Published online Apr 14, 2014. doi: 10.3748/wjg.v20.i14.4030
Revised: February 5, 2013
Accepted: March 6, 2013
Published online: April 14, 2014
Processing time: 506 Days and 8.3 Hours
AIM: To evaluate the safety and feasibility of laparoscopic spleen-preserving distal pancreatectomy (LSPDP) with autologous islet transplantation (AIT) for benign tumors of the pancreatic body-neck.
METHODS: Three non-diabetic, female patients (age 37, 44 and 35 years, respectively) were declared candidates for surgery, between May and September 2011, because of pancreatic body/neck cystic lesions. The planned operation was an LSPDP associated with AIT from the normal pancreas distal to the neoplasm. Islets isolation was performed on the residual pancreatic parenchyma after frozen section examination of the margin. Purified autologous islets were infused into the portal vein by a percutaneous transhepatic approach the day after surgery.
RESULTS: The procedure was performed successfully in all the three cases, and the spleen was preserved along with its vessels. Mean operation time was 283 ± 52 min and average blood loss was 133 ± 57 mL. Residual pancreas weights were 33, 22 and 30 g, and 105.200, 40.390 and 94.790 islet equivalents were isolated, respectively. Surgical complications occurred in one patient (grade A pancreatic fistula). Postoperative stays were 6, 6 and 7 d, respectively. Histopathological evaluation revealed mucinous cystic neoplasm in cases 1 and 3, and serous cystic neoplasm in patient 2. No postoperative insulin administration was required. One patient developed a transient partial portal thrombosis 2 mo after islet infusion. Patients are insulin independent at a mean follow up of 8 ± 2 mo.
CONCLUSION: Combination of LSPDP and AIT is feasible and could be effective to minimize the surgical impact for benign neoplasm of pancreatic body-neck.
Core tip: The article describes, for the first time, a combination of techniques to reduce all possible consequences of pancreatic resection for benign/borderline neoplasms located at the pancreatic body-neck. The procedure combines laparoscopy, spleen preservation and islet autotransplantation. The laparoscopic approach reduces the access trauma of an extensive surgery. The spleen preservation avoids infectious and hematological complications related to splenectomy. Islet autotransplantation could reduce the incidence of pancreatogenic diabetes after resection.
- Citation: Balzano G, Carvello M, Piemonti L, Nano R, Ariotti R, Mercalli A, Melzi R, Maffi P, Braga M, Staudacher C. Combined laparoscopic spleen-preserving distal pancreatectomy and islet autotransplantation for benign pancreatic neoplasm. World J Gastroenterol 2014; 20(14): 4030-4036
- URL: https://www.wjgnet.com/1007-9327/full/v20/i14/4030.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i14.4030
Distal pancreatectomy (DP) is the standard operation for borderline pancreatic tumors located in the body-neck of the pancreas[1,2]. Spleen preservation is indicated in such cases to reduce immunological and hematological impairment[3].
Although there are no prospective randomized studies, there is agreement regarding the feasibility and efficacy of the laparoscopic approach compared with the open procedure[3-10].
DP is associated with the risk of post-surgical diabetes, ranging from 5% to 42%, related with the amount of resected parenchyma[11-15]. Autologous islet transplantation (AIT) is effective in the prevention of post-surgical diabetes, by rescuing the endocrine component of the non-neoplastic resected pancreas[16,17].
Minimizing the surgical damage is a challenging goal, especially in patients with a long life expectancy. In the setting of a benign neoplasm of the pancreatic body-neck, this goal could be achieved completely by the combination of a laparoscopic technique, spleen preservation and AIT; such an approach has not been reported yet. In this report, we describe three patients affected by pancreatic cystic neoplasm of the body-neck, successfully treated by laparoscopic spleen-preserving distal pancreatectomy (LSPDP) and AIT.
