Published online Jul 14, 2014. doi: 10.3748/wjg.v20.i26.8449
Revised: February 14, 2014
Accepted: March 12, 2014
Published online: July 14, 2014
Processing time: 251 Days and 9.4 Hours
There have been major developments in endoscopic imaging techniques in recent years. Endoscopes with high definition and magnification can provide high quality images that allow for the histological estimation of lesions in vivo and in situ when combined with ancillary enhancement techniques such as chromoendoscopy (CE) and virtual CE (narrow band imaging fujinon intelligent chromoendoscopy, or i-Scan). Despite the enormous potential for these advanced techniques, their value and feasibility in the clinic are still doubted, particularly in cases of colonic polyps that are slated for removal, where in vivo characterization may be deemed unnecessary. However, there are several advantages offered by such advanced endoscopic imaging. CE with or without magnification demonstrates highly accurate histology and invasion depth prediction, and virtual CE is a feasible and less cumbersome alternative to CE in terms of histological estimation, though not sufficiently accurate for depth invasion prediction. Furthermore, the supplementary information provided by advanced imaging systems can assist the endoscopist in the selection of a strategic approach, such as in deciding whether a colonic lesion should be resected, left in situ, or requires more intensive surgical treatment. Lastly, advanced high-resolution imaging techniques may be more cost effective, such that histopathology of low-risk lesions following resection can be eliminated. The results of these evaluations and comparisons with traditional CE are presented and discussed. Taken together, the benefits provided by these advanced capabilities justify their development, and advocates their use for the treatment and management of colonic polyps.
Core tip: Endoscopic characterization of colonic polyps by “virtual histology” is a currently accessible tool for identification of lesion type, thus enabling endoscopists to determine optimal treatment strategies. Chromoendoscopy (CE) has shown high accuracy for differentiating polyp histologies (neoplastic vs non-neoplastic) and for estimating the depth of invasion. Furthermore, digital systems such as narrow band imaging are a viable alternative to CE regarding lesion characterization, ease of use, reversibility, and cleanliness.
- Citation: Lopez-Ceron M, Sanabria E, Pellise M. Colonic polyps: Is it useful to characterize them with advanced endoscopy? World J Gastroenterol 2014; 20(26): 8449-8457
- URL: https://www.wjgnet.com/1007-9327/full/v20/i26/8449.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i26.8449
Colorectal cancer (CRC) is the second most common cancer in women and the third most common in men from developed countries[1], which contrasts with the preventable nature of gastrointestinal cancers. While colorectal adenomas have long been considered as CRC precursors, recent evidence shows that that 20%-30% of all CRCs emerge from a distinct group of lesions, generically named serrated polyps, consisting of traditional serrated adenomas, sessile serrated adenomas (SSA) and hyperplastic polyps (HP)[2]. While HPs are generally understood to show little or no potential for degeneration, the other serrated polyps are capable of malignant transformation, similar to classic adenomas[3]. Currently, endoscopy is the most viable method for CRC prevention, which not only allows for diagnosis, but also for the removal of premalignant lesions. Furthermore, in addition to its preventative effects, endoscopic polypectomy has also been shown to reduce CRC mortality[4].
Substantial progress in the advancement of endoscopic imaging has been achieved in recent years. The acquisition of higher quality images allows for visualization of subtle details in the gastrointestinal mucosa. Prediction of lesion type prior to histological processing is made possible through examination of mucosal surface architecture and microvasculature, a method referred to as “virtual histology” or “optical biopsy.”
The prediction of polyp histology and degree of invasion depth that can be ascertained with virtual histology has important therapeutic implications. Before resecting a lesion, the endoscopist must determine if the lesion contains an invasive cancer, if it can be left in situ, and if required, what the optimal resection method is. Enhanced endoscopic imaging can assist the endoscopist in these determinations. Whereas resection of small HPs (≤ 5 mm) of the rectum and sigmoid colon, juvenile polyps and inflammatory polyps is not necessary considering their non-existent potential for degeneration, endoscopic resection is generally suitable for the remaining lesion types, excluding those containing cancer foci in deeper layers that require surgical management. Tumors with superficial submucosal invasion (< 1000 μm, Vienna classification sm-1)[5] have a negligible rate of lymph node metastasis. Hence, endoscopic resection of these lesions may be the definitive treatment provided they are well- or moderately-differentiated, do not show vascular or lymphatic invasion, and the resection margin is > 1 mm[6]. Importantly, these types of lesions can be accurately identified as a result of advancements in endoscopic imaging and ancillary techniques.
High-definition endoscopes display images of a superior quality, and the use of magnification devices allows for a highly detailed inspection of the mucosal surface. Whereas amplified digital images lack sharpness, these optical systems can achieve a × 150 magnification without loss of resolution[7]. In addition to these advances, there are auxiliary enhancement techniques that provide additional detail. The use of optical systems is generally implied in studies using magnification, yet endoscopes providing optical magnification are not available in the United States or in Europe (with some exceptions in the United Kingdom and a few other European centers). Thus, studies using this technology are mainly from Asian countries.
