INTRODUCTION
Gastric cancer is the second most common cause of cancer death, and the fourth most prevalent cancer worldwide (7.8%)[1]. Early gastric carcinoma (EGC) has been defined as gastric adenocarcinoma confined to either the mucosa or submucosa, irrespective of regional lymph node metastasis, whereas advanced gastric cancer has been defined as adenocarcinoma that has invaded into the muscularis propria or beyond[2]. The incidence of EGC differs between Eastern and Western nations. In South Korea, the proportion of gastric cancers diagnosed at an early stage increased from 28.6% in 1995 to 32.8% in 1999. In Japan, the proportion of gastric cancers diagnosed at an early stage has increased from 18% to 57% over the past 20 years[3,4]. These changes are mostly attributable to advances in diagnostic technologies, including radiologic and endoscopic modalities, which allow earlier detection of gastric cancer, as well as nationwide mass screening programs for gastric cancer[5,6].
With respect to therapeutic strategies for EGC, radical surgery with complete removal of the first and second tier lymph nodes can achieve 5-year survival rates in excess of 90%, and recurrence rates lower than 2%-3%[7,8]. Recently, endoscopic resection methods [such as endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD)] have become widely accepted as first-line therapies for EGC without lymph node (LN) metastasis. This acceptance partly results from the risks of gastrectomy and its negative effects on the patient’s quality of life[5,6]. Widely accepted selection criteria for EMR are the presence of an intramucosal and intestinal (differentiated) adenocarcinoma with a diameter of ≤ 2 cm, and the absence of lymphovascular invasion. With the development of ESD, broader indications have been established, including submucosal invasion to a maximal depth of 500 μm, and sizes up to 3 cm in diameter. These broader indications are a consequence of the wider resection of the mucosa and submucosa. For both EMR and ESD, the aforementioned indications are based on large Japanese datasets, which suggest a minimal risk of lymph node metastasis for lesions that fall within those criteria. Therefore, we believe that it is appropriate to review lymph node metastasis in EGC. Our review particularly focuses on methods of predicting lymph node metastasis in EGC.
LYMPH NODE METASTASIS OF EGC AND THE INDICATIONS OF EMR/ESD
In a meta-analysis of previously reported datasets[9], lymph node metastasis was evident in about 3.2% (0.0%-20.3%) of mucosal EGC and 19.2% (10.2%-33.3%) of submucosal EGC. Historically, Gotoda et al[10] reported that some groups of patients with EGC showed either no risk or minimal risks of lymph node metastasis, as compared with the risks of mortality from surgery. These 5 groups were characterized as follows: (1) intramucosal cancer, differentiated adenocarcinoma, and no lymphovascular invasion (irrespective of ulcer findings); (2) intramucosal cancer, differentiated adenocarcinoma, no lymphovascular invasion, and without ulcer findings (irrespective of tumor size); (3) undifferentiated intramucosal cancer, no lymphovascular invasion, without ulcer findings, and tumor less than 2 cm in size; and (4) minute submucosal penetration (SM1), differentiated adenocarcinoma, no lymphovascular invasion, and tumor less than 3 cm in size[10]. As a consequence of these findings, the guidelines for EMR and the extended criteria for ESD were based on these datasets. However, the validity of these criteria is currently under debate[11,12]. Kang et al[11] reported that there were 1.4% and 15% LN metastases for mucosal and submucosal EGCs, respectively, even though the lesions were well within the ESD criteria. However, Gotoda et al[10] reported that there were no LN metastases (95%CI: 0%-0.3%) among 1230 differentiated mucosal gastric cancers that were less than 3 cm in diameter and without lymphatic involvement, vessel involvement, or ulceration. Further, Gotoda et al[10] reported that there were no nodal metastases (95%CI: 0%-0.4%) among 929 differentiated EGCs that were of any size and without lymphatic involvement, involvement, or ulceration. Finally, Gotoda et al[10] reported that there were no LN metastases among 141 diffuse-type EGCs that were less than 3 cm and without lymphatic involvement, vessel involvement, or ulceration. In contrast to the results of Gotoda et al[10], Kang et al[11] reported that 1.6% (2/126), 1.4% (2/146), and 15.0% (3/20) of cases in the same respective categories involved lymph node metastasis. Additionally, Hölscher et al[12] reported that nodal metastasis was evident in ≥ 2 cm tumors as early as deep mucosal invasion, irrespective of their histologies. These observations could be explained by differences in the diagnostic criteria of gastric adenocarcinoma, and the likelihood that a diagnosis of adenocarcinoma, instead of high-grade dysplasia, could contribute to a lower rate of LN metastasis in some cohorts[13,14]. These reports inspire some concern regarding the extended criteria for ESD as a curative therapeutic modality, because their results suggest that a risk of positive nodal metastasis is still present in some cases. Therefore, a more accurate and consistent method of predicting lymph node metastasis is needed to support the use of EMR/ESD for curative resection.
CLINICOPATHOLOGICAL FEATURES FOR PREDICTING LYMPH NODE METASTASIS IN EGC, AND A RECOMMENDATION FOR A SCORING SYSTEM
Various attempts have been made to predict LN metastasis in EGC, on the basis of endoscopic findings and various clinicopathologic factors (depth of invasion, tumor size, macroscopic types, and histological differentiation). Kwee et al[9] reported that younger age, the location of the tumor in the middle stomach, larger tumor size, depressed tumor type, ulceration, diffuse histologic type, and lymphatic tumor invasion were associated with LN metastasis in mucosa-confined EGC. Further, female sex, location of the tumor in the lower stomach, larger tumor size, diffuse histologic type, increasing depth of submucosal invasion, and lymphovascular invasion were associated with LN metastasis in submucosal invasive EGCs[9].
In the course of investigating submucosal invasive EGC as an extended indication for ESD, a variety of studies have attempted to predict LN metastasis of submucosal gastric cancer[11,15-17]. An et al[15] reported that tumor size, histologic type, tumor depth, lymphatic invasion, and perineural invasion were associated with LN metastasis in submucosal EGCs. Among these factors, tumor size ≥ 2 cm, and the presence of lymphovascular tumor emboli were independent risk factors for LN metastasis in a multivariate analysis. Kurihara et al[16] reported that tumor diameter, lymphatic invasion, and depth of invasion were associated with lymph node metastasis. Kang et al[11] reported that tumor size, presence of ulceration, lymphovascular invasion, and depth of submucosal invasion were risk factors for LN metastasis in submucosal EGCs.
With respect to intestinal-type EGCs, lymphovascular invasion and depth of invasion have been reported to be independent risk factors for LN metastasis[11]. This same research group has also reported that the lateral extent of submucosal invasion is an important risk factor, in addition to depth of invasion and lymphovascular tumor emboli[17]. On the basis of the reported datasets, lymphovascular tumor emboli, tumor size, histologic type, and submucosal depth of invasion should be considered risk factors for predicting LN metastasis in submucosal invasive EGCs. The indication of ESD is largely dependent on these datasets, the analysis of which supported the extended criteria of < 500 μm depth of invasion, absence of lymphovascular invasion, tumor diameter < 3 cm, and intestinal histologic type.
As mentioned previously, lymphovascular tumor emboli, tumor size, histologic type, and depth of invasion were components of the extended ESD criteria for submucosal invasion EGC. Among these factors, tumor size and histologic type can be easily identified prior to endoscopic resection from endoscopic findings and biopsy specimens. However, it is difficult to detect the presence of submucosal invasion or lymphovascular tumor emboli using endoscopy and biopsy prior to endoscopic resection. Indeed, it is difficult to find evidence of submucosal invasion or lymphovascular tumor emboli on biopsy specimens, because of their small sizes and their paucity of submucosal tissue.