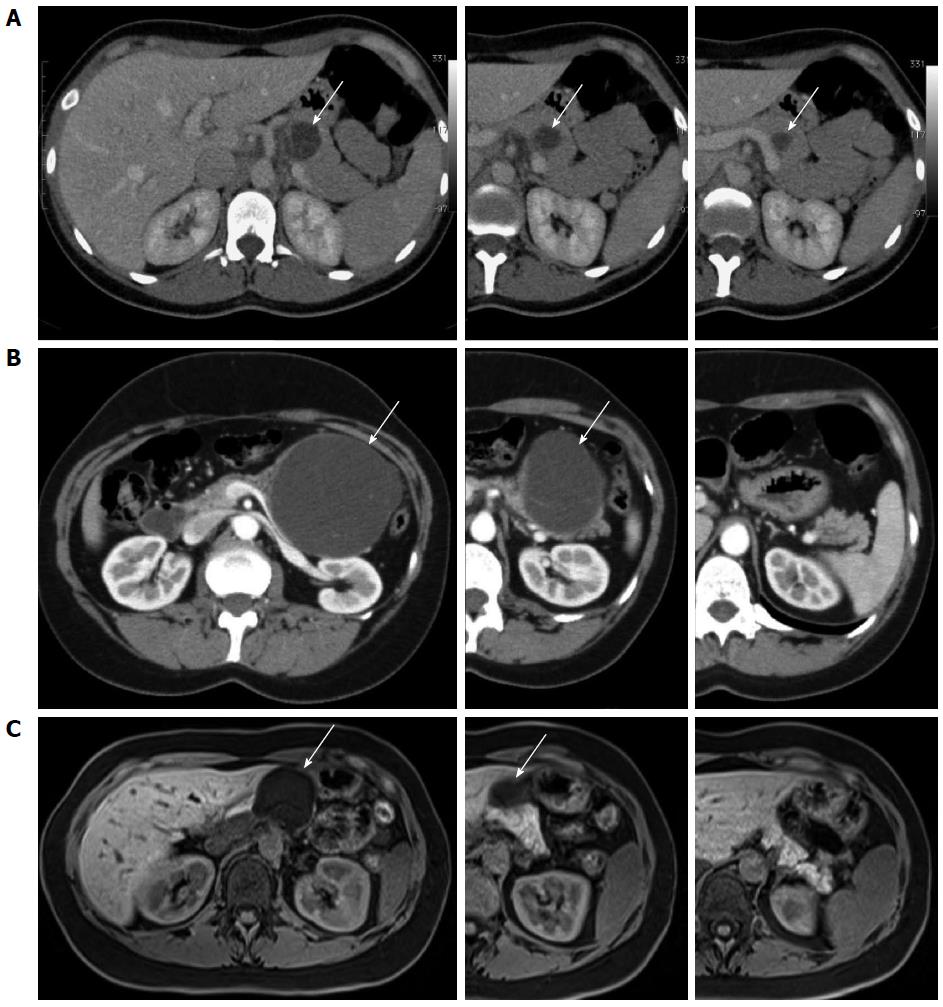
A 37-year-old woman underwent a computerized tomography (CT) scan because of persistent postprandial abdominal pain. The CT-scan revealed a 4 cm cystic lesion in the body of the pancreas, suspected as being a mucinous cistadenoma (Figure 1A). Plasma carcinoembryonic antigen (CEA) and carbohydrate antigen (CA) 19-9 levels were within the normal range. Fine needle biopsy, performed by endoscopic ultrasound, detected the presence of mucinous fluid associated with epithelial cells but without signs of dysplasia.
A 44-year-old woman presented with an abdominal epigastric mass associated with nausea and mild pain since 2 mo. A CT-scan showed a 10 cm cystic lesion of the pancreatic body (Figure 1B). There were no mural nodules or contrast-enhancement of the cyst wall. Plasma CEA and CA 19-9 values were normal.
A 35-year-old woman underwent a CT-scan because of left-sided renal colic. As a collateral finding, a pancreatic cystic lesion was described. Abdominal magnetic resonance imaging (MRI) showed a 5 cm unilocular cyst of the pancreatic neck suspected as being a mucinous cistadenoma, with a 1.5 cm intramural nodule (Figure 1C). Plasma CEA level was normal, while the CA 19-9 was elevated (421 UI/mL). A careful evaluation of the MR images showed no involvement of the surrounding pancreatic parenchyma, with a well-demarcated capsule of the cystic lesion and no dilation of the main pancreatic duct.
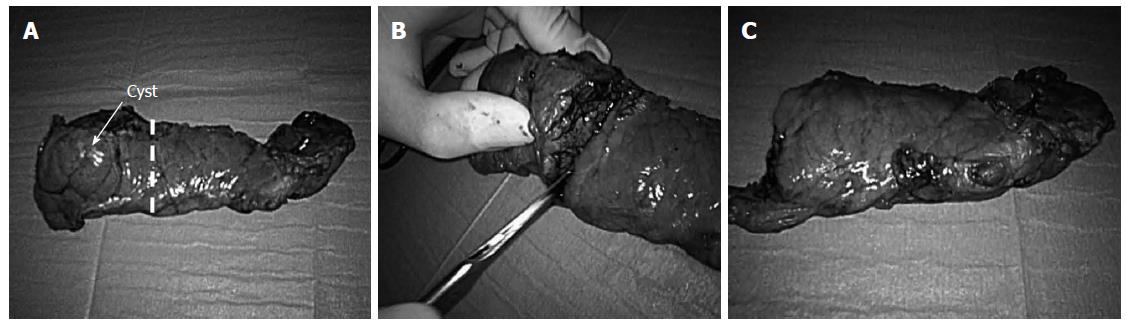
The patient is placed in a supine split legs position with the surgeon standing between the legs. Four trocars are used. After establishing laparoscopic access, the gastrocolic omentum is divided, accessing the lesser sac. In patient 2 (cyst diameter 10 cm) the cyst was emptied to allow adequate exposition of the splenic vessels. A retropancreatic tunnel is accomplished anterior to the superior mesenteric vein, exposing the splenic vein. The inferior border of the pancreatic body is mobilized from right to left. The splenic artery is identified close to its origin and freed from its pancreatic adhesions. The gland is transacted (about two centimeters to the right of the neoplasm) by a single application of a linear stapling device (ECHELON FLEXTM Powered ENDOPATH® Stapler). The splenic vessels are then skeletonized, proceeding towards the splenic hilum. The surgical specimen is extracted by endocatch through Pfannenstiel incision. The specimen is transected distal from the lesion with a 1 cm safety margin (Figure 2). A frozen section examination of this margin is performed. The spared pancreas is processed for islet isolation if the margin is negative.
The described surgical procedure was performed for all three patients.
Islets isolation process is performed by an automated method, as previously described[18,19]. Briefly, the pancreatic duct is perfused by a collagenase solution. After parenchymal distension, the organ is digested and the islets are freed from the exocrine tissue. Using a COBE machine the islets are then purified.
Under ultrasound guidance, using a percutaneous transhepatic approach, the islets are infused into the right portal vein.
Capillary blood glucose was measured four times per day during the postoperative hospital stay. A mixed meal tolerance test was performed 1 month after transplantation. Fasting serum C-peptide, insulin and hemoglobin A1c were measured at 15 d and at 1, 2, 3 and 6 mo after transplantation.
Operations were performed laparoscopically, with no conversion; the spleen was preserved along with the splenic vessels. The average blood loss was 133 ± 57 mL. The mean operative time was 283 ± 52 min. Intraoperative frozen section examination of the pancreatic margin was normal. In patient 3, because of the cyst’s characteristics and CA 19-9 elevation, an intraoperative frozen section examination of the cyst wall was performed to rule out malignancy. Pathological evaluation showed a mucinous cystoadenoma for patients 1 and 3, and a serous cystodenoma for patient 2. No major complication occurred during the operation or in the early postoperative period. Patient 3 developed a grade A pancreatic fistula (ISGPF definition[20]); the surgical drain was removed on postoperative day 36. Hospital stays were 6, 6 and 7 d, respectively.
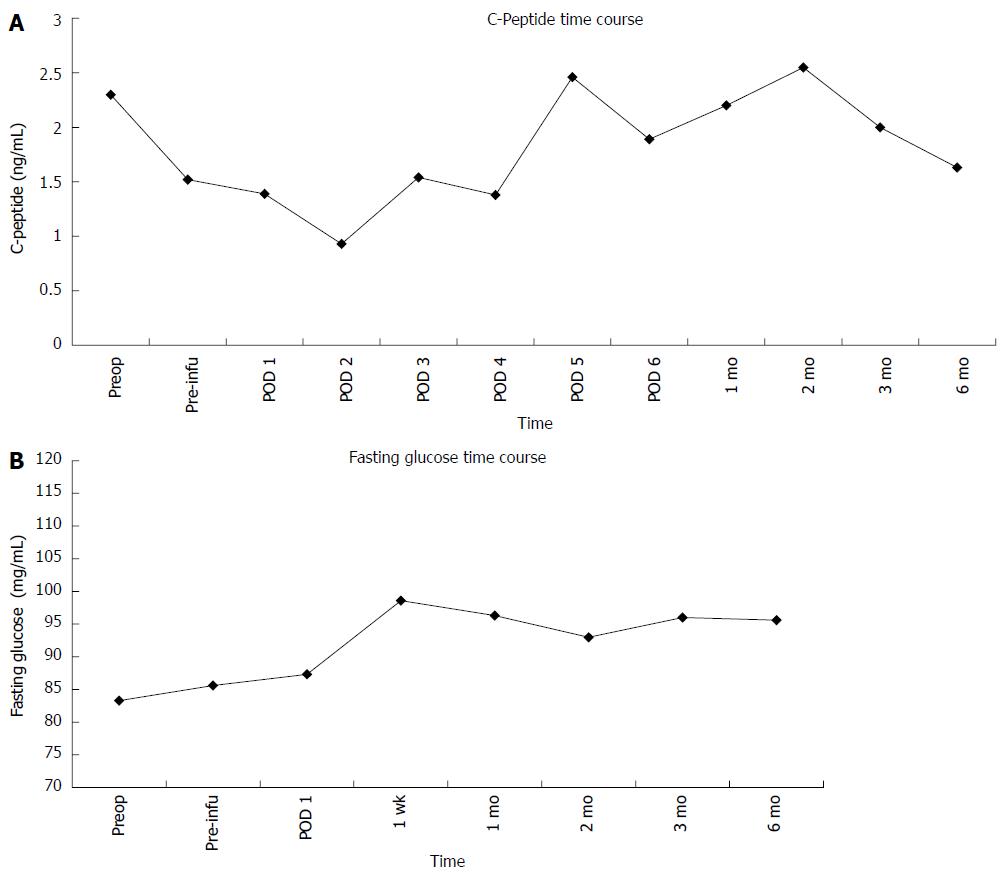
The weights of the processed pancreatic parenchymas were 30, 22 and 33 g for patients 1-3, respectively. After the isolation process, 94.790, 40.390 and 105.200 islet equivalents (IEs) were obtained at the final count, while absolute islet numbers were 268.000, 95.000 and 274.800, respectively. IE per kilogram of body weight (IE/kg) were 1528, 553 and 1696, respectively. Portal vein pressure remained stable after islet infusion. No bleeding occurred after islet infusion, and regular portal system patency was documented the day after reinfusion by ultrasound assessment. No postoperative insulin administration was required. One month after surgery, patient 3 was diagnosed with partial portal vein thrombosis by Doppler ultrasound, and was treated with low-molecular weight heparin. Metabolic assessment showed normal insulin production during follow-up. C-peptide values were comparable to preoperative values and remained stable during follow-up (Figure 3A). Fasting glucose remained stable after transplantation compared with the pre-transplant level (Figure 3B). The three patients were insulin independent at a median follow-up of 8 ± 2 mo.
Traditionally, DP is associated with splenectomy, and it is performed through a wide incision. For a borderline neoplasm of the body-neck, the removal of a significant healthy portion of the gland is required, with consequent risk of pancreatogenic diabetes.
LSPDP with AIT may be a paradigm of the surgical evolution towards minimizing the operative short- and long-term impact. The mainstays of this procedure are the reduction of access trauma through laparoscopy, the avoidance of immunological and hematological long-term consequences of splenectomy, and the improvement of postoperative glycemic control by AIT. The advantages of laparoscopy have not yet been proved by any randomized trial comparing laparoscopic versus laparotomic DP. However, several studies showed the benefits of the laparoscopic approach, and no difference between the two procedures in terms of complications have been reported[4-6,21-23].
In our case series, no conversion was required, the mean surgical time was 266 ± 37 min, and, despite the AIT procedure, hospital stay was 6-7 d. Grade A fistula occurred in one of the three patients.
Splenectomy may result in long-term immunological and hematological impairment[24-26]. Spleen-preservation is therefore indicated for patients undergoing distal pancreatic resection for benign and low-grade malignant lesions[23]. Moreover, after spleen preservation, a reduction in early postoperative complications, especially infective morbidity, has been reported[27].
The incidence of new-onset diabetes after DP is probably underestimated: a retrospective study on 125 non-diabetic patients reported a minimal rate of postoperative diabetes (7.5% when excluding patients with chronic pancreatitis)[15]. Patients with chronic pancreatitis have an increased risk (up to 42%) of developing diabetes after distal pancreatic resection, because the endocrine function of the organ is already impaired[12]. However, analysis of diabetes onset in hemipancreatectomized living-donors for pancreas transplantation showed an unexpectedly high rate of glucose metabolism impairment: 25% of donors had overt diabetes or glucose intolerance[28] and 40% had abnormalities of glucose metabolism 3-10 years after donation[29].
To solve this problem, median pancreatectomy has been proposed as a surgical strategy for the treatment of benign neoplasms of the pancreatic body-neck[30-33]. However, with respect to DP, median pancreatectomy has a higher risk of pancreatic fistula (50%)[32,34].
Islet autotransplantation is an alternative to median pancreatectomy to preserve the endocrine function of distal pancreas, without increasing the fistula risk. Ris et al[17] performed open DP and AIT successfully in 25 patients within a 17-year period. At a median follow-up of 90 mo, all the patients were insulin independent[17].
In our case series, we isolated pancreatic islets from the resected pancreases and re-infused them, preserving endocrine function and maintaining euglycemia in the early and late post-operative period. The expected complications of the transhepatic islet infusion are low, mainly related to minor intra-and perihepatic bleeding and transient portal thrombosis[35,36]. One of three patients was diagnosed with partial intrahepatic thrombosis one month after islet infusion, which was successfully treated by low molecular weight heparin therapy.
When a conservative operation is planned, accurate consideration has to be made concerning the possibility of unsuspected malignancy. Indeed, the reported rate of malignancy in three large series of spleen-preserving DP published in 2012, was < 1% (two out of 213 overall patients)[23,37,38]. In case of suspicion of malignancy, endoscopic ultrasonography can provide a more accurate description of the lesion morphology, allowing fine needle aspiration (FNA) to be performed for cytological evaluation of the cyst fluid or of the solid component of the cyst wall. The dissemination risk of endoscopic FNA in presumed benign lesions was never reported, but even in cases of pancreatic malignancy, endoscopic FNA is considered not to increase the risk of peritoneal dissemination[39].
A further concern regards the possibility of occult malignancy in the normal pancreas segment to be processed for AIT. Pre- and intra-operative work up is essential to select adequate cases for autotransplantation. In our protocol, the presence of any multifocal pancreatic neoplasm at preoperative imaging or intraoperative evaluation, including multifocal benign intraductal-papillary mucinous neoplasm or a diagnosis (suspected or ascertained) of multiple endocrine neoplasm is an exclusion criterion. In the retrospective study carried out by Ris et al[17], 3 mm was considered a suitable safety margin, as demonstrated by the postoperative follow up. In the 90 mo median follow-up, all the patients were disease free. In our short series, pancreatic specimens were sent for frozen section analysis of the margin, with a margin of 1 cm. Furthermore, in patient 3, because of the preoperative findings, a frozen section examination of the cyst wall was performed to look for mural nodules and rule out malignancy before islet infusion.
The ultimate goal of this technique is to reduce the morbidity of extended pancreatic resection, required for patients with benign pancreatic tumor, occurring at the body-neck site. The minimally invasive treatment, with the preservation of the spleen and the vessels in association with AIT, may provide an improvement in early and late postoperative quality of life. However, while laparoscopic DP is increasingly performed, very few institutions may offer an islet producing facility. Besides the referral of patients to specific institutions, the creation of a network with neighboring hospitals should be considered to provide access to AIT to a wider number of candidate patients to this procedure[17].
To the best of our knowledge, this is the first study demonstrating the feasibility and safety of the minimally invasive spleen preserving left pancreatectomy combined with AIT. A larger patient series is needed to confirm the metabolic advantages of the technique.
Benign pancreatic lesion of the pancreatic neck might require extensive surgery. The widespread use of laparoscopy has considerably reduced direct surgical trauma. Spleen preservation in such non-malignant cases has reduced the infectious morbidity of the standard procedure. However, with distal pancreatectomy, a considerable portion of the gland is removed, possibly leading to pancreatogenic diabetes. Islet auto transplantation is a promising strategy for reducing the risk of diabetes onset after pancreatic resection.
This article could be important for the future evolution of minimally invasive procedures in pancreatic surgery.
Laparoscopy has already reduced the surgical trauma to patients. However, there may be lifelong metabolic consequences (pancreatogenic diabetes) as a result of pancreatic resection. Islet autotransplantation has been demonstrated as effective in the control of metabolic impairment after pancreatic resection. The main goal of this technique is to provide a combination of minimally invasive treatment and improvement of long term metabolic outcome.
The procedure has been demonstrated as feasible. A larger patient series is needed to confirm the metabolic advantages of the technique.
Autologous islet transplantation is a procedure in which the endocrine component of the pancreas (islets of Langerhans) is preserved by a laboratory isolation process. The islets are then usually infused into the portal system by a percutaneous transhepatic approach.
Interesting small series of autotransplantation in minimally invasive partial resection of benign pancreatic neoplasms.
P- Reviewer: Merrett N S- Editor: Gou SX L- Editor: Stewart GJ E- Editor: Zhang DN
| 1. | Khalid A, Brugge W. ACG practice guidelines for the diagnosis and management of neoplastic pancreatic cysts. Am J Gastroenterol. 2007;102:2339-2349. [PubMed] |
| 2. | Sakorafas GH, Smyrniotis V, Reid-Lombardo KM, Sarr MG. Primary pancreatic cystic neoplasms revisited: part II. Mucinous cystic neoplasms. Surg Oncol. 2011;20:e93-101. [PubMed] |
| 3. | Ammori BJ, Ayiomamitis GD. Laparoscopic pancreaticoduodenectomy and distal pancreatectomy: a UK experience and a systematic review of the literature. Surg Endosc. 2011;25:2084-2099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 84] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 4. | Mehta SS, Doumane G, Mura T, Nocca D, Fabre JM. Laparoscopic versus open distal pancreatectomy: a single-institution case-control study. Surg Endosc. 2012;26:402-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 60] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 5. | Limongelli P, Belli A, Russo G, Cioffi L, D’Agostino A, Fantini C, Belli G. Laparoscopic and open surgical treatment of left-sided pancreatic lesions: clinical outcomes and cost-effectiveness analysis. Surg Endosc. 2012;26:1830-1836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 70] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 6. | Jusoh AC, Ammori BJ. Laparoscopic versus open distal pancreatectomy: a systematic review of comparative studies. Surg Endosc. 2012;26:904-913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 114] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 7. | Subhas G, Gupta N, Mittal VK, Jacobs MJ. Laparoscopic three-port distal pancreatectomy. HPB (Oxford). 2011;13:361-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Lillemoe KD, Kaushal S, Cameron JL, Sohn TA, Pitt HA, Yeo CJ. Distal pancreatectomy: indications and outcomes in 235 patients. Ann Surg. 1999;229:693-68; discussion 693-68;. [PubMed] |
| 9. | Asbun HJ, Stauffer JA. Laparoscopic approach to distal and subtotal pancreatectomy: a clockwise technique. Surg Endosc. 2011;25:2643-2649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 10. | Abu Hilal M, Hamdan M, Di Fabio F, Pearce NW, Johnson CD. Laparoscopic versus open distal pancreatectomy: a clinical and cost-effectiveness study. Surg Endosc. 2012;26:1670-1674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 101] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 11. | Yamaguchi K, Yokohata K, Ohkido M, Watanabe M, Ogawa Y, Chijiiwa K, Tanaka M. Which is less invasive--distal pancreatectomy or segmental resection. Int Surg. 2000;85:297-302. [PubMed] |
| 12. | Maeda H, Hanazaki K. Pancreatogenic diabetes after pancreatic resection. Pancreatology. 2011;11:268-276. [PubMed] |
| 13. | Kalil AN, Lichtenfels E, Fornari A, Rhoden E, Giovenardi R. Management of cystic neoplasms of the pancreas. Hepatogastroenterology. 2002;49:1432-1435. [PubMed] |
| 14. | Hutchins RR, Hart RS, Pacifico M, Bradley NJ, Williamson RC. Long-term results of distal pancreatectomy for chronic pancreatitis in 90 patients. Ann Surg. 2002;236:612-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 15. | King J, Kazanjian K, Matsumoto J, Reber HA, Yeh MW, Hines OJ, Eibl G. Distal pancreatectomy: incidence of postoperative diabetes. J Gastrointest Surg. 2008;12:1548-1553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 107] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 16. | Najarian JS, Sutherland DE, Baumgartner D, Burke B, Rynasiewicz JJ, Matas AJ, Goetz FC. Total or near total pancreatectomy and islet autotransplantation for treatment of chronic pancreatitis. Ann Surg. 1980;192:526-542. [PubMed] |
| 17. | Ris F, Niclauss N, Morel P, Demuylder-Mischler S, Muller Y, Meier R, Genevay M, Bosco D, Berney T. Islet autotransplantation after extended pancreatectomy for focal benign disease of the pancreas. Transplantation. 2011;91:895-901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 18. | Ricordi C, Lacy PE, Finke EH, Olack BJ, Scharp DW. Automated method for isolation of human pancreatic islets. Diabetes. 1988;37:413-420. [PubMed] |
| 19. | Shapiro AM, Ricordi C, Hering BJ, Auchincloss H, Lindblad R, Robertson RP, Secchi A, Brendel MD, Berney T, Brennan DC. International trial of the Edmonton protocol for islet transplantation. N Engl J Med. 2006;355:1318-1330. [PubMed] |
| 20. | Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J, Neoptolemos J, Sarr M, Traverso W, Buchler M. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 2005;138:8-13. [PubMed] |
| 21. | Venkat R, Edil BH, Schulick RD, Lidor AO, Makary MA, Wolfgang CL. Laparoscopic distal pancreatectomy is associated with significantly less overall morbidity compared to the open technique: a systematic review and meta-analysis. Ann Surg. 2012;255:1048-1059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 374] [Cited by in RCA: 381] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 22. | Boutros C, Ryan K, Katz S, Espat NJ, Somasundar P. Total laparoscopic distal pancreatectomy: beyond selected patients. Am Surg. 2011;77:1526-1530. [PubMed] |
| 23. | Butturini G, Inama M, Malleo G, Manfredi R, Melotti GL, Piccoli M, Perandini S, Pederzoli P, Bassi C. Perioperative and long-term results of laparoscopic spleen-preserving distal pancreatectomy with or without splenic vessels conservation: a retrospective analysis. J Surg Oncol. 2012;105:387-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 24. | Chaikof EL, McCabe CJ. Fatal overwhelming postsplenectomy infection. Am J Surg. 1985;149:534-539. [PubMed] |
| 25. | Leonard AS, Giebink GS, Baesl TJ, Krivit W. The overwhelming postsplenectomy sepsis problem. World J Surg. 1980;4:423-432. [PubMed] |
| 26. | Crary SE, Buchanan GR. Vascular complications after splenectomy for hematologic disorders. Blood. 2009;114:2861-2868. [PubMed] |
| 27. | Shoup M, Brennan MF, McWhite K, Leung DH, Klimstra D, Conlon KC. The value of splenic preservation with distal pancreatectomy. Arch Surg. 2002;137:164-168. [PubMed] [DOI] [Full Text] |
| 28. | Kendall DM, Sutherland DE, Najarian JS, Goetz FC, Robertson RP. Effects of hemipancreatectomy on insulin secretion and glucose tolerance in healthy humans. N Engl J Med. 1990;322:898-903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 180] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 29. | Kumar AF, Gruessner RW, Seaquist ER. Risk of glucose intolerance and diabetes in hemipancreatectomized donors selected for normal preoperative glucose metabolism. Diabetes Care. 2008;31:1639-1643. [PubMed] |
| 30. | Balzano G, Zerbi A, Veronesi P, Cristallo M, Di Carlo V. Surgical treatment of benign and borderline neoplasms of the pancreatic body. Dig Surg. 2003;20:506-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 59] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 31. | Orsenigo E, Baccari P, Bissolotti G, Staudacher C. Laparoscopic central pancreatectomy. Am J Surg. 2006;191:549-552. [PubMed] |
| 32. | Efron DT, Lillemoe KD, Cameron JL, Yeo CJ. Central pancreatectomy with pancreaticogastrostomy for benign pancreatic pathology. J Gastrointest Surg. 2004;8:532-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 82] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 33. | Hirono S, Tani M, Kawai M, Ina S, Nishioka R, Miyazawa M, Shimizu A, Uchiyama K, Yamaue H. A central pancreatectomy for benign or low-grade malignant neoplasms. J Gastrointest Surg. 2009;13:1659-1665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 62] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 34. | Balzano G, Zerbi A, Cristallo M, Di Carlo V. The unsolved problem of fistula after left pancreatectomy: the benefit of cautious drain management. J Gastrointest Surg. 2005;9:837-842. [PubMed] |
| 35. | Kawahara T, Kin T, Kashkoush S, Gala-Lopez B, Bigam DL, Kneteman NM, Koh A, Senior PA, Shapiro AM. Portal vein thrombosis is a potentially preventable complication in clinical islet transplantation. Am J Transplant. 2011;11:2700-2707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 76] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 36. | Villiger P, Ryan EA, Owen R, O’Kelly K, Oberholzer J, Al Saif F, Kin T, Wang H, Larsen I, Blitz SL. Prevention of bleeding after islet transplantation: lessons learned from a multivariate analysis of 132 cases at a single institution. Am J Transplant. 2005;5:2992-2998. [PubMed] |
| 37. | Adam1 JP, Jacquin A, Laurent C, Collet D, Masson B, Fernández-Cruz L, Sa-Cunha A. Laparoscopic spleen-preserving distal pancreatectomy: splenic vessel preservation compared with the Warshaw technique. JAMA Surg. 2013;148:246-252. [PubMed] |
| 38. | Hwang HK, Chung YE, Kim KA, Kang CM, Lee WJ. Revisiting vascular patency after spleen-preserving laparoscopic distal pancreatectomy with conservation of splenic vessels. Surg Endosc. 2012;26:1765-1771. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 39. | Ikezawa K, Uehara H, Sakai A, Fukutake N, Imanaka K, Ohkawa K, Tanakura R, Ioka T, Tanaka S, Ishikawa O. Risk of peritoneal carcinomatosis by endoscopic ultrasound-guided fine needle aspiration for pancreatic cancer. J Gastroenterol. 2013;48:966-972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |