Copyright
©2014 Baishideng Publishing Group Co.
World J Gastroenterol. Apr 28, 2014; 20(16): 4681-4691
Published online Apr 28, 2014. doi: 10.3748/wjg.v20.i16.4681
Published online Apr 28, 2014. doi: 10.3748/wjg.v20.i16.4681
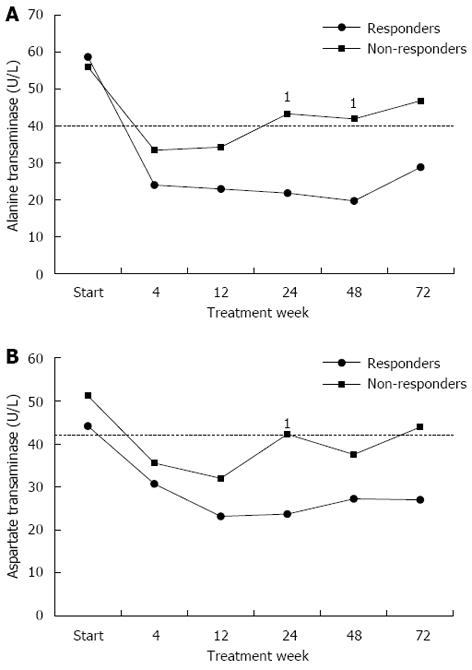
Figure 2 Alanine aminotransferase and aspartate aminotransferase mean levels during treatment in responders and non-responders.
A: Alanine aminotransferase (ALT) mean levels remained normal in responders all through the follow up period, while in non-responders, after starting as normal, they increased again to significantly higher levels than in responders from week 12 onwards, especially at weeks 24 and 48 (1P = 0.007, 0.003 respectively); B: Aspartate aminotransferase (AST) mean levels remained normal in responders all through the follow up period, while in non-responders, after starting as normal, they significantly increased again in week 24 (1P = 0.007) and once more in week 72. The dashed line represents the upper limit of normal.
-
Citation: Naghi SE, Abdel-Ghaffar TY, El-Karaksy H, Abdel-Aty EF, El-Raziky MS, Allam AA, Helmy H, El-Araby HA, Behairy BE, El-Guindi MA, El-Sebaie H, Abdel-Ghaffar AY, Ehsan NA, El-Hennawy AM, Sira MM. Safety and efficacy of
Hansenula -derived PEGylated-interferon alpha-2a and ribavirin combination in chronic hepatitis C Egyptian children. World J Gastroenterol 2014; 20(16): 4681-4691 - URL: https://www.wjgnet.com/1007-9327/full/v20/i16/4681.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i16.4681