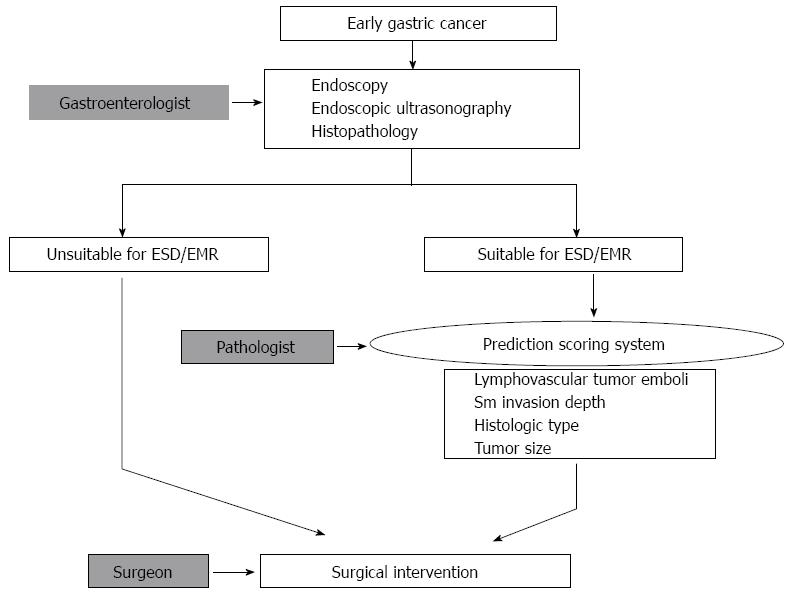
In consideration of these limitations, the management of patients who might undergo EMR/ESD could be performed using 2 processes, which have been described previously[18] (Figure 1). First, the selection of EGC patients for EMR/ESD could be performed on the basis of radiologic findings (endoscopic ultrasonography-based depth of invasion), endoscopic findings (size), and biopsy specimen findings (histopathological features). Second, endoscopic resection could be performed along with a comprehensive review of the EMR/ESD specimen, including examination for lymphovascular tumor emboli, tumor size, histologic type, and depth of invasion. The selection for further surgical resection after EMR/ESD could be determined from the results of this comprehensive review of the EMR/ESD specimen. Therefore, careful identification of the various histopathologic features of the EMR/ESD specimen is a mandatory component of the management of EMR/ESD patients. Various scoring systems have been developed for these purposes.
Figure 1 Workflow for deciding on a therapeutic method for cases of early gastric cancer.
The first step is the selection of suitable patients for endoscopic resection, based on endoscopic and histopathologic findings. After endoscopic resection, additional surgical intervention could be determined on the basis of a comprehensive review of the EMR/ESD specimen, including lymphovascular tumor emboli, tumor size, histologic type, and depth of invasion. ESD: Endoscopic submucosal dissection; EMR: Endoscopic mucosal resection; Sm: Submucosal.
Fujii et al[19] have demonstrated that lymphatic invasion, lymphocytic infiltration, poorly differentiated submucosal component, tumor size, depth of invasion, ulceration, and venous invasion are indications for surgical resection after ESD. They scored lymphatic invasion (+2), lymphocytic infiltration (-2), poorly differentiated component in the submucosa (+2), smallest diameter > 2 cm (+1), submucosal invasion depth > 2000 μm (+1), and venous invasion (+1) for each ESD specimen. The authors recommended that, after scoring each component, further surgical resection should be recommended if the sum of the scores is ≥ 4. They reported that their scoring system had 100% sensitivity, 68.0% specificity, and 73.7% diagnostic accuracy for predicting LN status in submucosal invasive EGC.
Kim et al[17] have developed the nodal prediction index formula, which is based on risk factors for LN metastasis in submucosal invasive EGCs. Their formula is NPI = (2.128 × lymphovascular tumor emboli) + (1.083 × submucosal invasion width ≥ 0.75 cm) + (0.507 × submucosal invasion depth ≥ 1000 μm + (0.515 × infiltrative growth pattern). Here, lymphovascular tumor emboli, submucosal invasion ≥ 1000 μm, submucosal width ≥ 0.75 cm, and infiltrative growth enter into the formula as either 0 (absent) or 1 (present). The nodal prediction index produces a greater area under the receiver operating characteristic (ROC) curve (0.809) than do individual evaluations of lymphovascular tumor emboli (0.744) or submucosal invasion width (0.689)[17]. Kim et al[17] reported that choosing a score of 1.8515 as the cutoff between the LN(+) and LN(-) groups yielded the ROC curve of optimal sensitivity and specificity. Particularly, using a 1.8515 cutoff score for predicting nodal metastasis resulted in a sensitivity, specificity, positive predictive value, negative predictive value, and accuracy of 70.4%, 80.1%, 41.3%, 93.5% and 79.3%, respectively. Although the exact results have varied across the different investigations, the use of a scoring system contributes an element of objectivity when deciding whether surgery is indicated after ESD.
PRACTICAL POINTS OF EVALUATION OF LYMPHOVASCULAR TUMOR EMBOLI AND DEPTH OF SUBMUCOSAL INVASION
As mentioned previously, lymphovascular tumor emboli and depth of submucosal invasion have been the strongest risk factors for lymph node metastasis in submucosal invasive EGCs. Therefore, careful identification of lymphovascular invasion and depth of submucosal invasion are crucial components of the management of patients who undergo EMR/ESD.
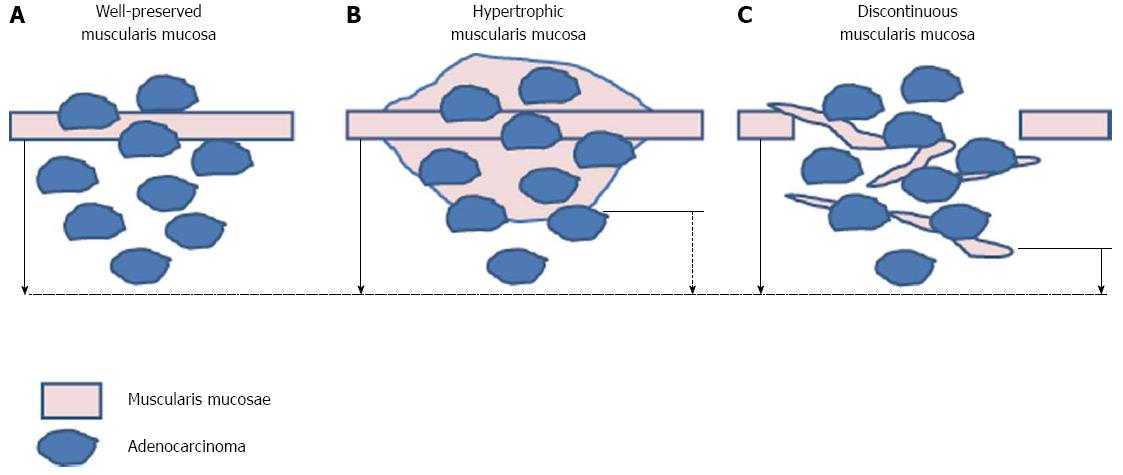
In the extended criteria for ESD, the cutoff value of submucosal invasion depth is 500 μm. However, the validity of 500 μm as an appropriate cutoff has been challenged. Specimen handling could artificially affect the depth of invasion[20]. During EMR/ESD specimen handling, the specimen could be stretched, reducing the thickness of the submucosa. Consequently, it has been suggested that the cutoff value for depth of submucosal invasion should be less than 500 μm, because the cutoff value has historically been determined from surgically resected lesions, instead of endoscopically resected lesions. Additionally, Kim et al[17] have argued that discrepancies in the measurement of depth of invasion could arise from differences between methods of measurement, as well as from the morphologic characteristics of the muscularis mucosae[17]. Indeed, Kim et al[17] revealed that there are differences in depth of invasion depending on whether the measurement is taken from the bottom of the muscularis mucosa, which has been suggested to be the standard method, or an imaginary line at the muscularis mucosa. In addition, the authors categorized the muscularis mucosa as normal, discontinuous, hypertrophic, or disappearing. They recommended measuring the submucosal depth of invasion from an imaginary line of the muscularis mucosae in patients with irregular muscularis mucosa (discontinuous, hypertrophic), and from the bottom of muscularis mucosa in patients with normal muscularis mucosa (Figure 2).
Figure 2 Recommendation of measurement of the submucosal depth of invasion.
Measuring from the bottom of muscularis mucosa in patients with normal muscularis mucosa (A) and from imaginary line of the muscularis mucosae in patients with irregular muscularis mucosa [hypertrophic (B), discontinuoud (C)] (solid arrow) is recommended rather than measuring from bottom of muscularis mucosae (broken arrow).
As mentioned previously, the presence of lymphovascular emboli is the most reliable risk factor for predicting LN metastasis in EGC. Therefore, careful identification of lymphovascular tumor emboli in endoscopically resected specimens is an important step for selecting suitable patients for further surgical intervention after endoscopic resection. Although the presence of lymphovascular emboli is defined by the presence of tumor cells within endothelial-lined vascular spaces, there are various debates about the recognition, diagnosis, and reporting of lymphovascular emboli in cancers[21-23]. Indeed, there are many mimics and artifacts of lymphovascular emboli, such as retraction artifacts around tumor cells, and intervening stroma-mimicking tumor cells in the lymphovascular spaces during histopathologic examination. Park et al[18] defined potential lymphovascular tumor emboli as being probable, suspicious, or definite. Specifically, potential lymphovascular tumor emboli were defined by “the presence of tumor cells within a vascular space,” in combination with the following criteria, which are based on previous publications[21,24]: (1) red cells or lymphocytes surrounding the tumor cells; (2) an endothelial cell lining; and (3) attachment to the vascular wall. Kim et al[17] recommend the use of strict lymphovascular tumor emboli criteria during the identification of lymphovascular tumor emboli in EMR/ESD specimens. Their recommendation proceeds from an analysis of their datasets that showed a greater the area under the ROC curve for definitive lymphovascular tumor emboli (as compared with the areas under the ROC curves for suspicious or probable cases) when predicting LN metastasis from endoscopically resection specimens. From a practical perspective, some reports have suggested that immunohistochemical staining (factor VIII-related antigen, CD31, and D2-40) is important for detecting lymphovascular emboli, because mimics and artifacts can otherwise lead to mistakes[22,25,26]. Jeon et al[26] reported that immunohistochemical staining resulted in better detection of lymphovascular emboli than the use of routine hematoxylin and eosin staining on ESD specimens. Additional prospective studies are needed to confirm the exact role of immunohistochemistry in the detection of lymphovascular tumor emboli in submucosal invasive EGCs.
RADIOLOGIC OR MOLECULAR BIOMARKERS TO PREDICT LYMPH NODE METASTASIS IN EARLY GASTRIC CANCER
At present, no imaging modalities are capable of reliably predicting LN status in cases of gastric cancer, especially those that involve EGC[9,27]. Imaging modalities that have been investigated include abdominal ultrasonography, endoscopic ultrasonography, computed tomography, magnetic resonance imaging, and positron emission tomography-computed tomography. Endoscopic ultrasonography and some biologic markers have been introduced as possible methods of predicting LN status in cases of gastric cancer, but the true reliabilities of these methods remain controversial. Endoscopic ultrasonography can be used as a screening method to select patients who are suitable for EMR/ESD, according to the measurement of depth of submucosal invasion[28]. Recently, laparoscopic sentinel node biopsy with endoscopic resection has been investigated as a promising method of predicting LN metastasis in EGC; although many clinical attempts regarding the use of sentinel nodes have been performed, this approach still has technical and clinical limitations[29,30].
The use of molecular biomarkers to predict LN metastasis in EGC also has limited clinical utility. Yoshii et al[31] reported that dual loss of membranous E-cadherin and beta-catenin was associated with LN metastasis in intestinal-type EGC. Tanaka et al[32] reported that loss of beta-catenin was associated with LN metastasis of EGC. Mucin-4 expression is also associated with LN metastasis in EGC[33]. The expression of vascular endothelial growth factors C and D is associated with micrometastasis in EGC[34]. Lymphatic vessel density-identified and microvessel density-identified immunohistochemistry of D2-40 and CD31 have been associated with LN metastasis in EGC[35]. Besides tissue biomarkers, preoperative serum angiopoietin-2 level has also been associated with LN metastasis in EGC[36].
CONCLUSION
We recommend 2 steps for the management of EGC using endoscopic resection. The first step is the selection of suitable patients for endoscopic resection, based on endoscopic and histopathologic findings. After endoscopic resection, additional surgical intervention could be determined on the basis of a comprehensive review of the EMR/ESD specimen, including lymphovascular tumor emboli, tumor size, histologic type, and depth of invasion. Gastroenterologists, surgeons, and pathologists should communicate closely during these decision-making processes.
P- Reviewers: Wang YD, Wu SM, Xuan SY S- Editor: Wen LL L- Editor: A E- Editor: Liu XM