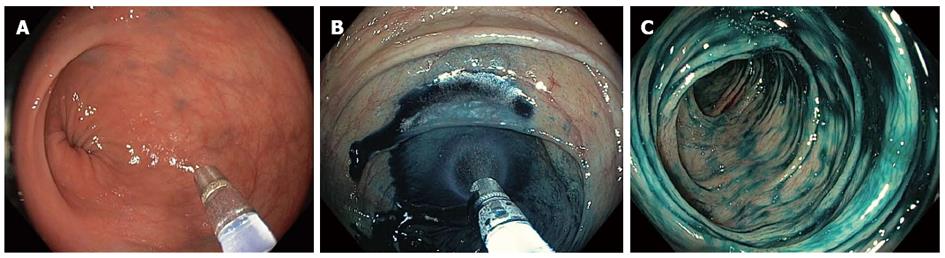
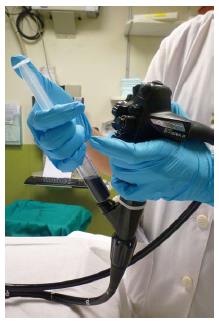
Chromoendoscopy (CE) is the topical instillation of dye through an endoscope channel to highlight small details of the mucosa. The two most commonly used dye types are vital or absorptive dyes that are taken up by intestinal cells, such as methylene blue and crystal violet, and contrast dyes such as indigo carmine, which are deposited in the mucosal grooves and enhance subtle mucosal unevenness. Before the dyes are administered, a solution containing mucolytic and de-foaming agents (for example, 300 mg of N-acetylcysteine and 100 mg of simethicone per liter of water) is pumped through the tissue to remove mucus and fecal remains. Dyes can be applied with a catheter for the staining of large mucosal areas (Figure 1), or directly through the endoscope channel with the aid of a syringe (Figure 2) (e.g., 5 cc of dye followed by 15 cc of air in a 20 cc syringe) for occasional application. Absorptive contrasts should be left to stand after instillation for one minute before rinsing. Generally, methylene blue is used at a concentration of 0.1%, while indigo carmine is used at concentrations of 0.1%-0.5% for large and small areas, respectively. Due to its potential toxicity[7], 0.05% crystal violet should be applied in small quantities with a special non-traumatic catheter combined with magnification devices.
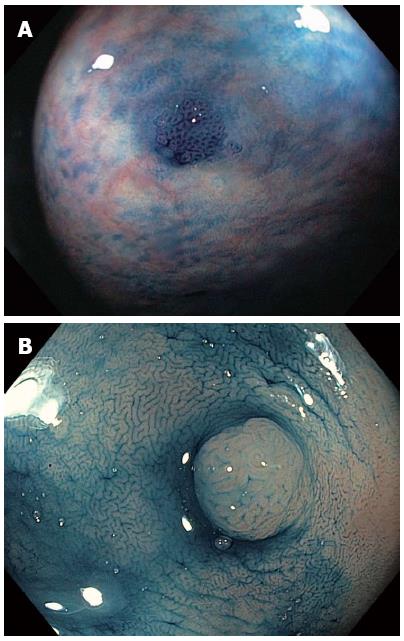
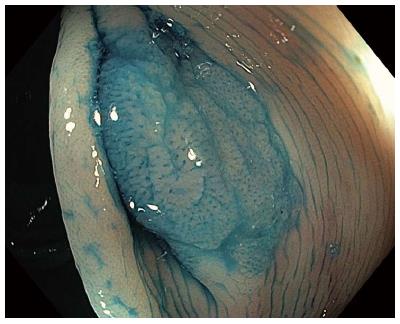
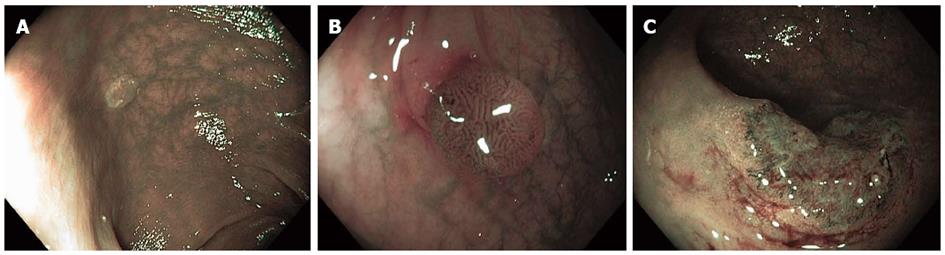
CE has been reliably used to distinguish neoplastic from non-neoplastic lesions. Additionally, it can predict the degree of invasion of colorectal tumors with high accuracy. Pit patterns of colonic lesions have been classified by Kudo et al[8] into seven categories (Table 1). To simply this complex classification for clinical use, pit patterns are grouped into three basic types: non-neoplastic lesions, Kudo I or II that do not require treatment (Figure 3A); Kudo non-invasive neoplasia, Kudo IIIs, IIIL, IV and selected cases of Vi, corresponding to adenomas and cancers with superficial submucosal invasion that are endoscopically treatable (Figure 3B); and invasive, Kudo Vn and some Vi, requiring surgical treatment due to deep submucosal invasion[9]. Invasive patterns are typically not evenly distributed throughout the lesion, and usually found in lesions located in confined areas with steep edges, depressions or nodules larger than 1 cm, thereby necessitating the use of magnification for identification. Using magnification endoscopy for pit pattern assessment, Japanese studies have demonstrated that neoplastic lesions can be distinguished from non-neoplastic lesions with an accuracy of up to 99.1%[10], and the degree of invasion (superficial vs deep) can be estimated in up to 98.8% of cases[11]. Furthermore, the application of these pattern assessments shows inter- and intra-observer agreement of 72%-86%[12]. CE using high-resolution endoscopes without magnification also allows for differentiation of neoplastic from non-neoplastic lesions, though with slightly reduced accuracy (87%-92%)[13-16].
| Kudo et al[8] class | Description | Most likely histology | Neoplastic/non-neoplastic | Treatment |
| I | Round crypts | Normal mucosa | Non-neoplastic | None |
| II | Regular wider or stellar crypts | Hyperplastic polyp (also sessile serrated adenomas) | Non-neoplastic | None if rectosigmoid and ≤ 5 mm/endoscopic resection |
| IIIL | Elongated or roundish crypts | Adenoma | Neoplastic | Endoscopic resection |
| IIIs | Tubular or roundish pits smaller than the normal crypts | Intramucosal/superficial invasive cancer | Neoplastic | Endoscopic resection |
| IV | Branch-like or gyrus-like crypts | Adenoma | Neoplastic | Endoscopic resection |
| Vi | Irregular crypts | Superficial invasive/deep invasive cancer | Neoplastic | Surgery/endoscopic resection in selected cases |
| Vn | Non-structural crypts | Deep submucosal invasive cancer | Neoplastic | Surgery |
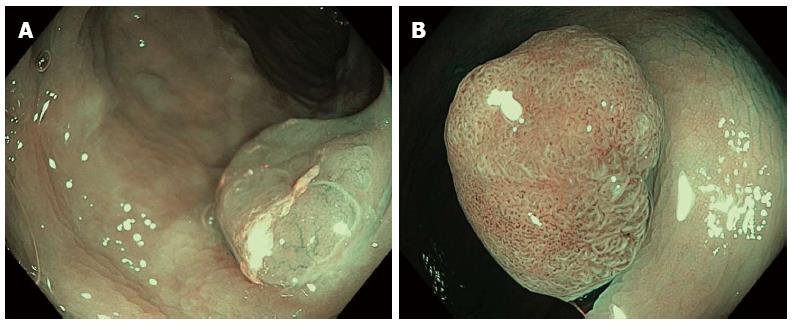
The recent rise in cases of serrated polyps (SP) highlights limitations in the simple categorization of neoplastic and non-neoplastic lesions for identification of malignant types. Although SPs are generally non-neoplastic and show no dysplasia, their resection is indicated considering their potential for malignancy[17], except in instances of diminutive (≤ 5 mm) HPs in the rectum or sigmoid colon. These HPs are not considered malignant and so can be left in situ. Consequently, the method for classification should be amended to accurately distinguish between adenoma, SSA and HP lesion types. Specifically, endoscopic differentiation between SSA and HP is indeed valuable as not all serrated lesions have the same malignant potential, with some indications that HPs can have a more benign behavior than SSAs and adenomas, though both HPs and diminutive SSAs can be located in the sigmoid colon and rectum. Although medical literature regarding endoscopic characterization of SPs is heterogeneous and often confusing, SSAs and HPs are typically described as pale and flat lesions, exhibiting poorly defined edges and often a layer of mucus or stool attached to their surface. The pit pattern in SSA was evaluated in a Japanese study with CE and magnification. A new category with wider and rounder crypts was thus described (type II-O) (Figure 4), which results in a more accurate (81%) diagnosis of SSA[18].
For its proven clinical applications, CE represents the benchmark for enhanced imaging techniques that have been subsequently developed. However, as the technique is labor- and time-intensive, CE has not been universally implemented, and thus has precipitated the development of several digital CE devices. Digital CE techniques provide similar information to standard CE, with the advantage of reversibility (turned on and off by pressing a button on the head of the endoscope), speed, and hygiene. All of them provide a high-resolution image. The first system marketed was narrow band imaging (NBI) by Olympus Medical Systems (Tokyo, Japan). Latter systems were designed and commercialized to provide a similar view via digital image post-processing, such as fujinon intelligent chromo endoscopy (FICE) from Fujinon (Saitama, Japan) and i-Scan from Pentax (Tokyo, Japan).
NBI is an endoscopic enhancement tool based on spectrum filtration to only allow the passage of green and blue light, thereby providing contrasted images that highlight subtle mucosal changes, revealing the inner structure and vascular pattern of the tissue. Thus, NBI provides information not only on the surface architecture as traditional CE, but also on vascularization, both of which are altered in neoplastic lesions of the gastrointestinal tract. Comparisons of Kudo’s pit patterns determined by NBI and CE show considerable but not total agreement[19-21]. NBI allows for the evaluation of vascularization intensities with relative ease, which are often not consistently reported, providing diagnostic accuracy equal or superior to the pit pattern[22,23]. Neoplastic lesions exhibit a dark color due to high vascularity, in contrast with lighter-colored non-neoplastic tissues. The combination of pit pattern and vascularization intensity is the most common diagnostic criterion, reaching a high diagnostic accuracy with (90%-91%)[22,23] and without (80%-95%)[24-28] magnification (Figure 5).
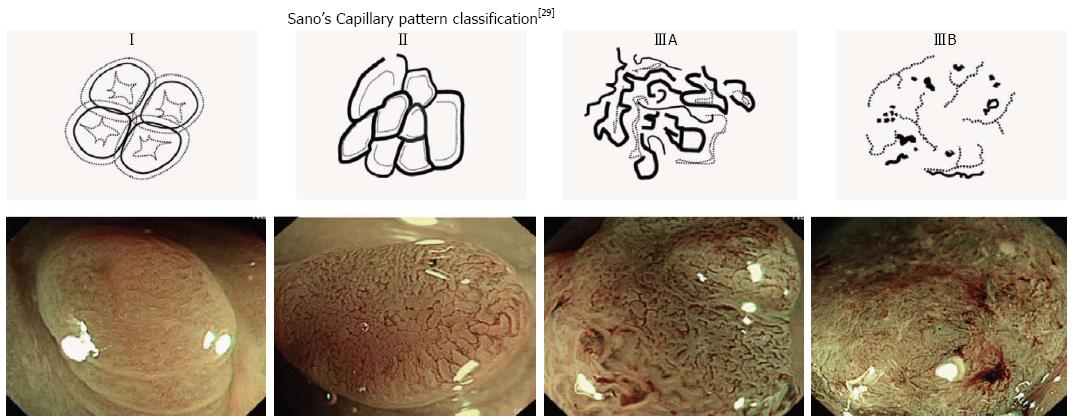
Several classification strategies have been documented in the literature for the assessment of NBI technology. One report evaluating the use of NBI without magnification classified results using a confidence measure, with an observation of at least one histological characteristic designated as a “high confidence” diagnosis prediction, and a “low confidence” prediction indicating there were questionable traits or features belonging to both neoplastic and non-neoplastic categories[28]. The determination of diagnostic predictions with high confidence allows therapeutic decisions to be made at the time of endoscopy. A classification based on capillary pattern (CP) has been introduced by Sano et al[29] for use with magnification endoscopes (Table 2, Figure 6). This classification method has demonstrated reliable discrimination of category CP I (non-neoplastic lesions), with correct classification of 95.3% of lesions[30]. Application of Sano’s classification without the use of magnification yields reduced but satisfactory accuracy (91%)[31]. Similar accuracies have also been reported by studies directly comparing the performance of NBI against CE to distinguish non-neoplastic from neoplastic lesions[19,21,32,33].
| Sano et al[29] classification | ||||
| CPI | CPII | CPIIIA | CPIIIB | |
| Capillary pattern | Absent | Present | Present | |
| Meshed capillary vessels with blind endings, branching and curtailed irregularity | ||||
| Capillary characteristics | - | Vessels surrounding glands | Lack of uniformity, high density of vessels | Nearly avascular or loose microvessels |
| Most likely histology | Normal or hyperplastic polyp | Adenoma or intramucosal carcinoma | Superficial submucosal invasive carcinoma (sm1) | Deep submucosal invasive carcinoma |
| Treatment | None | Polypectomy or endoscopic mucosal resection | Polypectomy or endoscopic mucosal resection/surgery | Surgery |
Recently, a study using high-resolution white-light images and NBI described several characteristic features of SSA, including a cloud-like surface, indistinct borders, irregular shape, and dark spots within the crypts. The presence of all these features was sufficient to distinguish SSA from HP with an accuracy of 93%[34]. Moreover, the ability to estimate the degree of invasion of colorectal neoplasms with NBI has also been assessed, largely in Asian studies using magnification with a focus on microvasculature characteristics. Application of Sano’s classification allowed for the discrimination of cancerous lesions with superficial submucosal invasion (sm1) from those with deeper invasion with an accuracy of 87.7% (sensitivity 84.8% and specificity 88.7%)[35], though accuracy obtained by CE with magnification is superior (98.8%)[11]. Hence, CE with magnification is preferred in cases with suspected invasive lesions.
Since the use of magnification is not widespread, a joint classification with Asian and Western endoscopists has been recently designed. NICE classification (NBI International Colorectal Endoscopic Classification)[36] is based on polyp color as well as surface and vessel patterns (Table 3, Figure 7). Although these features are best visualized with magnification, NICE categorization is still feasible without it, as preliminary studies differentiated Type 1 from Type 2 in polyps < 1 cm with a sensitivity of 98% and negative predictive value of 95%[37].
| Type 1 | Type 2 | Type 3 | |
| Color | Same or lighter than background | Brown | Brown or dark brown Occasional white patchy areas |
| Vessels | None or isolated lacy vessels | Brown vessels surrounding white structures | Areas of disrupted or missing vessels |
| Surface pattern | Dark or white spots of uniform size or homogeneous absence of pattern | Oval, tubular or branched white structures surrounded by brown vessels | Amorphous or absent surface pattern |
| Most likely histology | Hyperplastic polyp | Adenoma or intramucosal or superficial submucosal invasive carcinoma (sm1) | Deep submucosal invasive carcinoma |
| Treatment | None | Polypectomy or endoscopic mucosal resection | Surgery |
The characterization of lesions with NBI has been applied in clinical practice using a “Resect and Discard” concept[38]. Histological analysis and endoscopic resection can be avoided under certain conditions with this strategy, such as in cases with HPs smaller than 5 mm in the rectum and sigmoid. Furthermore, lesions with low carcinogenic potential (adenomas less than 10 mm) can be safely removed and discarded also without a need for histopathological analysis. In both cases, prediction of histology needs to be done with a high degree of confidence, and lesions greater than 10 mm should routinely be sent for histology. This strategy also allows for the establishment of a proper surveillance interval immediately following the colonoscopy, without a need to wait for the pathology report. Initial evaluations of this strategy demonstrated predictions with a high degree of confidence in 89% of polyps and allocation of proper surveillance intervals in 98% of cases[38].
It has been estimated that application of this strategy in the United States would save $33 million dollars annually[39]. The American Association of Gastrointestinal Endoscopy (ASGE) has subsequently stated that polyps ≤ 5 mm can safely be resected and discarded only after an optical diagnosis with a high degree of confidence has been performed. To determine if these smaller lesions in the sigmoid colon or rectum can be safely left in situ, optical diagnostic methods should provide a negative predictive value (NPV) for the diagnosis of adenoma of at least 90%. Moreover, in combination with routine histology of polyps > 5 mm, proper surveillance intervals should be achieved in at least 90% of cases[40]. NBI achieves these requirements when performed by proficient endoscopists, with one recent study demonstrating proper surveillance intervals in polyps ≤ 5 mm in 92%-99% of cases (according to American or European guidelines, respectively), and a 92% NPV for the diagnosis of rectosigmoid adenomas[41]. However, this threshold was not reached when performed by non-academic centers or non-tertiary hospitals[42-44]. Therefore, NBI interpretation should be applied with caution before making these results generalizable in clinical practice. Furthermore, the medicolegal consequences of the “Resect and Discard” strategy must be taken into account. While the resection and pathologic assessment of low risk lesions carries a huge time and cost burden, advanced histological features (i.e., villous component, high grade dysplasia, or cancer), though rare, can occur in lesions < 1 cm[45]. The guidelines of endoscopy societies should therefore provide legal standards and include recommendations regarding advanced imaging interpretation to support endoscopists. In this regard, it has been proposed that high quality pictures of discarded and un-resected lesions should be included in the endoscopic report[46].
Alternative digital CE techniques, such FICE and i-Scan, have not been as widely implemented as NBI, thus less evidence is available for evaluation and comparison. As FICE and i-SCAN enhance vascularization (with additional selectable modes in which the surface structure is also enhanced), studies to date have been based on inspection of the vascular pattern and/or pit pattern. FICE[47-53] and i-Scan[54-59] systems have been evaluated for the discrimination of neoplastic and non-neoplastic lesions, demonstrating similar results to NBI, although poorer than CE in some cases. FICE combined with magnification has also been tested for prediction of submucosal invasion depth, with accuracies similar to NBI, but significantly lower than CE[51].
The exponential increase in lesions detected in the bowel after the implementation of colorectal cancer screening programs along with the improvements in image quality have made the endoscopic characterization of polyps especially relevant. A rigorous estimation of the histologic lesion type facilitates the determination of appropriate endoscopic treatment, if needed, or to indicate surgery. The development of ancillary techniques to white-light endoscopy like CE has enabled accurate discrimination between neoplastic and non-neoplastic lesions along with an estimation of the degree of invasion when combined with magnification systems. Nevertheless, this technique has not been fully implemented in Western countries since it is cumbersome and time-consuming. Digital CE systems, such as NBI, have attempted to overcome these drawbacks with their ease of use, reversibility and cleanliness. Their capability to accurately distinguish non-neoplastic from neoplastic lesions has been demonstrated, though their use in estimating the degree of invasion still needs further evaluation. NBI, along with the “Resect and Discard” strategy, has proven to be accurate in academic centers and tertiary hospitals, with the potential for significant financial savings. The available evidence suggests that conventional CE is the most accurate technique for the characterization of colonic polyps, though NBI is a viable alternative for experienced endoscopists. Furthermore, inclusion of recommendations regarding advanced imaging interpretation in endoscopy society guidelines will minimize legal consequences of the “Resect and Discard” strategy.
P- Reviewers: Bujanda L, Kato J, Rosty C, Sabbagh LC S- Editor: Zhai HH L- Editor: A E- Editor: Zhang DN
| 1. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23762] [Cited by in RCA: 25504] [Article Influence: 1821.7] [Reference Citation Analysis (7)] |
| 2. | Leggett B, Whitehall V. Role of the serrated pathway in colorectal cancer pathogenesis. Gastroenterology. 2010;138:2088-2100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 677] [Cited by in RCA: 722] [Article Influence: 48.1] [Reference Citation Analysis (1)] |
| 3. | Rex DK, Ahnen DJ, Baron JA, Batts KP, Burke CA, Burt RW, Goldblum JR, Guillem JG, Kahi CJ, Kalady MF. Serrated lesions of the colorectum: review and recommendations from an expert panel. Am J Gastroenterol. 2012;107:1315-129; quiz 1314, 1330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 825] [Cited by in RCA: 828] [Article Influence: 63.7] [Reference Citation Analysis (0)] |
| 4. | Winawer SJ, Zauber AG, Ho MN, O’Brien MJ, Gottlieb LS, Sternberg SS, Waye JD, Schapiro M, Bond JH, Panish JF. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med. 1993;329:1977-1981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3107] [Cited by in RCA: 3115] [Article Influence: 97.3] [Reference Citation Analysis (1)] |
| 5. | Schlemper RJ, Riddell RH, Kato Y, Borchard F, Cooper HS, Dawsey SM, Dixon MF, Fenoglio-Preiser CM, Fléjou JF, Geboes K. The Vienna classification of gastrointestinal epithelial neoplasia. Gut. 2000;47:251-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1463] [Cited by in RCA: 1533] [Article Influence: 61.3] [Reference Citation Analysis (0)] |
| 6. | Castells A, Marzo-Castillejo M, Mascort JJ, Amador FJ, Andreu M, Bellas B, Ferrández A, Ferrándiz J, Giráldez M, Gonzalo V. [Clinical practice guideline. Prevention of colorectal cancer. 2009 update. Asociación Española de Gastroenterología]. Gastroenterol Hepatol. 2009;32:717.e1-717.58. [PubMed] |
| 7. | Pellisé M, Díaz Tasende J, Balaguer F, Bustamante-Balén M, Herráiz M, Herreros de Tejada A, Gimeno-García AZ, López-Cerón M, Marín JC, Parra Blanco A. [Technical review of advanced diagnostic endoscopy in patients at high risk of colorectal cancer]. Gastroenterol Hepatol. 2012;35:278-292. [PubMed] |
| 8. | Kudo S, Rubio CA, Teixeira CR, Kashida H, Kogure E. Pit pattern in colorectal neoplasia: endoscopic magnifying view. Endoscopy. 2001;33:367-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 300] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 9. | Fujii T, Hasegawa RT, Saitoh Y, Fleischer D, Saito Y, Sano Y, Kato S. Chromoscopy during colonoscopy. Endoscopy. 2001;33:1036-1041. [PubMed] |
| 10. | Kato S, Fu KI, Sano Y, Fujii T, Saito Y, Matsuda T, Koba I, Yoshida S, Fujimori T. Magnifying colonoscopy as a non-biopsy technique for differential diagnosis of non-neoplastic and neoplastic lesions. World J Gastroenterol. 2006;12:1416-1420. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 63] [Cited by in RCA: 52] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 11. | Matsuda T, Fujii T, Saito Y, Nakajima T, Uraoka T, Kobayashi N, Ikehara H, Ikematsu H, Fu KI, Emura F. Efficacy of the invasive/non-invasive pattern by magnifying chromoendoscopy to estimate the depth of invasion of early colorectal neoplasms. Am J Gastroenterol. 2008;103:2700-2706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 259] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 12. | Huang Q, Fukami N, Kashida H, Takeuchi T, Kogure E, Kurahashi T, Stahl E, Kudo Y, Kimata H, Kudo SE. Interobserver and intra-observer consistency in the endoscopic assessment of colonic pit patterns. Gastrointest Endosc. 2004;60:520-526. [PubMed] |
| 13. | Kiesslich R, von Bergh M, Hahn M, Hermann G, Jung M. Chromoendoscopy with indigocarmine improves the detection of adenomatous and nonadenomatous lesions in the colon. Endoscopy. 2001;33:1001-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 158] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 14. | Eisen GM, Kim CY, Fleischer DE, Kozarek RA, Carr-Locke DL, Li TC, Gostout CJ, Heller SJ, Montgomery EA, Al-Kawas FH. High-resolution chromoendoscopy for classifying colonic polyps: a multicenter study. Gastrointest Endosc. 2002;55:687-694. [PubMed] |
| 15. | Fu KI, Sano Y, Kato S, Fujii T, Nagashima F, Yoshino T, Okuno T, Yoshida S, Fujimori T. Chromoendoscopy using indigo carmine dye spraying with magnifying observation is the most reliable method for differential diagnosis between non-neoplastic and neoplastic colorectal lesions: a prospective study. Endoscopy. 2004;36:1089-1093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 182] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 16. | Sonwalkar S, Rotimi O, Rembacken BJ. Characterization of colonic polyps at conventional (nonmagnifying) colonoscopy after spraying with 0.2 % indigo carmine dye. Endoscopy. 2006;38:1218-1223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Snover DC. Update on the serrated pathway to colorectal carcinoma. Hum Pathol. 2011;42:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 439] [Cited by in RCA: 478] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 18. | Kimura T, Yamamoto E, Yamano HO, Suzuki H, Kamimae S, Nojima M, Sawada T, Ashida M, Yoshikawa K, Takagi R. A novel pit pattern identifies the precursor of colorectal cancer derived from sessile serrated adenoma. Am J Gastroenterol. 2012;107:460-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 129] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 19. | East JE, Suzuki N, Saunders BP. Comparison of magnified pit pattern interpretation with narrow band imaging versus chromoendoscopy for diminutive colonic polyps: a pilot study. Gastrointest Endosc. 2007;66:310-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 129] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 20. | Machida H, Sano Y, Hamamoto Y, Muto M, Kozu T, Tajiri H, Yoshida S. Narrow-band imaging in the diagnosis of colorectal mucosal lesions: a pilot study. Endoscopy. 2004;36:1094-1098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 387] [Cited by in RCA: 367] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 21. | Hirata M, Tanaka S, Oka S, Kaneko I, Yoshida S, Yoshihara M, Chayama K. Magnifying endoscopy with narrow band imaging for diagnosis of colorectal tumors. Gastrointest Endosc. 2007;65:988-995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 161] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 22. | East JE, Suzuki N, Bassett P, Stavrinidis M, Thomas HJ, Guenther T, Tekkis PP, Saunders BP. Narrow band imaging with magnification for the characterization of small and diminutive colonic polyps: pit pattern and vascular pattern intensity. Endoscopy. 2008;40:811-817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 104] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 23. | Tischendorf JJ, Wasmuth HE, Koch A, Hecker H, Trautwein C, Winograd R. Value of magnifying chromoendoscopy and narrow band imaging (NBI) in classifying colorectal polyps: a prospective controlled study. Endoscopy. 2007;39:1092-1096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 155] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 24. | Rogart JN, Jain D, Siddiqui UD, Oren T, Lim J, Jamidar P, Aslanian H. Narrow-band imaging without high magnification to differentiate polyps during real-time colonoscopy: improvement with experience. Gastrointest Endosc. 2008;68:1136-1145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 92] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 25. | Sikka S, Ringold DA, Jonnalagadda S, Banerjee B. Comparison of white light and narrow band high definition images in predicting colon polyp histology, using standard colonoscopes without optical magnification. Endoscopy. 2008;40:818-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 87] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 26. | Rastogi A, Keighley J, Singh V, Callahan P, Bansal A, Wani S, Sharma P. High accuracy of narrow band imaging without magnification for the real-time characterization of polyp histology and its comparison with high-definition white light colonoscopy: a prospective study. Am J Gastroenterol. 2009;104:2422-2430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 136] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 27. | Rastogi A, Early DS, Gupta N, Bansal A, Singh V, Ansstas M, Jonnalagadda SS, Hovis CE, Gaddam S, Wani SB. Randomized, controlled trial of standard-definition white-light, high-definition white-light, and narrow-band imaging colonoscopy for the detection of colon polyps and prediction of polyp histology. Gastrointest Endosc. 2011;74:593-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 117] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 28. | Rex DK. Narrow-band imaging without optical magnification for histologic analysis of colorectal polyps. Gastroenterology. 2009;136:1174-1181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 210] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 29. | Uraoka T, Saito Y, Ikematsu H, Yamamoto K, Sano Y. Sano’s capillary pattern classification for narrow-band imaging of early colorectal lesions. Dig Endosc. 2011;23 Suppl 1:112-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 71] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 30. | Sano Y, Ikematsu H, Fu KI, Emura F, Katagiri A, Horimatsu T, Kaneko K, Soetikno R, Yoshida S. Meshed capillary vessels by use of narrow-band imaging for differential diagnosis of small colorectal polyps. Gastrointest Endosc. 2009;69:278-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 196] [Article Influence: 12.3] [Reference Citation Analysis (1)] |
| 31. | Henry ZH, Yeaton P, Shami VM, Kahaleh M, Patrie JT, Cox DG, Peura DA, Emura F, Wang AY. Meshed capillary vessels found on narrow-band imaging without optical magnification effectively identifies colorectal neoplasia: a North American validation of the Japanese experience. Gastrointest Endosc. 2010;72:118-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 32. | Su MY, Hsu CM, Ho YP, Chen PC, Lin CJ, Chiu CT. Comparative study of conventional colonoscopy, chromoendoscopy, and narrow-band imaging systems in differential diagnosis of neoplastic and nonneoplastic colonic polyps. Am J Gastroenterol. 2006;101:2711-2716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 183] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 33. | Chiu HM, Chang CY, Chen CC, Lee YC, Wu MS, Lin JT, Shun CT, Wang HP. A prospective comparative study of narrow-band imaging, chromoendoscopy, and conventional colonoscopy in the diagnosis of colorectal neoplasia. Gut. 2007;56:373-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 242] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 34. | Hazewinkel Y, López-Cerón M, East JE, Rastogi A, Pellisé M, Nakajima T, van Eeden S, Tytgat KM, Fockens P, Dekker E. Endoscopic features of sessile serrated adenomas: validation by international experts using high-resolution white-light endoscopy and narrow-band imaging. Gastrointest Endosc. 2013;77:916-924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 152] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 35. | Ikematsu H, Matsuda T, Emura F, Saito Y, Uraoka T, Fu KI, Kaneko K, Ochiai A, Fujimori T, Sano Y. Efficacy of capillary pattern type IIIA/IIIB by magnifying narrow band imaging for estimating depth of invasion of early colorectal neoplasms. BMC Gastroenterol. 2010;10:33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 156] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 36. | Tanaka S, Sano Y. Aim to unify the narrow band imaging (NBI) magnifying classification for colorectal tumors: current status in Japan from a summary of the consensus symposium in the 79th Annual Meeting of the Japan Gastroenterological Endoscopy Society. Dig Endosc. 2011;23 Suppl 1:131-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 90] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 37. | Hewett DG, Kaltenbach T, Sano Y, Tanaka S, Saunders BP, Ponchon T, Soetikno R, Rex DK. Validation of a simple classification system for endoscopic diagnosis of small colorectal polyps using narrow-band imaging. Gastroenterology. 2012;143:599-607.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 376] [Cited by in RCA: 397] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 38. | Ignjatovic A, East JE, Suzuki N, Vance M, Guenther T, Saunders BP. Optical diagnosis of small colorectal polyps at routine colonoscopy (Detect InSpect ChAracterise Resect and Discard; DISCARD trial): a prospective cohort study. Lancet Oncol. 2009;10:1171-1178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 286] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 39. | Hassan C, Pickhardt PJ, Rex DK. A resect and discard strategy would improve cost-effectiveness of colorectal cancer screening. Clin Gastroenterol Hepatol. 2010;8:865-89, 865-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 219] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 40. | Rex DK, Kahi C, O’Brien M, Levin TR, Pohl H, Rastogi A, Burgart L, Imperiale T, Ladabaum U, Cohen J. The American Society for Gastrointestinal Endoscopy PIVI (Preservation and Incorporation of Valuable Endoscopic Innovations) on real-time endoscopic assessment of the histology of diminutive colorectal polyps. Gastrointest Endosc. 2011;73:419-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 406] [Cited by in RCA: 457] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 41. | Repici A, Hassan C, Radaelli F, Occhipinti P, De Angelis C, Romeo F, Paggi S, Saettone S, Cisarò F, Spaander M. Accuracy of narrow-band imaging in predicting colonoscopy surveillance intervals and histology of distal diminutive polyps: results from a multicenter, prospective trial. Gastrointest Endosc. 2013;78:106-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 42. | Ladabaum U, Fioritto A, Mitani A, Desai M, Kim JP, Rex DK, Imperiale T, Gunaratnam N. Real-time optical biopsy of colon polyps with narrow band imaging in community practice does not yet meet key thresholds for clinical decisions. Gastroenterology. 2013;144:81-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 185] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 43. | Kuiper T, Marsman WA, Jansen JM, van Soest EJ, Haan YC, Bakker GJ, Fockens P, Dekker E. Accuracy for optical diagnosis of small colorectal polyps in nonacademic settings. Clin Gastroenterol Hepatol. 2012;10:1016-120; quiz e79. [PubMed] |
| 44. | Paggi S, Rondonotti E, Amato A, Terruzzi V, Imperiali G, Mandelli G, Terreni N, Lenoci N, Spinzi G, Radaelli F. Resect and discard strategy in clinical practice: a prospective cohort study. Endoscopy. 2012;44:899-904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 45. | Gupta N, Bansal A, Rao D, Early DS, Jonnalagadda S, Wani SB, Edmundowicz SA, Sharma P, Rastogi A. Prevalence of advanced histological features in diminutive and small colon polyps. Gastrointest Endosc. 2012;75:1022-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 142] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 46. | Rastogi A. Optical diagnosis of small colorectal polyp histology with high-definition colonoscopy using narrow band imaging. Clin Endosc. 2013;46:120-129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 47. | Pohl J, Nguyen-Tat M, Pech O, May A, Rabenstein T, Ell C. Computed virtual chromoendoscopy for classification of small colorectal lesions: a prospective comparative study. Am J Gastroenterol. 2008;103:562-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 102] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 48. | Pohl J, Lotterer E, Balzer C, Sackmann M, Schmidt KD, Gossner L, Schaab C, Frieling T, Medve M, Mayer G. Computed virtual chromoendoscopy versus standard colonoscopy with targeted indigocarmine chromoscopy: a randomised multicentre trial. Gut. 2009;58:73-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 104] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 49. | Togashi K, Osawa H, Koinuma K, Hayashi Y, Miyata T, Sunada K, Nokubi M, Horie H, Yamamoto H. A comparison of conventional endoscopy, chromoendoscopy, and the optimal-band imaging system for the differentiation of neoplastic and non-neoplastic colonic polyps. Gastrointest Endosc. 2009;69:734-741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 65] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 50. | dos Santos CE, Lima JC, Lopes CV, Malaman D, Salomão AD, Garcia AC, Teixeira CR. Computerized virtual chromoendoscopy versus indigo carmine chromoendoscopy combined with magnification for diagnosis of small colorectal lesions: a randomized and prospective study. Eur J Gastroenterol Hepatol. 2010;22:1364-1371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 51. | Yoshida N, Naito Y, Kugai M, Inoue K, Uchiyama K, Takagi T, Ishikawa T, Handa O, Konishi H, Wakabayashi N. Efficacy of magnifying endoscopy with flexible spectral imaging color enhancement in the diagnosis of colorectal tumors. J Gastroenterol. 2011;46:65-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 52. | Longcroft-Wheaton GR, Higgins B, Bhandari P. Flexible spectral imaging color enhancement and indigo carmine in neoplasia diagnosis during colonoscopy: a large prospective UK series. Eur J Gastroenterol Hepatol. 2011;23:903-911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 53. | Yoshida N, Naito Y, Inada Y, Kugai M, Inoue K, Uchiyama K, Handa O, Takagi T, Konishi H, Yagi N. The detection of surface patterns by flexible spectral imaging color enhancement without magnification for diagnosis of colorectal polyps. Int J Colorectal Dis. 2012;27:605-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 54. | Hoffman A, Sar F, Goetz M, Tresch A, Mudter J, Biesterfeld S, Galle PR, Neurath MF, Kiesslich R. High definition colonoscopy combined with i-Scan is superior in the detection of colorectal neoplasias compared with standard video colonoscopy: a prospective randomized controlled trial. Endoscopy. 2010;42:827-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 128] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 55. | Lee CK, Lee SH, Hwangbo Y. Narrow-band imaging versus I-Scan for the real-time histological prediction of diminutive colonic polyps: a prospective comparative study by using the simple unified endoscopic classification. Gastrointest Endosc. 2011;74:603-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 63] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 56. | Hoffman A, Kagel C, Goetz M, Tresch A, Mudter J, Biesterfeld S, Galle PR, Neurath MF, Kiesslich R. Recognition and characterization of small colonic neoplasia with high-definition colonoscopy using i-Scan is as precise as chromoendoscopy. Dig Liver Dis. 2010;42:45-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 86] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 57. | Chan JL, Lin L, Feiler M, Wolf AI, Cardona DM, Gellad ZF. Comparative effectiveness of i-SCAN™ and high-definition white light characterizing small colonic polyps. World J Gastroenterol. 2012;18:5905-5911. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 58. | Neumann H, Vieth M, Fry LC, Günther C, Atreya R, Neurath MF, Mönkemüller K. Learning curve of virtual chromoendoscopy for the prediction of hyperplastic and adenomatous colorectal lesions: a prospective 2-center study. Gastrointest Endosc. 2013;78:115-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 59. | Pigò F, Bertani H, Manno M, Mirante V, Caruso A, Barbera C, Manta R, Bassotti G, Olivetti G, Conigliaro RL. i-Scan high-definition white light endoscopy and colorectal polyps: prediction of histology, interobserver and intraobserver agreement. Int J Colorectal Dis. 2013;28:399-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |