biol. 2012;13:743-747. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 204] [Cited by in F6Publishing: 178] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 92. | Castoria G, Giovannelli P, Di Donato M, Hayashi R, Arra C, Appella E, Auricchio F, Migliaccio A. Targeting androgen receptor/Src complex impairs the aggressive phenotype of human fibrosarcoma cells. PLoS One. 2013;8:e76899. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 93. | Kanda T, Yokosuka O, Omata M. Androgen Receptor and Hepatocellular Carcinoma. J Gastroint Dig Syst. 2013;S12:012. [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |