Published online Jun 7, 2014. doi: 10.3748/wjg.v20.i21.6534
Revised: December 5, 2013
Accepted: January 8, 2014
Published online: June 7, 2014
Processing time: 264 Days and 4.2 Hours
AIM: To describe the variation that divalent metal transporter 1 (DMT1) shows in patients after Roux-en-Y gastric bypass (RYGB) surgery.
METHODS: Prospective and analytical study of DMT1 level at the brush border of proximal jejunum in patients having undergone RYGB surgery. The mucosa of proximal jejunum forming the gastrojejunal anastomosis was biopsied during surgery and after 6 mo later with an endoscopic biopsy. All the patients received precise instructions regarding feeding and nutritional supplementation. Both samples were processed at the same time by immunohistochemistry and western blot. Samples were analysed by a pathologist. For statistical analysis, the χ2 and Wilcoxon tests were used.
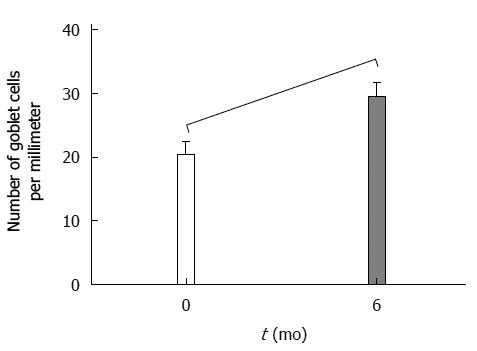
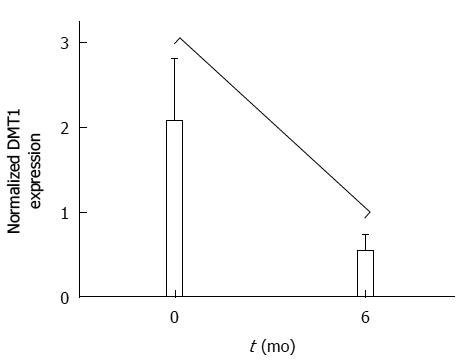
RESULTS: Sixteen patients were recruited, 13 of whom completed the study. Twelve were women. Average age and body mass index (BMI) were 44.1 and 40.4, respectively. Both body weight and BMI decreased significantly during the study period, with an average percent excess weight loss (%EWL) of 60% ± 13.3% and an average percent excess BMI loss (%EBMIL) of 79.6% ± 21.6%. Only two patients presented with mild anaemia 6 mo after surgery, but their ferritin levels stayed within normal ranges. Staining for DMT1 showed a significant increase in the cytoplasm of enterocytes located at the tips of the villi (χ2 = 6.03; P = 0.049). Nevertheless, the total quantity of DMT1 decreased significantly (Z = 2.04; P = 0.04). Associated with these results, we observed a significant increase in goblet cells in the villi 6 mo postoperatively (Z = -2.47; P = 0.013).
CONCLUSION: Six months after RYGB surgery, patients exhibit an increase in DMT1 expression in the enterocytes of the tips of the villi at the proximal jejunum.
Core tip: Anaemia after Roux-en-Y gastric bypass surgery is one of the most common nutritional deficiencies. Different nutritional supplementation strategies have been developed to prevent this complication, but a subset of patients still develop it. This study brings readers the first report on the molecular changes that occur in the physiology of iron absorption in these patients.
- Citation: Marambio A, Watkins G, Castro F, Riffo A, Zúñiga R, Jans J, Villanueva ME, Díaz G. Changes in iron transporter divalent metal transporter 1 in proximal jejunum after gastric bypass. World J Gastroenterol 2014; 20(21): 6534-6540
- URL: https://www.wjgnet.com/1007-9327/full/v20/i21/6534.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i21.6534
Nutritional deficiencies are common complications of bariatric surgery, especially in association with Roux-en-Y gastric bypass (RYGB). Of particular importance are iron deficiency and anaemia. It is accepted that such patients must receive vitamin supplements perpetually, and iron supplements depending on the presence or absence of factors that influence the development of anaemia, such as gender or age. In spite of this, a fraction of the patients will still develop iron deficiency and in some cases will require the administration of intravenous iron supplementation[1].
The mechanisms that result in iron deficiency are mainly related to a lack of gastric acidity and the exclusion of the duodenum and part of the jejunum, the main sites of iron absorption[2]. The iron transporter divalent metal transporter 1 (DMT1) has been detected in these regions. DMT1 is a transmembrane protein found on the apical membrane of the enterocyte that, by the proton-motive force, transports ferrous iron (Fe2+) into the cell[3].
DMT1 is mainly found in the duodenum, and its expression decreases along the digestive tract. In conditions of iron deficiency, the duodenum is capable of adapting by overexpressing DMT1, and in overload conditions by diminishing its expression[4]. In humans there are no reports of the presence or absence of this phenomenon at the proximal jejunum. Moreover, there are no reports concerning over-expression in patients undergoing RYGB surgery, which is an iron deficiency model involving the described mechanism.
The aim of the current study is to describe the changes in DMT1 expression in a group of patients having undergone RYGB surgery. Because to our knowledge, no research has been carried out on this topic, the intention is to start a new line of research addressing the molecular aspects that regulate the occurrence of anaemia in such patients.
For this prospective and analytical study, patients from the Surgery Department of the University of Chile Clinical Hospital were recruited. To be enrolled, patients had to be scheduled for RYGB surgery. All patients were informed of the nature of the study and authorised their inclusion by signing an informed consent. The study was approved by the ethics committee of our hospital. The design consisted of an initial assessment that included anamnesis and full physical examination. Age, weight, height, body mass index (BMI), and medical, surgical and family history were documented. All patients underwent a complete blood count and a serum ferritin test. Exclusion criteria were preoperative iron deficiency, previous use of iron supplements, poor tolerance to iron supplements, the presence of diseases that affect the red series or jejunum, habitual smoking (> 1 pack per week) and current pregnancy. In addition, patients who subsequently presented significant early or late postoperative complications (leak, haemorrhage, stenosis, etc.) were excluded from the final analysis.
All patients underwent RYGB surgery by a standard technique[5]. During the surgery, a sample of jejunal mucosa was collected at the level of the gastrojejunal anastomosis (GJA) for histological analysis (jejunum approximately 25 cm distal to Treitz’s angle). Once discharged, all the patients received precise indications concerning their feeding (similar guidelines for all patients having undergone RYGB surgery) and vitamin supplement intake. The latter consisted of Maltofer vitaminado® (multivitamin complex with minerals, trace elements and iron as iron III 60 mg from Andrómaco laboratory, Santiago, Chile), 1 tablet per day for 1 mo, which was then changed to Berocca Plus® (B complex, calcium and magnesium from Bayer laboratory, Santiago, Chile), 1 tablet per day permanently. In addition to the former, patients were prescribed Neurobionta® (B complex, 10000 U, from Merck laboratory, Santiago, Chile), 1 intramuscular ampoule per month for 3 mo, and then every 3 or 6 mo for women or men respectively.
Six months after surgery, patients underwent a complete blood count, a serum ferritin test and an upper digestive endoscopy with biopsy of the jejunal mucosa at the alimentary limb (approximately 10 cm distal to the GJA). The histological specimens were fixed in 4% formaldehyde (for haematoxylin-eosin staining and immunohistochemistry analysis) and stored in liquid nitrogen at -80 °C (for western blot analysis).
Immediately upon collection, the tissue specimens were fixed in 4% formaldehyde in phosphate-buffered saline (PBS) for 24 h. The specimens were subsequently dehydrated and embedded in paraffin in an automatic processor to create paraffin blocks in standard plastic cassettes. Sections 5 μm thick were obtained using a rotation microtome with disposable blades. These were mounted on xylan-coated glass slides and dried in an oven at 60 °C for 24 h.
Sections for haematoxylin and eosin (H and E) staining were deparaffinised in xylene and hydrated in a sequence of ethanol in decreasing concentrations ending in distilled water. Nuclear contrast was obtained with Harris haematoxylin for 3 min. The sections were then washed under running water to turn the nuclei blue. Cytoplasmic staining with aqueous 0.5% eosin Y was applied for 30 s. Finally, sections were dehydrated with ethanol, clarified with xylene and mounted with permanent synthetic medium.
Immunohistochemistry (IHQ) sections were processed in the same way as for H and E staining but then rehydrated and pretreated with 1 mmol/L ethylenediaminetetraacetic acid (EDTA), pH 8.0 for 25 min at 96 °C in a steamer for antigen retrieval. The slides were washed in distilled water, followed by incubation with 3% aqueous hydrogen peroxide to block the endogenous peroxidase. The slides were then washed 3 times for 2 minutes with 0.01 mol/L Tris-buffered saline with 200 ìL/L Tween 20 (TBST), pH 7.6, and blockage of unspecific reactivity was carried out with horse serum for 10 min at room temperature (RT).
Once the blockage was finished, sections were incubated with anti-DMT1 antibody (pan-DMT1 rabbit polyclonal antibody, which recognises the amino-terminal sequence MVLGPEQKMSDDSVSGDH present in all the isoforms of human DMT1 and which was prepared by the Immunology Service of the School of Sciences of the University of Chile) in 1:200 dilution in TBST, for 45 min at 37 °C in a humid chamber. In parallel, sections were incubated with horse serum under the same conditions, as a negative control. After incubation with the antibody, sections were washed with TBST and treated with the detection kit Vector PK 7200 (Vector Laboratories Inc. 30 Ingold Road, Burlingame, CA 94010 United States). Sections were incubated for 25 min at RT with biotinylated secondary antibody from the kit, followed by a wash with TBST and finally incubation with developing solution (1 mg/mL dimethylaminobenzidine in 0.01 mol/L saline phosphate buffer pH 7.6 with 0.003% hydrogen peroxide) for 2 min at RT. The reaction was interrupted with distilled water. Nuclear contrast was obtained with Harris haematoxylin for 1 min. Finally, the sections were dehydrated with ethanol, clarified with xylene and mounted with permanent synthetic medium.
The western blot protocol consisted of (1) initial homogenisation of the specimens with a tissue lysis buffer solution in presence of enzymatic inhibitors (RIPA solution) in a glass-glass Wheaton® homogeniser; sonication of the sample for 7 min; further homogenisation to ensure its total distribution; and then centrifugation of the sample at 10000 rpm at 4 °C for 10 min and extraction of the supernatant that contained the protein of interest; (2) quantification of the protein in the extract by Lowry's method; (3) electrophoretic separation of the proteins (according to their molecular weight), carried out in acrylamide/polyacrylamide gels (8%); (4) electrotransfer of the proteins to a nitrocellulose membrane; (5) incubation of membranes with a specific antibody against the protein of interest (anti-DMT1) and a secondary horseradish peroxidase-conjugated antibody, followed by immunodetection with the method of chemoluminescence and its subsequent detection with auto radiographic film; and (6) quantification of protein bands by appropriate software showing the results as total pixels per quantifiable band.
The samples were analysed by a single pathologist (MV) who was blinded to the groups (preoperative vs postoperative). For morphological analysis of the villi and goblet cells, haematoxylin and eosin staining was used. For the analysis of DMT1 staining, the enterocyte was divided into cytoplasm and brush border. Qualitative analysis was performed to compare the intensity of the staining at 0 and 6 mo after surgery. The staining was classified into three levels: (+) mild, (++) moderate and (+++) intense.
All data are presented as the mean ± SD. Stata 8.1 software (Stata Corp., Lakeway Drive, TX, United States) was used for statistical analysis. Because the data were normally distributed, we applied Wilcoxon’s test and the χ2 test, considering P≤ 0.05 statistically significant.
We recruited 16 patients who met the inclusion/exclusion criteria, but 3 patients were lost from the study because they failed to complete the 6-mo post-surgery follow-up control. There were no postoperative complications. Among the 13 patients who were analysed, the average age was 44.1 ± 12.7 years (20-66), and only one patient was a male. Four of the patients had type 2 diabetes mellitus, 6 had arterial hypertension, and 6 had dyslipidaemia. Twelve patients underwent non-resective laparoscopic RYGB, whereas the patient with the highest BMI (50.9) underwent an open technique with resection of the gastric remnant.
Both weight and BMI decreased significantly (P = 0.001 for both variables), for an average percent excess weight loss (%EWL) of 60% ± 13,3% and an average percent excess BMI loss (%EBMIL) of 79.6% ± 21.6%. Two patients presented with mild anaemia six months after surgery (haemoglobin: 11.2 g/dL and 11.4 g/dL, respectively). Nevertheless, their ferritin levels stayed within normal ranges. When the group was analysed as a whole, there was not a significant decrease in the level of haemoglobin or ferritin. Table 1 shows the evolution of anthropometric and haematologic variables.
| Variable (averages) | Preoperative | 6 mo postop. | P1 |
| Weight (kg) | 99.8 ± 17.7 | 71.7 ± 12.3 | 0.001 |
| BMI | 40.4 ± 5.7 | 29.1 ± 4 | 0.001 |
| Haematocrit (%) | 41.4 ± 2.7 | 39.7 ± 3.5 | 0.272 |
| Haemoglobin (g/dL) | 13.7 ± 1 | 13.1 ± 1.1 | 0.071 |
| Ferritin (ng/mL) | 85.2 ± 76 | 69.3 ± 58.7 | 0.208 |
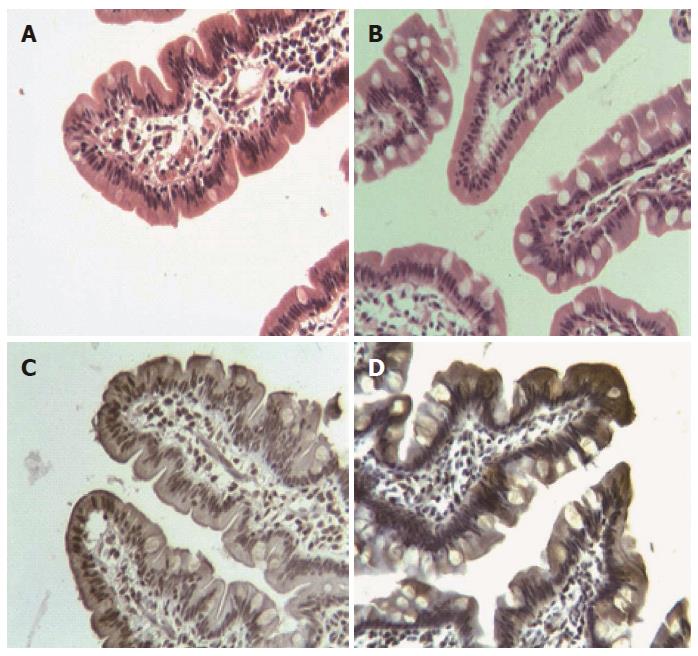
During histological analysis, most of the villi and crypts showed preservation of the normal morphologic pattern 6 mo after surgery (Figure 1A, B). Villi showed an increase in the number of goblet cells per millimetre of epithelium (P = 0.013) (Figure 2). By IHC, DMT1 was present in the whole epithelium, mainly concentrated at level of the brush border.
At 6 mo there was a significant increase of DMT1 in the cytoplasm of epithelial cells (χ2 = 6.03; P = 0.049) at the apex of the villi (Figure 1C, D). At level of the brush border, the expression of DMT1 did not show significant variation (Table 2). In spite of these findings and having analysed the total amount of the receptor in the jejunal mucosa by western blot, we found a significant decrease of DMT1 (P = 0.040) 6 mo post-surgery (Figure 3).
| Patients | Cytoplasm | P1 | Brush border | P1 | ||
| 0 mo | 6 mo | 0.049 | 0 mo | 6 mo | 0.218 | |
| 1 | ++ | +++ | ++ | ++ | ||
| 2 | ++ | + | ++ | ++ | ||
| 3 | + | + | ++ | ++ | ||
| 4 | ++ | + | ++ | ++ | ||
| 5 | ++ | ++ | ++ | ++ | ||
| 6 | + | ++ | + | ++ | ||
| 7 | ++ | ++ | ++ | ++ | ||
| 8 | ++ | +++ | ++ | ++ | ||
| 9 | ++ | ++ | ++ | ++ | ||
| 10 | + | +++ | ++ | +++ | ||
| 11 | + | + | + | ++ | ||
| 12 | ++ | + | ++ | ++ | ||
| 13 | ++ | +++ | ++ | ++ | ||
The number of bariatric surgeries has progressively increased given the obesity epidemic that affects the population of many countries, especially in the West[6]. RYGB is one of the most frequent bariatric surgeries due to its excellent results, mainly in relation to the resolution of comorbidities[7] and its low rate of complications[8]. Nevertheless, as the quantity of operated patients increases, so do the long-term complications, some of the most important of which are nutritional deficiencies. The most frequent nutritional deficiencies after bariatric surgery is iron deficiency and secondary anaemia[9-11], although in many cases it follows the deficit of other minerals or vitamins, such as vitamin B12 and the folates. This is why there are established follow-up and nutritional supplementation protocols, usually with multivitamin and iron oral intake. However, a subset of patients still will still manifest the deficit.
No randomised and controlled studies have addressed the iron deficiency in such patients. There are only retrospective data and case series. From the latter, it is possible to estimate that between 30% and 50%[11-15] of patients will show iron deficiency or anaemia, depending on the study. These might manifest months or even years after the surgery, but in most cases the disorder appears within the first 12 mo[16]. Thus, these proportions might vary depending on the moment at which the measurement is carried out. Some patients have a higher tendency than others to present this complication because it is more common in women of fertile age (due to increased blood loss), adolescents and pregnant women (the latter due to higher iron requirements). This disorder is seen frequently before surgery, as a result of the eating and living habits of such patients[17,18].
There are various reasons for the greater tendency towards iron deficiency and anaemia among bariatric surgery patients, mainly the lack of gastric acidity, the exclusion of the main absorption site (duodenum), decreased ingestion, postoperative bleeding and the presence of marginal ulcers[2]. The longer the alimentary limb, the higher the incidence of this pathology[11], because of the eventual increase of malabsorption. It must be taken into account that these patients are also at risk of presenting other mineral or vitamin deficiencies that might also cause anaemia, such as vitamins from the B complex, copper, vitamin C and protein deficiencies, although these are less frequent reasons.
The iron in our diet comes in two forms, either as haeme iron or as inorganic iron. The former is highly bioavailable and is mainly found in red meats. Its absorption mechanism is not completely clear, but haeme carrier protein 1 (HCP1) has been suggested as the iron transporter at the brush border[19]. The non-haeme iron is widely distributed and is absorbed in its ferrous state (Fe2+) by DMT1, which has the ability to transport non-specific metals such as manganese, lead, cadmium, zinc and copper[3]. In its ferric form (Fe3+), iron is absorbed in parallel by mobilferrin[20]. However, the presence of an apical ferric reductase (Dcytb) has been described at the duodenal mucosa, where it reduces Fe+3 to facilitate its absorption.
In conditions of iron deficiency, the duodenal mucosa is the mucosa that adapts the most. The amount of DMT1 mRNA in the duodenum increases in iron deficiency[4,21-24], as does Dcytb[4,23]. The molecular mechanisms for this adaptation are not well known, although it has been suggested that the apical transporters are regulated by local signals and the basolateral ones by systemic signals[25]. We have demonstrated that in patients undergoing RYGB, DMT1 expression in the cytoplasm of enterocytes located in the apex of the villi of the proximal jejunum is increased 6 mo after surgery. Nevertheless, we were surprised that the total quantity of the receptor in the same area decreased. To reduce the possibility of error in the measurements and in the handling of samples, biopsies at 0 and 6 mo were kept under the same conditions and finally processed simultaneously under the same laboratory conditions and by the same staff. While the biopsies varied in size, western blots were performed by normalising protein levels to the tissue size, thereby eliminating the variable amount of tissue as a bias.
Therefore, a possible explanation for our protein expression findings are the cellular changes seen in the villi in such patients, such as the significant increase in the number of goblet cells that displace enterocytes which have greater absorptive capacity than goblet cells. This might be triggered by the physical/chemical stimulus of food, now undigested by the exclusion of the stomach and the duodenum, a mechanism that acts within these cells[26,27]. This might be the beginning of a compensation mechanism between the enterocyte decrease and the increased DMT1 in the remaining enterocytes. This adaptation would allow our patients to maintain their iron reserves at normal levels despite all the side effects of the RYGB that affect iron absorption, which after 6 mo has already decreased[28]. These changes might explain the high rate of iron metabolism disorders observed in all these patients during a longer-term follow-up, keeping in mind that many other individual factors may influence the development of anaemia. Undoubtedly, further cellular research is needed, prospective in nature and for longer periods, in patients undergoing RYGB, including other molecules involved in iron metabolism, such as Dcytb, ferroportin, hepcidin and hephaestin. This would improve our understanding of the mechanisms that produce iron metabolism disorders in bariatric surgery patients.
In conclusion, 6 mo after RYGB surgery, patients showed increased expression of DMT1 in the cytoplasm of the enterocytes located in the apex of the villi of the proximal jejunum. This could be a compensation mechanism because it is associated with a decrease of the total quantity of the receptor in the mucosa, most likely following the cellular changes experienced by the intestinal villi in RYGB patients.
This investigation was undertaken by a multidisciplinary team with patients from the Surgery Department of the University of Chile Clinical Hospital. We are thankful to the invaluable help from Drs Enrique Lanzarini, Attila Csendes, Jorge Rojas, Luis Gutiérrez, Juan Carlos Díaz, Maher Musleh, Héctor Valladares and Zoltan Berger.
Iron deficiency and anaemia are common complications affecting patients after Roux-en-Y gastric bypass (RYGB) surgery. Various mechanisms could explain this, especially the exclusion of the duodenum, the main site of iron absorption effected by the divalent metal transporter 1 (DMT1). In spite of various protocols of supplementation and nutritional management, iron deficiency is still present in a proportion of patients.
DMT1 is a transmembrane protein found on the apical membrane of the enterocyte that, by the proton-motive force, transports ferrous iron (Fe2+) into the cell. In conditions of iron deficiency, the duodenum is capable of adapting by overexpressing the DMT1 transporter, and in overload conditions by downregulating it. In this study, the authors demonstrate that 6 mo after RYGB surgery, patients exhibit an increase in DMT1 expression in the enterocytes of the tips of the villi at the proximal jejunum.
Several studies have highlighted the importance of nutritional deficiencies in patients subjected to RYGB surgery, in particular iron deficiency, which occurs in 30%-50% of patients. In this study, the authors demonstrate that the variations in the iron receptor DMT1 may help to explain why some patients develop anaemia despite being supplemented.
Understanding the changes experienced by these patients in terms of iron absorption mechanisms will help to create future strategies to prevent the development of anaemia.
The authors examined the variation that DMT1 shows in patients after Roux-en-Y gastric bypass surgery. This study reveals that 6 mo after surgery, patients exhibit an increase in the expression of DMT1 in the cytoplasm of enterocytes of the tips of the villi at the proximal jejunum. This could be a compensation mechanism because it is associated with a decrease in the total quantity of the transporter in the mucosa, most likely as a result of the cellular changes present at the intestinal villi of such patients. The results are interesting and represent the first report on the molecular changes that occur in the physiology of iron absorption in these patients.
P- Reviewers: Hedberg J, Kalpesh J S- Editor: Ma YJ L- Editor: A E- Editor: Liu XM
| 1. | Varma S, Baz W, Badine E, Nakhl F, McMullen H, Nicastro J, Forte F, Terjanian T, Dai Q. Need for parenteral iron therapy after bariatric surgery. Surg Obes Relat Dis. 2008;4:715-719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 2. | Love AL, Billett HH. Obesity, bariatric surgery, and iron deficiency: true, true, true and related. Am J Hematol. 2008;83:403-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 103] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 3. | Miret S, Simpson RJ, McKie AT. Physiology and molecular biology of dietary iron absorption. Annu Rev Nutr. 2003;23:283-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 179] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 4. | Zoller H, Koch RO, Theurl I, Obrist P, Pietrangelo A, Montosi G, Haile DJ, Vogel W, Weiss G. Expression of the duodenal iron transporters divalent-metal transporter 1 and ferroportin 1 in iron deficiency and iron overload. Gastroenterology. 2001;120:1412-1419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 211] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 5. | Csendes A, Burgos AM, Burdiles P. Incidence of anastomotic strictures after gastric bypass: a prospective consecutive routine endoscopic study 1 month and 17 months after surgery in 441 patients with morbid obesity. Obes Surg. 2009;19:269-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 50] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 6. | Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010. JAMA. 2012;307:491-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3909] [Cited by in RCA: 3840] [Article Influence: 295.4] [Reference Citation Analysis (0)] |
| 7. | Papapietro K, Díaz E, Csendes A, Díaz JC, Braghetto I, Burdiles P, Maluenda F, Rojas J. [Effects of gastric bypass on weight, blood glucose, serum lipid levels and arterial blood pressure in obese patients]. Rev Med Chil. 2005;133:511-516. [PubMed] |
| 8. | Guzmán S, Manrique M, Raddatz A, Norero E, Salinas J, Achurra P, Funke R, Boza C, Crovari F, Escalona A. [Results of bariatric surgery. Experience over 18 years]. Rev Med Chil. 2013;141:553-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Davies DJ, Baxter JM, Baxter JN. Nutritional deficiencies after bariatric surgery. Obes Surg. 2007;17:1150-1158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 76] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 10. | Kaidar-Person O, Person B, Szomstein S, Rosenthal RJ. Nutritional deficiencies in morbidly obese patients: a new form of malnutrition? Part B: minerals. Obes Surg. 2008;18:1028-1034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 122] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 11. | Marinella MA. Anemia following Roux-en-Y surgery for morbid obesity: a review. South Med J. 2008;101:1024-1031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 12. | Vargas-Ruiz AG, Hernández-Rivera G, Herrera MF. Prevalence of iron, folate, and vitamin B12 deficiency anemia after laparoscopic Roux-en-Y gastric bypass. Obes Surg. 2008;18:288-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 139] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 13. | Bloomberg RD, Fleishman A, Nalle JE, Herron DM, Kini S. Nutritional deficiencies following bariatric surgery: what have we learned? Obes Surg. 2005;15:145-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 339] [Cited by in RCA: 291] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 14. | Gasteyger C, Suter M, Gaillard RC, Giusti V. Nutritional deficiencies after Roux-en-Y gastric bypass for morbid obesity often cannot be prevented by standard multivitamin supplementation. Am J Clin Nutr. 2008;87:1128-1133. [PubMed] |
| 15. | Dalcanale L, Oliveira CP, Faintuch J, Nogueira MA, Rondó P, Lima VM, Mendonça S, Pajecki D, Mancini M, Carrilho FJ. Long-term nutritional outcome after gastric bypass. Obes Surg. 2010;20:181-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 112] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 16. | Brolin RE, Gorman JH, Gorman RC, Petschenik AJ, Bradley LB, Kenler HA, Cody RP. Prophylactic iron supplementation after Roux-en-Y gastric bypass: a prospective, double-blind, randomized study. Arch Surg. 1998;133:740-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 117] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 17. | Schweiger C, Weiss R, Berry E, Keidar A. Nutritional deficiencies in bariatric surgery candidates. Obes Surg. 2010;20:193-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 97] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 18. | Bavaresco M, Paganini S, Lima TP, Salgado W, Ceneviva R, Dos Santos JE, Nonino-Borges CB. Nutritional course of patients submitted to bariatric surgery. Obes Surg. 2010;20:716-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 104] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 19. | Anderson GJ, Frazer DM, McLaren GD. Iron absorption and metabolism. Curr Opin Gastroenterol. 2009;25:129-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 131] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 20. | Conrad ME, Umbreit JN, Moore EG, Hainsworth LN, Porubcin M, Simovich MJ, Nakada MT, Dolan K, Garrick MD. Separate pathways for cellular uptake of ferric and ferrous iron. Am J Physiol Gastrointest Liver Physiol. 2000;279:G767-G774. [PubMed] |
| 21. | Barisani D, Parafioriti A, Bardella MT, Zoller H, Conte D, Armiraglio E, Trovato C, Koch RO, Weiss G. Adaptive changes of duodenal iron transport proteins in celiac disease. Physiol Genomics. 2004;17:316-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 22. | Stuart KA, Anderson GJ, Frazer DM, Powell LW, McCullen M, Fletcher LM, Crawford DH. Duodenal expression of iron transport molecules in untreated haemochromatosis subjects. Gut. 2003;52:953-959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 46] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 23. | Dupic F, Fruchon S, Bensaid M, Loreal O, Brissot P, Borot N, Roth MP, Coppin H. Duodenal mRNA expression of iron related genes in response to iron loading and iron deficiency in four strains of mice. Gut. 2002;51:648-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 94] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 24. | Simovich M, Hainsworth LN, Fields PA, Umbreit JN, Conrad ME. Localization of the iron transport proteins Mobilferrin and DMT-1 in the duodenum: the surprising role of mucin. Am J Hematol. 2003;74:32-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 25. | Frazer DM, Wilkins SJ, Becker EM, Murphy TL, Vulpe CD, McKie AT, Anderson GJ. A rapid decrease in the expression of DMT1 and Dcytb but not Ireg1 or hephaestin explains the mucosal block phenomenon of iron absorption. Gut. 2003;52:340-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 133] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 26. | Specian RD, Oliver MG. Functional biology of intestinal goblet cells. Am J Physiol. 1991;260:C183-C193. [PubMed] |
| 27. | Kim YS, Ho SB. Intestinal goblet cells and mucins in health and disease: recent insights and progress. Curr Gastroenterol Rep. 2010;12:319-330. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 821] [Cited by in RCA: 953] [Article Influence: 68.1] [Reference Citation Analysis (0)] |
| 28. | Ruz M, Carrasco F, Rojas P, Codoceo J, Inostroza J, Rebolledo A, Basfi-fer K, Csendes A, Papapietro K, Pizarro F. Iron absorption and iron status are reduced after Roux-en-Y gastric bypass. Am J Clin Nutr. 2009;90:527-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 76] [Article Influence: 4.8] [Reference Citation Analysis (0)] |