Published online Jul 21, 2014. doi: 10.3748/wjg.v20.i27.9138
Revised: March 10, 2014
Accepted: April 15, 2014
Published online: July 21, 2014
Processing time: 176 Days and 16.8 Hours
AIM: To evaluate whether an abdominoperineal excision (APE) is associated with increased local recurrence (LR) and shortened disease-free survival (DFS) in mid-low rectal cancer with a negative circumferential resection margin (CRM).
METHODS: 283 consecutive cases of mid-low rectal cancer underwent preoperative 30 Gy/10 F radiotherapy and surgery in Peking University Cancer Hospital between August 2003 and August 2009. Patients with positive CRM and intraoperative distant metastasis were precluded according to exclusion criteria. Survival analyses were performed in patients with APE or non-APE procedures.
RESULTS: 256 of the 283 (90.5%) cases were enrolled in the analysis, including 78 (30.5%) and 178 (69.5%) cases who received APE and non-APE procedures. Fewer female patients (P = 0.016), lower level of tumor (P = 0.000) and higher body mass index (P = 0.006) were found in the APE group. On univariate analysis, the APE group had a higher LR rate (5.1% vs 1.1%, P = 0.036) and decreased DFS (73.1% vs 83.4%, P = 0.021). On multivariate analysis, APE procedure was also an independent risk factor for LR (HR = 5.960, 1.085-32.728, P = 0.040) and decreased DFS (HR = 2.304, 1.298-4.092, P = 0.004). In stratified analysis for lower rectal cancer, APE procedure was still an independent risk factor for higher LR rate (5.6% vs 0%, P = 0.024) and shortened DFS (91.5% vs 73.6%, P = 0.002).
CONCLUSION: Following preoperative 30 Gy/10 F radiotherapy, APE procedure was still a predictor for LR and decreased DFS even with negative CRM. More intensive preoperative treatment should be planned for the candidates who are scheduled to receive APE with optimal imaging assessment.
Core tip: The present study focused on survival differences between rectal cancer treated with abdominoperineal excision or non-abdominoperineal excision (APE) following preoperative radiotherapy, with the adjustments of the circumferential resection margin (CRM) to preclude the influence of surgical radicality. The results revealed the more aggressive oncological behavior of low-lying or fixed tumors, which were unavailable for the sphincter preservation procedure even with negative CRM. We also emphasized the importance of preoperative staging and decision-making before APE procedure, and reviewed the related hypotheses for the unfavorable local control of APE in the discussion.
- Citation: Wang L, Gu GL, Li ZW, Peng YF, Gu J. Abdominoperineal excision following preoperative radiotherapy for rectal cancer: Unfavorable prognosis even with negative circumferential resection margin. World J Gastroenterol 2014; 20(27): 9138-9145
- URL: https://www.wjgnet.com/1007-9327/full/v20/i27/9138.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i27.9138
Treatment for mid-low rectal cancer has substantially improved in the last few decades with the introduction of total mesorectal excision (TME) combined with neoadjuvant radiotherapy (nRT). Locally advanced rectal cancer could have better local control with a combination treatment of nRT and TME, which had been proven to be effective in many large-scale trials[1-5]. With the downstaging and downsize effect of nRT and a better understanding of tumor spread, sphincter-preserving surgery for low-lying tumor now can be safely performed with 1-cm distal margin[6]. Nevertheless, abdominoperineal excision (APE) still has indications for low-lying tumors which invade levator ani or are resistant to nRT[7].
The current literature reports a poorer outcome following APE than non-APE surgery[7-12]. High frequency of the circumferential resection margin (CRM) in patients who underwent APE was identified in series of studies, and it had been suggested as the main reason for worse outcome after APE[11,13-18]. In the results of a 12 year period follow-up of the Dutch TME trial, short-course nRT was not effective in patients with a positive CRM[1]. However, the unfavorable oncological results of APE might be multifactorial, excluding the increased CRM involvement rate. For instance, bulkier tumors with locally aggressive characteristics received more APE. In a previous report[9], a worse prognosis after APE was observed in patients even with clear CRM in the subgroups analyses. It is unclear whether surgical quality, APE procedure itself, or tumor biological behavior is responsible for the higher rate of treatment failure. There is currently little research comparing outcomes after APE or non-APE procedures with the adjustment of surgical radicality following nRT.
In our unit, nRT was a modified short-course regimen as 30 Gy in 10 fractions (biological equivalent dose: 36 Gy) with a prolonged interval of 2-4 wk to surgery, which has been promoted by the Committee of the Chinese Anti-Cancer Association (CACA) from 2001[19-22].
The present retrospective study was designed to address the following question: in patients with pathologically identified negative CRM, is the APE procedure is still relevant with an unfavorable prognosis when compared with non-APE surgery following 30 Gy/10 F nRT?
We retrospectively reviewed the data of 283 rectal cancer patients who received 30 Gy/10 F neoadjuvant radiotherapy (nRT) and total mesorectal excision at Peking University Cancer Hospital between August 2003 and August 2009.
All involved patients received preoperative neoadjuvant radiotherapy followed by TME. The radiotherapy regimen consisted of a 30 Gy dose delivered in 10 fractions for 2 wk. The biological equivalent dose of this regimen is 36 Gy, according to linear-quadratic formula. Three-dimensional conformal radiotherapy (3D-CRT) was employed routinely.
Surgery was performed 2-4 wk after following the principle of total mesorectal excision[23]. The decision to perform APE was made before surgery for low-lying or levator threatening rectal cancer, but made intraoperatively for mid rectal cancer depending on whether sufficient length of muscle tube could be mobilized for a tumor-free anastomosis. The abdominoperineal excision was performed in the lithotomy position without an extended resection of levator ani or coccyx. After surgery, 5-fluorouracil-based chemotherapy was administered for 6 mo if patients could tolerate.
The 7th edition of the American Joint Committee on the Cancer TNM system was used for tumor staging. Following nRT and surgery, the results of histopathologic examination of the specimens were reviewed by the same group of experienced pathologists; CRM involvement was assessed following the protocol laid out by Quirke et al[24-26]. Tumor regression grade (TRG) was evaluated by a 3-points system assessing residual tumor cell and fibrosis, with less than 5% residual tumor being identified as major regression[27,28].
Each subject conformed to the following entry criteria: (1) patient was diagnosed as having rectal adenocarcinoma by biopsy; (2) cancerous lesion was located within 10 cm from the anal verge; (3) the cancer was staged as T3-4 or any T, N+ by endorectal ultrasound, pelvic magnetic resonance imaging (MRI), or computed tomography (CT); and (4) presence of distant metastasis excluded by imaging exams.
Patients with the following characteristics were excluded: (1) previous chemotherapy, or pelvic radiation; (2) previous history (within 5 years) of malignant tumor; (3) intraoperative confirmed metastasis; and (4) pathologically-confirmed circumferential resection margin (CRM) by Quirke’s protocol[24,29].
Patients were followed at three-month intervals for the first two years and then at six-month intervals for the next three years. Evaluations consisted of physical examination, serum CEA, a complete blood count, and blood chemical analysis. Proctoscopy, abdominal ultrasonography, CT of the abdomen and pelvis, and chest radiography was also routinely performed every 6-12 mo.
Endpoints of the research were 3-year disease-free survival (3 years DFS)[30] and local recurrence (LR) rate.
The categorical variables were analyzed with the Pearson chi-squared or Fisher’s exact test, and the level of significance was set at 0.05. DFS curves were compared among groups. The Kaplan-Meier survival curve (method: log-rank test) was used for time-to-event parameters. Multivariate Cox proportional hazards regression was used to analyze the major factors affecting DFS and LR, with the level of significance set at 0.1. The software IBM SPSS Statistics for Mac, Version 22.0 (Armonk, NY: IBM Corp.) was used for the analyses.
The records of 283 patients were reviewed. Positive CRM was identified in 26 of 283 patients (9.2%). The incidence of CRM involvement had no statistical difference between the APE and non-APE groups [10.3% (9/87) vs 8.7% (17/196), P = 0.653]. Intraoperative liver metastasis occurred in one patient (0.3%).
There were 256 cases with negative CRM and without synchronous distant metastasis that were entered into the analysis following the inclusion and exclusion criteria. The median age was 58 years (range: 22-85 years), with 56.7% (145/256) male patients. The median body mass index (BMI) was 23.5 (range: 15.6-36.3). The median distance of tumors from the anal verge was 5.0 cm (range: 1-10 cm).
Initial clinical pre-staging was cIIin 18.8% (48/256) and cIII in 81.2% (208/256) of cases. The median operation time was 120 min (range: 60-240 min) and the median blood loss was 200 mL (range: 50-4000 mL). 30.5% (78/256), 67.6% (173/256), and 1.9% (5/256) of patients received APE, LAR, and the Hartmann procedure.
The distribution of ypTNM stages was: complete response (no microscopic residual tumor cell), 6.3% (16/256); stage I, 32.4% (83/256); stage II, 23.4% (60/256); and stage III, 37.8% (97/256).
Patients were divided into APE and non-APE groups according to types of surgery. The characteristics of the two groups, including preoperative variables and postoperative histologic/pathologic stages, are listed in Table 1.
| Variates | Non-APE (n =178) | APE (n = 78) | P value | |
| Gender | ||||
| Male | 92 (51.7) | 53 (67.9) | 0.016 | |
| Female | 86 (48.3) | 25 (32.1) | ||
| Age (yr) | ||||
| < 65 | 119 (66.9) | 56 (71.8) | 0.434 | |
| ≥ 65 | 59 (33.1) | 22 (28.2) | ||
| Level of tumor | ||||
| ≤ 5 cm | 82 (46.1) | 72 (92.3) | 0.000 | |
| > 5 cm | 96 (53.9) | 6 (7.7) | ||
| BMI (kg/m2) | ||||
| ≤ 23.5 | 97 (54.5) | 28 (35.9) | 0.006 | |
| > 23.5 | 81 (45.5) | 50 (64.1) | ||
| Pre-operative CEA1 | ||||
| ≤ 5 ng/mL | 97 (63.0) | 41 (66.1) | 0.664 | |
| > 5 ng/mL | 57 (37.0) | 21 (33.9) | ||
| Differentiation | ||||
| ypCR | 12 (6.7) | 6 (7.7) | 0.651 | |
| G1-2 | 120 (67.4) | 56 (71.8) | ||
| G3-4 | 46 (25.8) | 16 (20.5) | ||
| TRG | ||||
| Complete regression | 12 (6.7) | 6 (7.7) | 0.402 | |
| Major regression | 27 (15.2) | 7 (9.0) | ||
| Minor regression | 139 (78.1) | 65 (83.3) | ||
| ypT | ||||
| T0 | 12 (6.7) | 6 (7.7) | 0.511 | |
| T1 | 11 (6.2) | 5 (6.4) | ||
| T2 | 56 (31.5) | 29 (37.2) | ||
| T3 | 98 (551) | 36 (46.2) | ||
| T4 | 1 (0.6) | 2 (2.6) | ||
| ypN | ||||
| N0 | 114 (64.0) | 45 (57.7) | 0.209 | |
| N1/N1c | 37 (20.8) | 24 (30.8) | ||
| N2a/b | 27 (15.2) | 9 (11.5) | ||
| ypTNM stage | ||||
| ypCR | 12 (6.7) | 4 (5.1) | 0.642 | |
| I | 57 (32.0) | 26 (33.3) | ||
| II | 45 (25.3) | 15 (19.2) | ||
| III | 64 (36.0) | 33 (42.3) |
Statistical analysis showed that fewer female patients (P = 0.016), lower level of tumors (P = 0.000), and higher median BMI (P = 0.006) were found in the APE group compared to the non-APE group. Other patient characteristics showed no statistical significance between these two groups.
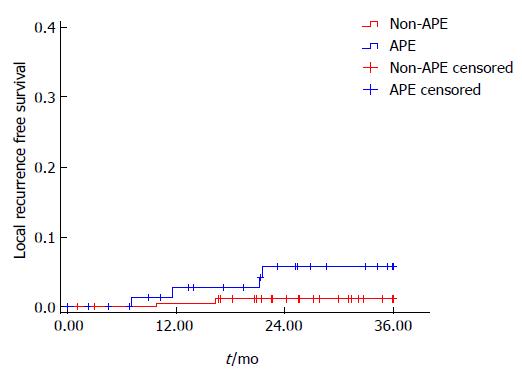
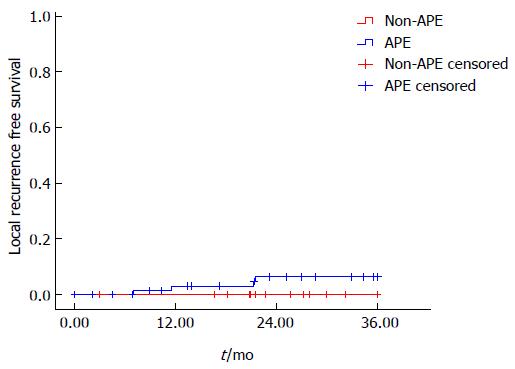
The LR rate was 5.1% (4/78) and 1.1% (2/178) in the APE and non-APE groups, respectively, with a significant difference in univariate analysis (P = 0.036) (Figure 1). On stratified analysis in the low rectal cancer group (level of tumor ≤ 5 cm), the LR rate was also significantly higher in the APE group than the non-APE group [5.6% (4/72) vs 0% (0/72), P = 0.024]. Multivariate analysis was not performed for limited events of local failure (Figure 2).
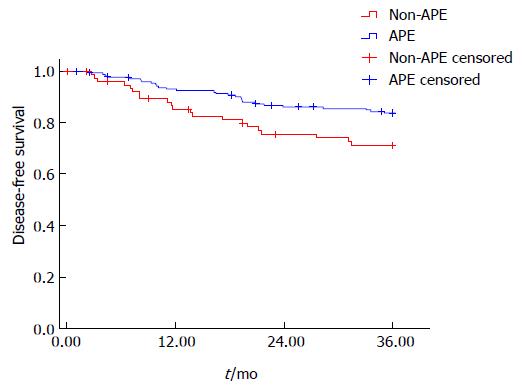
On univariate analysis, there was a significantly shortened 3 years DFS in the APE group compared to the non-APE group (74.1% vs 84.3%, P = 0.021) (Figure 3). On the multivariate COX regression model, APE procedure (HR = 2.304, 1.298-4.092, P = 0.004), ypN stage (HR = 2.288, 1.593-3.284, P = 0.000), and tumor differentiation (HR = 2.044, 1.178-3.545, P = 0.011) were independently associated DFS.
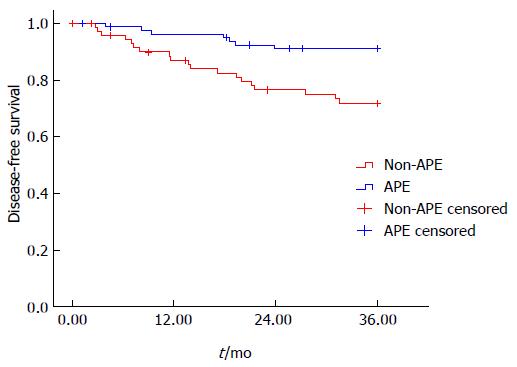
On stratified analysis in the group of low rectal cancer (level of tumor ≤ 5 cm), the 3 years DFS was also significantly lower in the APE group than the non-APE group (91.5% vs 73.6%, P = 0.002) (Figure 4). On the multivariate COX regression model, APE procedure (HR = 3.810, 1.544-9.403, P = 0.004), gender (HR = 2.318, 0.983-5.464, P = 0.055), ypT stage (HR = 2.671, 1.303-5.474, P = 0.007), and ypN stage (HR = 3.839, 2.181-6.757, P = 0.000) were independently associated DFS in lower rectal cancer.
APE has been the standard surgery for the lower rectum. In last few decades, more sphincter preserving surgeries could be performed with a negative distal margin following the introduction of total mesorectal excision and novel stapling devices. Furthermore, the rate of APE has decreased with the application of nRT, which caused tumor regression and the downstaging effect. Good response to nRT enhances anal sphincter preservation in rectal cancer patients[4,31-34]. Nevertheless, for distal bulky tumors which invade the levator ani or sphincter, APE remains the first choice for treatment.
Previous studies concluded that APE was associated with a higher local recurrence rate[8,10,12,13,15,35,36] and decreased disease-free or cancer-specific survival than the non-APE procedure[10,12,15,35,37]. In many studies, the unfavorable prognosis of APE compared with non-APE could be explained by the high incidence of CRM involvement. Wibe et al[13] reported that the APE procedure was associated with a significantly higher incidence of positive CRM involvement (12% vs 5%, P = 0.01) when compared with anterior resection (AR). Consequently, patients who received APE had worse local control (10% vs 15%, P = 0.008) and poorer overall survival (55% vs 68%, P < 0.001). Nagtegaal et al[14] also investigated the results from the Dutch TME trial and found more positive CRMs presented in the patients operated with APE compared to AR (30.4% vs 10.7%, P = 0.002). Overall survival differed greatly between APE and AR (38.5% vs 57.6%, P = 0.008). Similar results were reported in many other studies[11,15-17].
Despite the higher involved CRM rate being accepted as the main cause for the poorer prognosis of APE, the strategy for performing APE is still multifactorial. Apparently lower and advanced tumors are treated by APE rather than non-APE. Some authors indicated that patients who received APE had more advanced T stage than those received non-APE[7,37], while others found more advanced TNM stage in the APE group[13,15,18,38-40]. Consequently, neoadjuvant radiotherapy was more frequently adopted in these high-risk patients for better local control, even without the expectation of sphincter preservation[10,13,18,40]. For the heterogeneous factors above that might induce selection bias, it was difficult to analyze whether the technique of the APE procedure or pre-existing oncological risks would be responsible for the poorer outcome of APE.
The present study was designed to analyze the prognostic value of type of surgery in patients following nRT when excluding CRM positive cases. In our study, a significant higher incidence of lower tumor (92.3% vs 46.1%, P = 0.000), higher median BMI (64.1% vs 45.5%, P = 0.006), and male patients (51.7% vs 67.9%, P = 0.016) was observed in the APE group compared to the non-APE group. It is not surprising that the location of tumor predominates over other factors when making a decision regarding type of surgery. Male gender and a higher BMI were mainly associated with increased difficulty to perform sphincter preservation procedure with a safe margin. Tumor-related factors such as tumor differentiation, TRG, LVI, T stage, N stage, and TNM stage were not significantly different between the APE and non-APE groups following nRT.
Our results revealed that APE itself was an independent risk factor in patients with a negative margin. Although with pathological confirmed surgical radicality, the local recurrence rate in the APE group was statistically higher than the non-APE group (5.1% vs 1.1%, P = 0.040). On survival analysis, a difference of 3 years DFS was also significant between the APE and non-APE groups. In stratified analysis of lower rectal cancer, APE procedure is also a risk factor for local failure and disease relapse.
Reshef et al[9] reported the similar phenomenon that a higher local recurrence rate (7% vs 3%, P = 0.02) and shortened disease-free survival (54% vs 70%, P < 0.001) after APE, rather than non-APE, persisted in patients with clear CRM. Meanwhile, the proportion of patients who underwent nRT was significant higher in the APE group. Patients in the APE group also more frequently had tumors with an advanced TNM stage.
In our study, we eliminated the bias of tumor biological factors and CRM involvement for pertinent analyses. Following short-course nRT and surgery with negative CRM, the prognosis of APE was still less favorable than the non-APE group. In the Dutch TME and CR07 trial[1-3,5], the local recurrence rate of patients with negative CRM was 3.2% (22/691) and 3.3% (22/674), respectively, following short-course nRT, which was closely consistent with our results (3.3%, 6/256).
Some authors advocate that candidates for APE procedure should receive long-course chemoradiation to enhance downstaging and the downsize effect. Nevertheless, Bujko et al[6] found there was no significant difference in local recurrence (15.6% vs 10.6%, P = 0.210), despite patients having a higher incidence of complete response (16.1% vs 0.7%, P value not given) and lesser CRM involvement (4.4% vs 12.9%, P = 0.017) following long-course chemoradiotherapy than short-course nRT. New nRT regimens which have been reported with the addition of oxaliplatin or induction chemotherapy were more intensive, but failed to reveal oncological superiority[41-44].
Radiotherapy regimen in the present study was 30 Gy in 10 fractions, which was designed to have a similar biological equivalent dose as 5 × 5 Gy regimen and a prolonged interval of 2-4 wk prior to surgery to increase response rate and clinical efficiency[19-22]. Our results revealed objective pathologic downstaging and response rate, which was higher than that of published data of the traditional short-course regimen. This combination of short-course regimen and delayed surgery was also adopted in newly published studies with 8%-10% ypCR rate[45,46].
Apart from nRT, surgical technique was another factor that might influence patient prognosis. Heald et al[8] postulated that a lack of precisely definable planes for perineal dissection increased the incidence of implantation of shed cancer cells on large areas of raw surfaces and soft tissue residues in APE procedure.
In this study, APE was conventionally performed without extended resection of levator ani and coccyx. Recently, Holm et al[47,48] introduced extended APE in a prone jack-knife position and performed more cylindrical resection of low rectal cancer. With this technique, the amount of tissue beyond the internal sphincter or muscularis propria significantly increased.
Some authors recommended preoperative decision-making of type of surgery by MRI to avoid intraoperative “coning-in” of the mesorectum for those due to receive APE[48,49]. Bebenek et al[50] also evaluated low rectal specimens and preliminarily revealed extramesorectal lymphatic drainage besides the mesorectal route. These findings may elucidate the cause of local recurrence in patients with negative CRM following APE procedure. However, extended APE is still controversial in the literature[51,52].
MRI has shown a diagnostic advantage over other radiological methods[53]. In the Mercury trial, patients were classified to have a good or bad prognosis by mesorectal invasion on MRI[54]. By this means, patients with low rectal cancer should be recognized as a high-risk group because the mesorectum gradually tapers at the anorectal junction. When staging rectal cancer, MRI can visualize the construction of the anal-canal region and provide a more accurate description of the pelvic structures than was previously available[55]. Patients should also be re-staged by MRI following neoadjuvant therapy for reassessment before making surgical plan[53].
In summary, our study proved the unfavorable prognosis of rectal cancer following 30 Gy/10 F nRT and APE, even with negative CRM. More intensive neoadjuvant regimen, improved surgical technique, and accurate preoperative assessment should be recommended for low rectal cancer in the future.
The main limitation of this study was that the pathology department in our hospital did not routinely perform macroscopic assessment of resected mesorectum or record tumor perforation. Therefore, it is limited to surgical quality that was comprehensively revealed, except for CRM assessment. Despite this limitation, our data was obtained from a high volume hospital with large number of cases. We believe these findings would benefit clinical practice.
Rectal cancer following abdominoperineal excision (APE) was thought to be associated with a poorer outcome for the high incidence of positive circumferential resection margin (CRM). However, the poorer outcome of low rectal cancer following abdominoperineal excision is multifactorial. The inherent biological behavior of low rectal cancer should be evaluated with the adjustment of surgical radicality.
The research hotspot in this field is whether the inferior prognosis of low rectal cancer treated with APE is caused by the surgical procedure itself, is attributable to other factors as inherent biological behavior, or both.
The innovation and breakthrough of the present study is that we precluded CRM positive cases and compared local recurrent rate and disease-free survival in patients received APE or non-APE procedures, mitigated the influence by surgical technique related bias in analyses, and revealed the inherent biological behavior of low-lying or levator threatening rectal cancer.
The results of the present study proved the unfavorable survival of low-lying or levator threatening rectal cancer following APE and preoperative radiotherapy. More intensive neoadjuvant treatment and optimized pathways for performing APE might be needed in order to improve treatment outcomes for these patients.
This is a retrospective study focusing on the outcomes in pre-radiated rectal cancer followed by APE or non-APE. The authors precluded margin positive cases and revealed the biological behavior of low rectal cancer without the influence of technique-related factors. Their results prove that local control and disease-free survival in APE cases is worse than in non-APE cases, even with neoadjuvant radiotherapy and R0 resection.
P- Reviewers: Kang SB, Lam AK, Riss S, Zhou PH S- Editor: Qi Y L- Editor: Rutherford A E- Editor: Liu XM
| 1. | van Gijn W, Marijnen CA, Nagtegaal ID, Kranenbarg EM, Putter H, Wiggers T, Rutten HJ, Påhlman L, Glimelius B, van de Velde CJ. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12-year follow-up of the multicentre, randomised controlled TME trial. Lancet Oncol. 2011;12:575-582. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1138] [Cited by in F6Publishing: 1276] [Article Influence: 98.2] [Reference Citation Analysis (0)] |
| 2. | Sebag-Montefiore D, Stephens RJ, Steele R, Monson J, Grieve R, Khanna S, Quirke P, Couture J, de Metz C, Myint AS. Preoperative radiotherapy versus selective postoperative chemoradiotherapy in patients with rectal cancer (MRC CR07 and NCIC-CTG C016): a multicentre, randomised trial. Lancet. 2009;373:811-820. [PubMed] [Cited in This Article: ] |
| 3. | Peeters KC, Marijnen CA, Nagtegaal ID, Kranenbarg EK, Putter H, Wiggers T, Rutten H, Pahlman L, Glimelius B, Leer JW. The TME trial after a median follow-up of 6 years: increased local control but no survival benefit in irradiated patients with resectable rectal carcinoma. Ann Surg. 2007;246:693-701. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 853] [Cited by in F6Publishing: 841] [Article Influence: 49.5] [Reference Citation Analysis (0)] |
| 4. | Sauer R, Becker H, Hohenberger W, Rödel C, Wittekind C, Fietkau R, Martus P, Tschmelitsch J, Hager E, Hess CF. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731-1740. [PubMed] [Cited in This Article: ] |
| 5. | Kapiteijn E, Marijnen CA, Nagtegaal ID, Putter H, Steup WH, Wiggers T, Rutten HJ, Pahlman L, Glimelius B, van Krieken JH. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med. 2001;345:638-646. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3104] [Cited by in F6Publishing: 3030] [Article Influence: 131.7] [Reference Citation Analysis (0)] |
| 6. | Bujko K, Rutkowski A, Chang GJ, Michalski W, Chmielik E, Kusnierz J. Is the 1-cm rule of distal bowel resection margin in rectal cancer based on clinical evidence? A systematic review. Ann Surg Oncol. 2012;19:801-808. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 94] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 7. | Chuwa EW, Seow-Choen F. Outcomes for abdominoperineal resections are not worse than those of anterior resections. Dis Colon Rectum. 2006;49:41-49. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 50] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 8. | Heald RJ, Smedh RK, Kald A, Sexton R, Moran BJ. Abdominoperineal excision of the rectum--an endangered operation. Norman Nigro Lectureship. Dis Colon Rectum. 1997;40:747-751. [PubMed] [Cited in This Article: ] |
| 9. | Reshef A, Lavery I, Kiran RP. Factors associated with oncologic outcomes after abdominoperineal resection compared with restorative resection for low rectal cancer: patient- and tumor-related or technical factors only? Dis Colon Rectum. 2012;55:51-58. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 10. | Law WL, Chu KW. Abdominoperineal resection is associated with poor oncological outcome. Br J Surg. 2004;91:1493-1499. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 74] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 11. | Marr R, Birbeck K, Garvican J, Macklin CP, Tiffin NJ, Parsons WJ, Dixon MF, Mapstone NP, Sebag-Montefiore D, Scott N. The modern abdominoperineal excision: the next challenge after total mesorectal excision. Ann Surg. 2005;242:74-82. [PubMed] [Cited in This Article: ] |
| 12. | den Dulk M, Putter H, Collette L, Marijnen CA, Folkesson J, Bosset JF, Rödel C, Bujko K, Påhlman L, van de Velde CJ. The abdominoperineal resection itself is associated with an adverse outcome: the European experience based on a pooled analysis of five European randomised clinical trials on rectal cancer. Eur J Cancer. 2009;45:1175-1183. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 146] [Cited by in F6Publishing: 156] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 13. | Wibe A, Syse A, Andersen E, Tretli S, Myrvold HE, Søreide O. Oncological outcomes after total mesorectal excision for cure for cancer of the lower rectum: anterior vs. abdominoperineal resection. Dis Colon Rectum. 2004;47:48-58. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 340] [Cited by in F6Publishing: 326] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 14. | Nagtegaal ID, van de Velde CJ, Marijnen CA, van Krieken JH, Quirke P. Low rectal cancer: a call for a change of approach in abdominoperineal resection. J Clin Oncol. 2005;23:9257-9264. [PubMed] [Cited in This Article: ] |
| 15. | Ptok H, Marusch F, Kuhn R, Gastinger I, Lippert H. Influence of hospital volume on the frequency of abdominoperineal resection and long-term oncological outcomes in low rectal cancer. Eur J Surg Oncol. 2007;33:854-861. [PubMed] [Cited in This Article: ] |
| 16. | Tilney HS, Tekkis PP, Sains PS, Constantinides VA, Heriot AG. Factors affecting circumferential resection margin involvement after rectal cancer excision. Dis Colon Rectum. 2007;50:29-36. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 35] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 17. | Youssef H, Collantes EC, Rashid SH, Wong LS, Baragwanath P. Rectal cancer: involved circumferential resection margin - a root cause analysis. Colorectal Dis. 2009;11:470-474. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 18. | Shihab OC, Brown G, Daniels IR, Heald RJ, Quirke P, Moran BJ. Patients with low rectal cancer treated by abdominoperineal excision have worse tumors and higher involved margin rates compared with patients treated by anterior resection. Dis Colon Rectum. 2010;53:53-56. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 94] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 19. | Du CZ, Xue WC, Cai Y, Li M, Gu J. Lymphovascular invasion in rectal cancer following neoadjuvant radiotherapy: a retrospective cohort study. World J Gastroenterol. 2009;15:3793-3798. [PubMed] [Cited in This Article: ] |
| 20. | Wang L, Gu J. Risk factors for symptomatic anastomotic leakage after low anterior resection for rectal cancer with 30 Gy/10 f/2 w preoperative radiotherapy. World J Surg. 2010;34:1080-1085. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 21. | Du C, Xue W, Li J, Cai Y, Gu J. Morphology and prognostic value of tumor budding in rectal cancer after neoadjuvant radiotherapy. Hum Pathol. 2012;43:1061-1067. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 22. | Peng Y, Wang L, Du C, Gu J. Expression of vascular endothelial growth factor can predict distant metastasis and disease-free survival for clinical stage III rectal cancer following 30-Gy/10-f preoperative radiotherapy. Int J Colorectal Dis. 2012;27:1555-1560. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 23. | Heald RJ. Total mesorectal excision is optimal surgery for rectal cancer: a Scandinavian consensus. Br J Surg. 1995;82:1297-1299. [PubMed] [Cited in This Article: ] |
| 24. | Nagtegaal ID, van de Velde CJ, van der Worp E, Kapiteijn E, Quirke P, van Krieken JH. Macroscopic evaluation of rectal cancer resection specimen: clinical significance of the pathologist in quality control. J Clin Oncol. 2002;20:1729-1734. [PubMed] [Cited in This Article: ] |
| 25. | Quirke P. Training and quality assurance for rectal cancer: 20 years of data is enough. Lancet Oncol. 2003;4:695-702. [PubMed] [Cited in This Article: ] |
| 26. | Quirke P, Steele R, Monson J, Grieve R, Khanna S, Couture J, O’Callaghan C, Myint AS, Bessell E, Thompson LC. Effect of the plane of surgery achieved on local recurrence in patients with operable rectal cancer: a prospective study using data from the MRC CR07 and NCIC-CTG CO16 randomised clinical trial. Lancet. 2009;373:821-828. [PubMed] [Cited in This Article: ] |
| 27. | Wheeler JM, Warren BF, Mortensen NJ, Ekanyaka N, Kulacoglu H, Jones AC, George BD, Kettlewell MG. Quantification of histologic regression of rectal cancer after irradiation: a proposal for a modified staging system. Dis Colon Rectum. 2002;45:1051-1056. [PubMed] [Cited in This Article: ] |
| 28. | Wheeler JM, Dodds E, Warren BF, Cunningham C, George BD, Jones AC, Mortensen NJ. Preoperative chemoradiotherapy and total mesorectal excision surgery for locally advanced rectal cancer: correlation with rectal cancer regression grade. Dis Colon Rectum. 2004;47:2025-2031. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 100] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 29. | Nagtegaal ID, Marijnen CA, Kranenbarg EK, van de Velde CJ, van Krieken JH. Circumferential margin involvement is still an important predictor of local recurrence in rectal carcinoma: not one millimeter but two millimeters is the limit. Am J Surg Pathol. 2002;26:350-357. [PubMed] [Cited in This Article: ] |
| 30. | de Gramont A, Hubbard J, Shi Q, O’Connell MJ, Buyse M, Benedetti J, Bot B, O’Callaghan C, Yothers G, Goldberg RM. Association between disease-free survival and overall survival when survival is prolonged after recurrence in patients receiving cytotoxic adjuvant therapy for colon cancer: simulations based on the 20,800 patient ACCENT data set. J Clin Oncol. 2010;28:460-465. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 31. | Janjan NA, Khoo VS, Abbruzzese J, Pazdur R, Dubrow R, Cleary KR, Allen PK, Lynch PM, Glober G, Wolff R. Tumor downstaging and sphincter preservation with preoperative chemoradiation in locally advanced rectal cancer: the M. D. Anderson Cancer Center experience. Int J Radiat Oncol Biol Phys. 1999;44:1027-1038. [PubMed] [Cited in This Article: ] |
| 32. | Luna-Pérez P, Rodríguez-Ramírez S, Rodriguez-Coria DF, Fernández A, Labastida S, Silva A, López MJ. Preoperative chemoradiation therapy and anal sphincter preservation with locally advanced rectal adenocarcinoma. World J Surg. 2001;25:1006-1011. [PubMed] [Cited in This Article: ] |
| 33. | Gavioli M, Losi L, Luppi G, Iacchetta F, Zironi S, Bertolini F, Falchi AM, Bertoni F, Natalini G. Preoperative therapy for lower rectal cancer and modifications in distance from anal sphincter. Int J Radiat Oncol Biol Phys. 2007;69:370-375. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 34. | Kim NK, Baik SH, Min BS, Pyo HR, Choi YJ, Kim H, Seong J, Keum KC, Rha SY, Chung HC. A comparative study of volumetric analysis, histopathologic downstaging, and tumor regression grade in evaluating tumor response in locally advanced rectal cancer following preoperative chemoradiation. Int J Radiat Oncol Biol Phys. 2007;67:204-210. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 52] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 35. | Bonadeo FA, Vaccaro CA, Benati ML, Quintana GM, Garione XE, Telenta MT. Rectal cancer: local recurrence after surgery without radiotherapy. Dis Colon Rectum. 2001;44:374-379. [PubMed] [Cited in This Article: ] |
| 36. | García-Granero E, Martí-Obiol R, Gómez-Barbadillo J, García-Armengol J, Esclapez P, Espí A, Jiménez E, Millán M, Lledó S. Impact of surgeon organization and specialization in rectal cancer outcome. Colorectal Dis. 2001;3:179-184. [PubMed] [Cited in This Article: ] |
| 37. | Weiser MR, Quah HM, Shia J, Guillem JG, Paty PB, Temple LK, Goodman KA, Minsky BD, Wong WD. Sphincter preservation in low rectal cancer is facilitated by preoperative chemoradiation and intersphincteric dissection. Ann Surg. 2009;249:236-242. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 169] [Cited by in F6Publishing: 172] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 38. | Nakagoe T, Ishikawa H, Sawai T, Tsuji T, Tanaka K, Hidaka S, Nanashima A, Yamaguchi H, Yasutake T. Survival and recurrence after a sphincter-saving resection and abdominoperineal resection for adenocarcinoma of the rectum at or below the peritoneal reflection: a multivariate analysis. Surg Today. 2004;34:32-39. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 39. | Chiappa A, Biffi R, Bertani E, Zbar AP, Pace U, Crotti C, Biella F, Viale G, Orecchia R, Pruneri G. Surgical outcomes after total mesorectal excision for rectal cancer. J Surg Oncol. 2006;94:182-193; discussion 181. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 33] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 40. | Chambers W, Hancock L, McKenzie R, Buchel O, Lindsey I, Cunningham C, George B, Mortensen N. Changes in the management and outcome of rectal cancer over a 10-year period in Oxford. Colorectal Dis. 2011;13:1004-1008. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 41. | Aschele C, Cionini L, Lonardi S, Pinto C, Cordio S, Rosati G, Artale S, Tagliagambe A, Ambrosini G, Rosetti P. Primary tumor response to preoperative chemoradiation with or without oxaliplatin in locally advanced rectal cancer: pathologic results of the STAR-01 randomized phase III trial. J Clin Oncol. 2011;29:2773-2780. [PubMed] [Cited in This Article: ] |
| 42. | Fernández-Martos C, Pericay C, Aparicio J, Salud A, Safont M, Massuti B, Vera R, Escudero P, Maurel J, Marcuello E. Phase II, randomized study of concomitant chemoradiotherapy followed by surgery and adjuvant capecitabine plus oxaliplatin (CAPOX) compared with induction CAPOX followed by concomitant chemoradiotherapy and surgery in magnetic resonance imaging-defined, locally advanced rectal cancer: Grupo cancer de recto 3 study. J Clin Oncol. 2010;28:859-865. [PubMed] [Cited in This Article: ] |
| 43. | Gérard JP, Azria D, Gourgou-Bourgade S, Martel-Laffay I, Hennequin C, Etienne PL, Vendrely V, François E, de La Roche G, Bouché O. Comparison of two neoadjuvant chemoradiotherapy regimens for locally advanced rectal cancer: results of the phase III trial ACCORD 12/0405-Prodige 2. J Clin Oncol. 2010;28:1638-1644. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 547] [Cited by in F6Publishing: 559] [Article Influence: 39.9] [Reference Citation Analysis (0)] |
| 44. | Gérard JP, Azria D, Gourgou-Bourgade S, Martel-Lafay I, Hennequin C, Etienne PL, Vendrely V, François E, de La Roche G, Bouché O. Clinical outcome of the ACCORD 12/0405 PRODIGE 2 randomized trial in rectal cancer. J Clin Oncol. 2012;30:4558-4565. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 289] [Cited by in F6Publishing: 286] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 45. | Faria S, Kopek N, Hijal T, Liberman S, Charlebois P, Stein B, Meterissian S, Meguerditchian A, Fawaz Z, Artho G. Phase II trial of short-course radiotherapy followed by delayed surgery for locoregionally advanced rectal cancer. Colorectal Dis. 2014;16:O66-O70. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 46. | Pettersson D, Holm T, Iversen H, Blomqvist L, Glimelius B, Martling A. Preoperative short-course radiotherapy with delayed surgery in primary rectal cancer. Br J Surg. 2012;99:577-583. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 123] [Cited by in F6Publishing: 128] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 47. | West NP, Finan PJ, Anderin C, Lindholm J, Holm T, Quirke P. Evidence of the oncologic superiority of cylindrical abdominoperineal excision for low rectal cancer. J Clin Oncol. 2008;26:3517-3522. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 304] [Cited by in F6Publishing: 321] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 48. | Holm T, Ljung A, Häggmark T, Jurell G, Lagergren J. Extended abdominoperineal resection with gluteus maximus flap reconstruction of the pelvic floor for rectal cancer. Br J Surg. 2007;94:232-238. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 497] [Cited by in F6Publishing: 429] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 49. | Shihab OC, Heald RJ, Holm T, How PD, Brown G, Quirke P, Moran BJ. A pictorial description of extralevator abdominoperineal excision for low rectal cancer. Colorectal Dis. 2012;14:e655-e660. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 50. | Bebenek M, Wojnar A. Infralevator lymphatic drainage of low-rectal cancers: preliminary results. Ann Surg Oncol. 2009;16:887-892. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 51. | Moore TJ, Moran BJ. Precision surgery, precision terminology: the origins and meaning of ELAPE. Colorectal Dis. 2012;14:1173-1174. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 52. | Asplund D, Haglind E, Angenete E. Outcome of extralevator abdominoperineal excision compared with standard surgery: results from a single centre. Colorectal Dis. 2012;14:1191-1196. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 66] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 53. | MERCURY Study Group. Extramural depth of tumor invasion at thin-section MR in patients with rectal cancer: results of the MERCURY study. Radiology. 2007;243:132-139. [PubMed] [Cited in This Article: ] |
| 54. | Taylor FG, Quirke P, Heald RJ, Moran B, Blomqvist L, Swift I, Sebag-Montefiore DJ, Tekkis P, Brown G. Preoperative high-resolution magnetic resonance imaging can identify good prognosis stage I, II, and III rectal cancer best managed by surgery alone: a prospective, multicenter, European study. Ann Surg. 2011;253:711-719. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 429] [Cited by in F6Publishing: 421] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 55. | Guo M, Gao C, Li D, Guo W, Shafik AA, Zbar AP, Pescatori M. MRI anatomy of the anal region. Dis Colon Rectum. 2010;53:1542-1548. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |