Published online Mar 14, 2014. doi: 10.3748/wjg.v20.i10.2586
Revised: January 5, 2014
Accepted: January 20, 2014
Published online: March 14, 2014
Hepatopulmonary syndrome (HPS) is characterized as a triad: liver disease, intrapulmonary vascular dilatation and arterial hypoxemia. HPS is reported to be present in 4% to 32% of adult patients with end-stage liver disease and in 9%-20% of children. The pathogenesis of HPS has not been clearly identified. Portal hypertension causes impairment in the perfusion of the bowel and increases the enteral translocation of Gram (-) bacteria and endotoxins. This stimulates the release of vasoactive mediators, such as tumor necrosis factor-alpha, heme oxygenase-derived carbon monoxide and nitric oxide. Genetic alterations have not been associated with this syndrome yet; however, cytokines and chemokines have been suggested to play a role. Recently, it was reported that cumulated monocytes lead to the activation of vascular endothelial growth factor-dependent signaling pathways and pulmonary angiogenesis, which plays an important role in HPS pathogenesis. At present, the most effective and only radical treatment is a liver transplant (LT). Cirrhotic patients who are on the waiting list for an LT have a shorter survival period if they develop HPS. Therefore, it is suggested that all cirrhotic cases should be followed closely for HPS and they should have priority in the waiting list.
Core tip: Hepatopulmonary syndrome (HPS) is an important complication of cirrhosis. HPS is a significant factor in dyspnea and cyanosis in cirrhotic cases. At present, the most effective and only radical treatment is a liver transplant. Cirrhotic patients who are on the waiting list for a liver transplant have a shorter survival period if they develop HPS. Therefore, it is suggested that all cirrhotic cases should be followed closely for HPS and they should have priority in the waiting list. This review aims to reevaluate the recent progress in the diagnosis, pathophysiology and treatment of HPS.
- Citation: Tumgor G. Cirrhosis and hepatopulmonary syndrome. World J Gastroenterol 2014; 20(10): 2586-2594
- URL: https://www.wjgnet.com/1007-9327/full/v20/i10/2586.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i10.2586
Pulmonary complications related to chronic liver diseases are frequently observed. The two most significant complications among them are hepatopulmonary syndrome (HPS) and portopulmonary hypertension (POPH). HPS is observed more frequently than POPH in patients with chronic liver diseases. HPS has been reported to be present in 4% to 32% of adult patients with end-stage liver disease[1,2], and in 9%-20% of children[3-5].
Kennedy et al[6] first defined HPS in 1977. HPS is characterized as a triad: liver disease, intrapulmonary vascular dilatation and arterial hypoxemia[7]. Cirrhosis is the most common condition associated with HPS. The cause of liver disease leading to portal hypertension does not seem to affect the development of HPS. HPS has been reported in patients with prehepatic portal hypertension in the absence of chronic liver disease, in Budd-Chiari syndrome and even in patients with acute or chronic inflammatory liver disease without evidence of cirrhosis or portal hypertension[8-12].
Prognosis is poor with the development of HPS in patients waiting for a liver transplant (LT). Therefore, these patients should be followed closely. This review aims to reevaluate the recent progress in the diagnosis, pathophysiology and treatment of HPS.
The pathogenesis of HPS has not been clearly identified. However, there are some important clinical clues. Although it has been observed in cases without cirrhosis and portal hypertension, most patients with HPS have cirrhosis and portal hypertension[4,13].
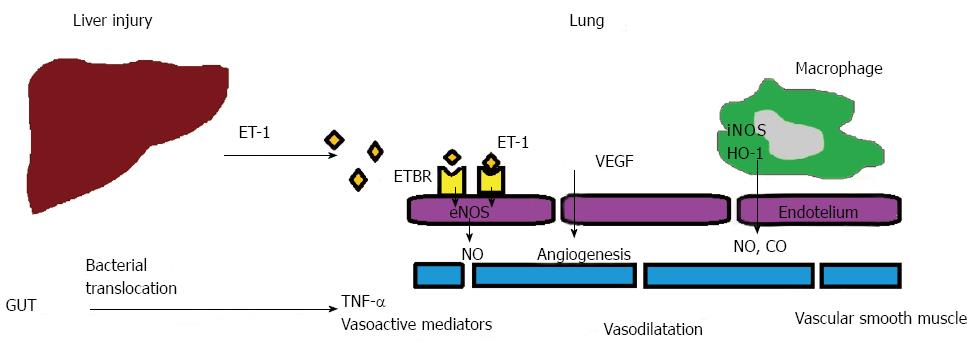
Portal hypertension causes impairment in the perfusion of the bowel and increases the enteral translocation of Gram (-) bacteria and endotoxins. This stimulates the release of vasoactive mediators, such as tumor necrosis factor-alpha (TNF-α), heme oxygenase (HO)-derived carbon monoxide (CO) and nitric oxide (NO)[14-18] (Figure 1).
In clinical studies, the increase of nitric oxide production in the lung plays a role in HPS pathogenesis[16-23]. When compared with cirrhotic control patients, exhalation NO levels increase in the cases with cirrhotic HPS. Plasma endothelin-1 (ET-1) levels increase in cases with cirrhosis and intrapulmonary vascular dilatation[24-26]. ET-1 causes NO-related vasodilatation with the activation of endothelin B receptors (ETBR) on endothelial cells[27,28]. In addition, increased phagocytosis of bacterial endotoxin in the lung promotes activation of inducible NO synthase (iNOS), which also contributes toward increased NO production. Bacterial translocation, and subsequent monocyte accumulation, may also stimulate pulmonary angiogenesis in HPS, which may partly be controlled by genetic factors[13].
Genetic alterations have not been associated with HPS yet; however, cytokines and chemokines have been suggested to play a role. It is more common in patients carrying the monocyte chemoattractant protein-1 (MCP-1) 2518G gene; conversely it is less frequent in patients with the endothelial NO synthase (eNOS) 298Asp allele and those carrying eNOS 298Asp[29]. These results suggest that the eNOS 298Asp polymorphism can prevent the development of HPS in cirrhotic patients. In addition, the G allele may be associated with higher MCP-1 expression in certain inflammatory conditions. The effect of the G allele appears to be dose dependent: cells from individuals homozygous for G at -2518 produced more MCP-1 than cells from G/A heterozygotes[30]. Higher levels of MCP-1, an inflammation marker, in HPS suggest the role of inflammation in the development of pulmonary shunts. Moreover, macrophages produce HO-1, which leads an increase in the production of CO and contributes to the vasodilatation[15]. CO is produced by the catabolism of heme with heme oxygenase[15,31].
Recently, cumulated monocytes have been observed to lead to the activation of vascular endothelial growth factor-dependent signaling pathways and pulmonary angiogenesis, which plays an important role in HPS pathogenesis[32]. Gene polymorphisms involved in the regulation of angiogenesis have also been associated with the risk of developing HPS[33].
Although other mediators, such as somatostatin analogue (octreotide), glucagon, prostacyclin, angiotensin-2, vasoactive intestinal peptide, calcitonin, substance P, atrial natriuretic factor and platelet-activating factor, may play a role in the pathogenesis of vascular changes in HPS, no clear relation was found between any of these mediators and vascular dilatation[34-39].
Important mechanisms mentioned above result in ventilation-perfusion (V/Q) mismatch, diffusion limitation of oxygen and, less commonly, direct arteriovenous connections[40,41]. The capillaries dilate to 15-500 μm (n: 8-15 μm) in HPS[42].
The hypoxia occurs because of the increased cardiac output caused by pulmonary vasodilatation and the inadequate oxygenation of the blood, which runs through enlarged pulmonary microcapillaries (Figure 2). In addition, hypoxia worsens as a result of the vasoconstrictor response to hypoxia, especially in the lower zones where there is less ventilation[43]. Lowered V/Q ratios result in elevated ΔP (A-a) O2, which is correctable only by 100% oxygen inhalation. However, hypoxemia caused by larger arteriovenous shunts (AVS) does not respond to inhalation of 100% oxygen[44].
Previous studies have shown that certain changes occur in lungs of animals with HPS. Melo-Silva et al[45] showed that in rat models, the tidal volume, minute ventilation and mean inspiratory flow were significantly reduced, chest wall pressure dissipation against the resistive and viscoelastic components and elasticity were increased, and the lung resistive pressure dissipation was lower; however, the viscoelastic pressure was higher in the HPS group. The proportion of collagen volume in the vasculature increased by 29% in the HPS animals.
Furthermore, patients with hepatic cirrhosis have an elevated plasma level of lipopolysacchride (LPS)[46]. Extra LPS was given to rats with cirrhosis, which resulted in further widening of the alveoli wall, a decreased density of cells, narrowed alveoli space and destruction of the integrity of type I cell membrane, with infiltration of polymorphs and fibrinous exudates, indicating interstitial pulmonary edema and an inflammatory reaction. There was severe stasis of the blood in alveolar walls and numerous red cells extravasated the airspace, resulting in the widespread dilatation of alveolar capillaries and the augmentation of the permeability of the microvasculature[18].
Cases of HPS can be asymptomatic or they can present with growth retardation, cyanoses, dyspnea, platypnea, orthopnea, spider angioma or finger clubbing. In a study conducted in The Mayo Clinic, 82% of 22 patients with HPS had symptoms and findings related to liver disease, and the time period from the respiratory complaints to the diagnosis of HPS was an average of 4.8 ± 2.5 years. In the same study, 18% of the patients complained of labored breathing and the liver disease was diagnosed after further tests[47]. The most frequent symptom was progressive dyspnea. Platypnea is defined as dyspnea of a patient when he/she is in an upright position. Digital clubbing and cyanosis are also frequent in HPS patients[29,48,49]. Spider angioma is not specific for the diagnosis of HPS[50]. The findings of chest radiography and decreased CO diffusion capacity determined by pulmonary function tests for the patients with HPS were nonspecific[51].
There is a poor correlation between liver disease and the level of oxygenation. While it has been reported mostly in patients with severely decompensated end-stage liver disease, namely child C patients, it has been also observed in patients with child A and child B cirrhosis[13,52].
In cirrhotic cases with suspicion of HPS, other cardiopulmonary diseases, such as pulmonary atelectasis, ascites, chronic obstructive pulmonary diseases, hepatic hydrothorax and infections should be ruled out.
Two types of HPS have been defined. While type 1 is related to precapillary dilatations, AVS is associated with type 2 HPS. In addition, while type 1 HPS responds to oxygen support, type 2 does not[53]. If PO2≥ 600 mmHg when the patient is given 100% oxygen, it is considered that it is probably not AVS. If the PO2 fails rise to 150-200 mmHg or over, then the hypoxia may be considered to be caused by AVS[54].
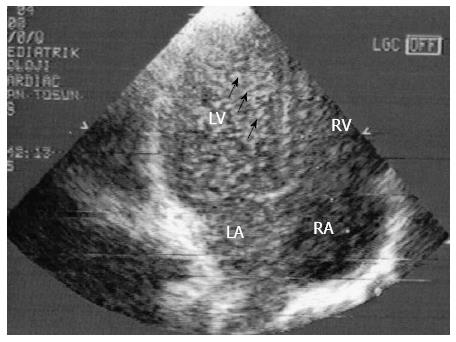
HPS is a serious complication frequently seen in cirrhotic patients and results from hypoxemia. The clinical findings in HPS are the same as those of hypoxemia. Contrast echocardiography (CEE) is accepted as a sensitive screening test for HPS. Other techniques, such as technetium 99m-labeled macroaggregated albumin (MAA) scan, computed tomography or pulmonary angiography, have also been used to diagnose HPS[55]. Saline microbubbles are used for CCE. A transpulmonary passage was considered present if microbubbles appeared in the left atrium at least three heartbeats after the initial appearance of contrast in the right side of the heart[56-59] (Figure 3).
Transesophageal CEE increases the imaging quality of the heart; therefore, it is more sensitive than transthoracic CEE to identify intrapulmonary vasodilatation and may detect HPS, which can go unnoticed by transthoracic CEE[60]. For patients with hypoxemic HPS, there is no study that shows it is more superior. The detection of intrapulmonary shunts by this method is not sufficient to diagnose HPS and impaired oxygenation is required to be shown.
In addition, in cases with HPS, left ventricle enlargement and higher systolic velocity in the mitral valve represent satisfactory indirect markers of HPS[41], in addition to CEE[61].
Orthodeoxia, positional modification of abnormal shunting, was defined as a fall in PaO2≥ 5% when upright, or 4 mmHg. While the PaO2 is normal in the horizontal position, it decreases in the upright position, depending on the increase of blood flow velocity in arteriovenous anastomosis in the basal segments of the lungs, caused by the effect of the gravity. This increases the ventilation-perfusion mismatch and hypoxia becomes apparent[62]. Orthodeoxia has been reported to be present in 20% to 80% of patients with HPS[63,64]. Hypoxemia is defined as PaO2 < 70 mmHg, and severe hypoxemia is defined as PaO2 < 50 mmHg. At sea level, and while breathing room air, a resting PA-aO2 of ≥ 2.0 kPa (≥ 15 mmHg) can be considered abnormal. For the people over 64, it should be evaluated as PA-aO2≥ 20 mmHg. Accordingly, the PA-aO2vs PaO2 calculation is done to grade the severity of HPS[58] (Table 1).
| Stage | PA-aO2 mmHg | PaO2 mmHg |
| Mild | ≥ 15 | ≥ 80 |
| Moderate | ≥ 15 | < 80 - ≥ 60 |
| Severe | ≥ 15 | < 60 - ≥ 50 |
| Very severe | ≥ 15 | < 50 (< 300 on 100% O2) |
In recent years, transcutaneous oxygen saturation measurement with pulse oximetry has emerged a simple, low cost, and widely available technique to screen for HPS. With a threshold value of < 96%, pulse oximetry has a sensitivity and specificity of 100% and 88%, respectively, for detecting patients with a PaO2 < 60 mmHg. A pulse oximetry value of < 94% detected all patients with a PaO2 < 60 mmHg with an increased specificity of 93%[65,66]. Contrary to these findings, CEE may be positive despite normal arterial blood gases. In a prospective study of candidates for LT, Krowka et al[67], found that 9.7% of 31 normoxemic patients had positive CEE. These findings suggested that mild or subclinical intrapulmonary vasodilatations insufficiency in cirrhotic patients may not alter gas exchange.
In the Technetium 99m-labeled MAA scan, MAA particles are given intravenously. The diameter of the marked particles is 20-50 μm and normally they cannot pass through pulmonary veins, which have a diameter of 8-15 μm. However, in the presence of an intrapulmonary shunt, these marked particles enter the circulatory system and appear in the kidneys and the brain. In the diagnosis of HPS, a value greater than 6% is significant and specific for HPS. However, as MAA provides positive results in the presence of intracardiac shunts as well; therefore, its sensitivity is low[41,68].
Unless there is an accompanying pulmonary disease, the spirometric tests in HPS are not impaired. However, abnormal diffusion capacity for carbon monoxide (DLCO) is frequently observed in patients with HPS[69]. In one study, the DLCO was decreased in 80% of the cases[70]. However, its specificity is low; therefore, it is not used in practice.
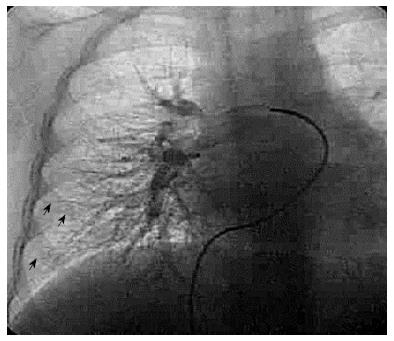
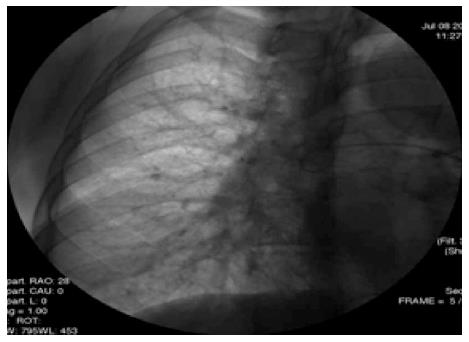
Pulmonary angiography is more invasive and less sensitive compared with high resolution chest computed tomography CEE[71] (Figures 4 and 5).
Currently, there are no effective medical therapies for HPS. In the past, HPS was considered as a contraindication for LT because of serious operative and perioperative complications in adults[72]. Today, LT is the only effective treatment option for patients with this condition, because of the underlying liver disease.
Although several investigations have been performed, no effective medical treatment has been found. Several attempts have been made to inhibit the development of HPS by administering nitric oxide, using diets low in L-arginine using methylene blue, which is an inhibitor of guanylate cyclase[73], aspirin[36], somatostatin[49,74], almitrine[75], N-acetylcysteine[76], indomethacin[51], garlic[77,78], mycophenolate mofetil (an inhibitor of angiogenesis and nitric oxide production)[79], pentoxifylline[80], decreasing the increased portal pressure by transjugular portosystemic shunt[81-83], and using antibiotics to decrease bacterial translocation in the bowel[84]. However, a role for any of these drugs in the long-term treatment of HPS has not been demonstrated. Currently, the most effective treatment is LT.
In cirrhotic cases with HPS, survival was significantly decreased compared with cirrhotic cases without HPS. In one study, the five-year survival rate was determined as 23% for cases with HPS and 67% for patients without HPS[85]. In studies performed at The Mayo Clinic, 33%-40% of patients with HPS died within 2.5-4 years. Most of the patients having clinically stable hepatic function worsened with the development of HPS[47]. In our center, eleven of 16 patients (68.7%) with HPS died before LT. The main causes of deaths were variceal bleeding accompanying multiorgan failure, pneumonia and sepsis[4].
Therefore, in addition to their Model for End-Stage Liver Disease (MELD) scores, it is very important for patients on the waiting list to be diagnosed as having HPS or not. However, many clinical features of HPS that might influence exception to the MELD scoring system, including standardized diagnostic criteria, pre- and post-LT mortality rates, and the rate of progression of hypoxemia, are not fully characterized[86]. In the United States, UNOS allows patients with confirmed HPS to be listed for an LT. For this reason, it is suggested that the patients with PaO2 < 60 mmHg on room air in the sitting position, should receive an increase in their MELD scores, and during their waiting period they should get a 2- to 3-point increase in their MELD scores every three months[86]. The indications are that patients who had an LT this way showed better survival. Iyer et al[87] observed a trend (without reaching statistical significance) for better 5-year survival in the MELD exception era (since 2002) as compared to earlier HPS transplants.
The severity of preoperative hypoxemia, underlying liver disease and comorbidities appear to be factors that increase postoperative mortality[88,89]. While some studies show that the mortality rate of cases with HPS are 29%-38.5%[4,90], there are other studies that show the survival after an LT is not different to that od the cirrhotic cases without HPS[84,89]. In fact Iyer et al[87] suggested that survival post-LT was not dependent on baseline PaO2 values obtained at the time of HPS diagnosis.
85%-100% of patients have an improvement in oxygenation within 1 year of an LT[4,53]. However, in some severe cases with hypoxemia, lung function may not recover up to one year after the operation. Most of these cases need oxygen for 5 to 700 d, and spend longer in hospital[89].
After liver transplantation and after all the factors that led to HPS have disappeared, patients recover from HPS[91,92]. In a single post mortem study, the changes after the transplantation in lungs in HPS could be related to collagen tissue deposition in pulmonary capillary and venule walls[93]. However, the recovery from HPS after transplantation shows that the pathological changes in the lungs in Type I HPS are reversible.
In our center, the perioperative and postoperative outcomes were uneventful in all patients. Even if previous reports indicated that patients with HPS require ventilatory support after an LT[67,94], two patients remained on mechanical ventilation and they were extubated two and five days post-transplant, respectively. If the post-operative hypoxemia is refractory to standard treatments, NO and trendelenburg positioning (supine position with feet elevated 15°-30° higher) and oscillator ventilation therapy are reported to be effective[89]. Experiences related with outcome of HPS after an LT are limited; portal venous thrombosis, intracranial events and multiorgan failure are seen more commonly in patients with HPS, and these complications are associated with a higher mortality rate[95]. In our series, we did not observe these complications, except for one case of acute respiratory distress syndrome, which was observed on postoperative day 1. This was probably related to systemic inflammatory response syndrome, because the patient had arterial hypoxemia, fever and ground glass opacity on the chest radiography.
In conclusion, HPS is an important complication of cirrhosis, causing dyspnea and cyanosis in cirrhotic cases. There is no relation between the development of HPS and the severity of cirrhosis. At present, the most effective and only radical treatment is an LT. Cirrhotic patients who are on the waiting list for an LT have a shorter survival period if they develop HPS. Therefore, it is suggested that all cirrhotic cases should be followed closely for HPS and should have priority in the waiting list.
P- Reviewers: Castellote J, Takagi H S- Editor: Wen LL L- Editor: Stewart G E- Editor: Liu XM
| 1. | Schenk P, Fuhrmann V, Madl C, Funk G, Lehr S, Kandel O, Müller C. Hepatopulmonary syndrome: prevalence and predictive value of various cut offs for arterial oxygenation and their clinical consequences. Gut. 2002;51:853-859. [PubMed] [DOI] [Cited in This Article: ] |
| 2. | Varghese J, Ilias-basha H, Dhanasekaran R, Singh S, Venkataraman J. Hepatopulmonary syndrome - past to present. Ann Hepatol. 2007;6:135-142. [PubMed] [Cited in This Article: ] |
| 3. | Noli K, Solomon M, Golding F, Charron M, Ling SC. Prevalence of hepatopulmonary syndrome in children. Pediatrics. 2008;121:e522-e527. [PubMed] [DOI] [Cited in This Article: ] |
| 4. | Tumgor G, Arikan C, Yuksekkaya HA, Cakir M, Levent E, Yagci RV, Kilic M, Aydogdu S. Childhood cirrhosis, hepatopulmonary syndrome and liver transplantation. Pediatr Transplant. 2008;12:353-357. [PubMed] [DOI] [Cited in This Article: ] |
| 5. | Sari S, Oguz D, Sucak T, Dalgic B, Atasever T. Hepatopulmonary syndrome in children with cirrhotic and non-cirrhotic portal hypertension: a single-center experience. Dig Dis Sci. 2012;57:175-181. [PubMed] [DOI] [Cited in This Article: ] |
| 6. | Kennedy TC, Knudson RJ. Exercise-aggravated hypoxemia and orthodeoxia in cirrhosis. Chest. 1977;72:305-309. [PubMed] [DOI] [Cited in This Article: ] |
| 7. | Krowka MJ. Hepatopulmonary syndrome versus portopulmonary hypertension: distinctions and dilemmas. Hepatology. 1997;25:1282-1284. [PubMed] [DOI] [Cited in This Article: ] |
| 8. | Hoeper MM, Krowka MJ, Strassburg CP. Portopulmonary hypertension and hepatopulmonary syndrome. Lancet. 2004;363:1461-1468. [PubMed] [DOI] [Cited in This Article: ] |
| 9. | Abrams GA, Fallon MB. The hepatopulmonary syndrome. Clin Liver Dis. 1997;1:185-200, xiii. [PubMed] [DOI] [Cited in This Article: ] |
| 10. | De BK, Sen S, Biswas PK, Sanyal R, Majumdar D, Biswas J. Hepatopulmonary syndrome in inferior vena cava obstruction responding to cavoplasty. Gastroenterology. 2000;118:192-196. [PubMed] [Cited in This Article: ] |
| 11. | Gupta D, Vijaya DR, Gupta R, Dhiman RK, Bhargava M, Verma J, Chawla YK. Prevalence of hepatopulmonary syndrome in cirrhosis and extrahepatic portal venous obstruction. Am J Gastroenterol. 2001;96:3395-3399. [PubMed] [DOI] [Cited in This Article: ] |
| 12. | Tzovaras N, Stefos A, Georgiadou SP, Gatselis N, Papadamou G, Rigopoulou E, Ioannou M, Skoularigis I, Dalekos GN. Reversion of severe hepatopulmonary syndrome in a non cirrhotic patient after corticosteroid treatment for granulomatous hepatitis: a case report and review of the literature. World J Gastroenterol. 2006;12:336-339. [PubMed] [Cited in This Article: ] |
| 13. | Grace JA, Angus PW. Hepatopulmonary syndrome: update on recent advances in pathophysiology, investigation, and treatment. J Gastroenterol Hepatol. 2013;28:213-219. [PubMed] [DOI] [Cited in This Article: ] |
| 14. | Nunes H, Lebrec D, Mazmanian M, Capron F, Heller J, Tazi KA, Zerbib E, Dulmet E, Moreau R, Dinh-Xuan AT. Role of nitric oxide in hepatopulmonary syndrome in cirrhotic rats. Am J Respir Crit Care Med. 2001;164:879-885. [PubMed] [Cited in This Article: ] |
| 15. | Carter EP, Hartsfield CL, Miyazono M, Jakkula M, Morris KG, McMurtry IF. Regulation of heme oxygenase-1 by nitric oxide during hepatopulmonary syndrome. Am J Physiol Lung Cell Mol Physiol. 2002;283:L346-L353. [PubMed] [DOI] [Cited in This Article: ] |
| 16. | Strassburg CP. Gastrointestinal disorders of the critically ill. Shock liver. Best Pract Res Clin Gastroenterol. 2003;17:369-381. [PubMed] [DOI] [Cited in This Article: ] |
| 17. | Zhang HY, Han DW, Wang XG, Zhao YC, Zhou X, Zhao HZ. Experimental study on the role of endotoxin in the development of hepatopulmonary syndrome. World J Gastroenterol. 2005;11:567-572. [PubMed] [Cited in This Article: ] |
| 18. | Zhang HY, Han DW, Zhao ZF, Liu MS, Wu YJ, Chen XM, Ji C. Multiple pathogenic factor-induced complications of cirrhosis in rats: a new model of hepatopulmonary syndrome with intestinal endotoxemia. World J Gastroenterol. 2007;13:3500-3507. [PubMed] [Cited in This Article: ] |
| 19. | Vallance P, Moncada S. Hyperdynamic circulation in cirrhosis: a role for nitric oxide? Lancet. 1991;337:776-778. [PubMed] [DOI] [Cited in This Article: ] |
| 20. | Fallon MB, Abrams GA, Luo B, Hou Z, Dai J, Ku DD. The role of endothelial nitric oxide synthase in the pathogenesis of a rat model of hepatopulmonary syndrome. Gastroenterology. 1997;113:606-614. [PubMed] [DOI] [Cited in This Article: ] |
| 21. | Liu L, Zhang M, Luo B, Abrams GA, Fallon MB. Biliary cyst fluid from common bile duct-ligated rats stimulates endothelial nitric oxide synthase in pulmonary artery endothelial cells: a potential role in hepatopulmonary syndrome. Hepatology. 2001;33:722-727. [PubMed] [Cited in This Article: ] |
| 22. | Rolla G, Brussino L, Colagrande P, Scappaticci E, Morello M, Bergerone S, Ottobrelli A, Cerutti E, Polizzi S, Bucca C. Exhaled nitric oxide and impaired oxygenation in cirrhotic patients before and after liver transplantation. Ann Intern Med. 1998;129:375-378. [PubMed] [DOI] [Cited in This Article: ] |
| 23. | Schroeder RA, Ewing CA, Sitzmann JV, Kuo PC. Pulmonary expression of iNOS and HO-1 protein is upregulated in a rat model of prehepatic portal hypertension. Dig Dis Sci. 2000;45:2405-2410. [PubMed] [Cited in This Article: ] |
| 24. | Asbert M, Ginès A, Ginès P, Jiménez W, Clària J, Saló J, Arroyo V, Rivera F, Rodés J. Circulating levels of endothelin in cirrhosis. Gastroenterology. 1993;104:1485-1491. [PubMed] [Cited in This Article: ] |
| 25. | Luo B, Abrams GA, Fallon MB. Endothelin-1 in the rat bile duct ligation model of hepatopulmonary syndrome: correlation with pulmonary dysfunction. J Hepatol. 1998;29:571-578. [PubMed] [DOI] [Cited in This Article: ] |
| 26. | Koch DG, Bogatkevich G, Ramshesh V, Lemasters JJ, Uflacker R, Reuben A. Elevated levels of endothelin-1 in hepatic venous blood are associated with intrapulmonary vasodilatation in humans. Dig Dis Sci. 2012;57:516-523. [PubMed] [DOI] [Cited in This Article: ] |
| 27. | Sato K, Oka M, Hasunuma K, Ohnishi M, Sato K, Kira S. Effects of separate and combined ETA and ETB blockade on ET-1-induced constriction in perfused rat lungs. Am J Physiol. 1995;269:L668-L672. [PubMed] [Cited in This Article: ] |
| 28. | Davenport AP, O’Reilly G, Molenaar P, Maguire JJ, Kuc RE, Sharkey A, Bacon CR, Ferro A. Human endothelin receptors characterized using reverse transcriptase-polymerase chain reaction, in situ hybridization, and subtype-selective ligands BQ123 and BQ3020: evidence for expression of ETB receptors in human vascular smooth muscle. J Cardiovasc Pharmacol. 1993;22 Suppl 8:S22-S25. [PubMed] [Cited in This Article: ] |
| 29. | Tumgor G, Berdeli A, Arikan C, Levent E, Aydogdu S. Mcp-1, eNOS, tPA and PAI-1 gene polymorphism and correlation of genotypes and phenotypes in hepatopulmonary syndrome. Dig Dis Sci. 2008;53:1345-1351. [PubMed] [Cited in This Article: ] |
| 30. | Rovin BH, Yoshiumura T, Tan L. Cytokine-induced production of monocyte chemoattractant protein-1 by cultured human mesangial cells. J Immunol. 1992;148:2148-2153. [PubMed] [Cited in This Article: ] |
| 31. | Arguedas MR, Drake BB, Kapoor A, Fallon MB. Carboxyhemoglobin levels in cirrhotic patients with and without hepatopulmonary syndrome. Gastroenterology. 2005;128:328-333. [PubMed] [DOI] [Cited in This Article: ] |
| 32. | Zhang J, Luo B, Tang L, Wang Y, Stockard CR, Kadish I, Van Groen T, Grizzle WE, Ponnazhagan S, Fallon MB. Pulmonary angiogenesis in a rat model of hepatopulmonary syndrome. Gastroenterology. 2009;136:1070-1080. [PubMed] [DOI] [Cited in This Article: ] |
| 33. | Roberts KE, Kawut SM, Krowka MJ, Brown RS, Trotter JF, Shah V, Peter I, Tighiouart H, Mitra N, Handorf E. Genetic risk factors for hepatopulmonary syndrome in patients with advanced liver disease. Gastroenterology. 2010;139:130-139.e24. [PubMed] [DOI] [Cited in This Article: ] |
| 34. | King PD, Rumbaut R, Sanchez C. Pulmonary manifestations of chronic liver disease. Dig Dis. 1996;14:73-82. [PubMed] [DOI] [Cited in This Article: ] |
| 35. | Panos RJ, Baker SK. Mediators, cytokines, and growth factors in liver-lung interactions. Clin Chest Med. 1996;17:151-169. [PubMed] [Cited in This Article: ] |
| 36. | Song JY, Choi JY, Ko JT, Bae EJ, Kim HS, Noh CI, Yoon YS. Long-term aspirin therapy for hepatopulmonary syndrome. Pediatrics. 1996;97:917-920. [PubMed] [Cited in This Article: ] |
| 37. | Ebeid AM, Escourrou J, Soeters PB, Murray P, Fischer JE. Hepatic inactivation of vasoactive intestinal peptide in man and dog. Ann Surg. 1978;188:28-33. [PubMed] [Cited in This Article: ] |
| 38. | Hörtnagl H, Singer EA, Lenz K, Kleinberger G, Lochs H. Substance P is markedly increased in plasma of patients with hepatic coma. Lancet. 1984;1:480-483. [PubMed] [DOI] [Cited in This Article: ] |
| 39. | Caramelo C, Fernández-Gallardo S, Santos JC, Iñarrea P, Sánchez-Crespo M, López-Novoa JM, Hernando L. Increased levels of platelet-activating factor in blood from patients with cirrhosis of the liver. Eur J Clin Invest. 1987;17:7-11. [PubMed] [DOI] [Cited in This Article: ] |
| 40. | Berthelot P, Walker JG, Sherlock S, Reid L. Arterial changes in the lungs in cirrhosis of the liver--lung spider nevi. N Engl J Med. 1966;274:291-298. [PubMed] [DOI] [Cited in This Article: ] |
| 41. | Genovesi MG, Tierney DF, Taplin GV, Eisenberg H. An intravenous radionuclide method to evaluate hypoxemia caused by abnormal alveolar vessels. Limitation of conventional techniques. Am Rev Respir Dis. 1976;114:59-65. [PubMed] [Cited in This Article: ] |
| 42. | Castro M, Krowka MJ. Hepatopulmonary syndrome. A pulmonary vascular complication of liver disease. Clin Chest Med. 1996;17:35-48. [PubMed] [Cited in This Article: ] |
| 43. | Carter EP, Sato K, Morio Y, McMurtry IF. Inhibition of K(Ca) channels restores blunted hypoxic pulmonary vasoconstriction in rats with cirrhosis. Am J Physiol Lung Cell Mol Physiol. 2000;279:L903-L910. [PubMed] [Cited in This Article: ] |
| 44. | Khan AN, Al-Jahdali H, Abdullah K, Irion KL, Sabih Q, Gouda A. Pulmonary vascular complications of chronic liver disease: Pathophysiology, imaging, and treatment. Ann Thorac Med. 2011;6:57-65. [PubMed] [DOI] [Cited in This Article: ] |
| 45. | Melo-Silva CA, Gaio E, Trevizoli JE, Souza CS, Gonçalves AS, Sousa GC, Takano G, Tavares P, Amado VM. Respiratory mechanics and lung tissue remodeling in a hepatopulmonary syndrome rat model. Respir Physiol Neurobiol. 2011;179:326-333. [PubMed] [DOI] [Cited in This Article: ] |
| 46. | Zhao LF, Han DW. Clinical significance of endotoxemia in patients with liver disease. Shijie Huaren Xiaohua Zazhi. 1999;7:391-393. [Cited in This Article: ] |
| 47. | Krowka MJ, Dickson ER, Cortese DA. Hepatopulmonary syndrome. Clinical observations and lack of therapeutic response to somatostatin analogue. Chest. 1993;104:515-521. [PubMed] [DOI] [Cited in This Article: ] |
| 48. | Martínez GP, Barberà JA, Visa J, Rimola A, Paré JC, Roca J, Navasa M, Rodés J, Rodriguez-Roisin R. Hepatopulmonary syndrome in candidates for liver transplantation. J Hepatol. 2001;34:651-657. [PubMed] [DOI] [Cited in This Article: ] |
| 49. | Mohammad Alizadeh AH, Fatemi SR, Mirzaee V, Khoshbaten M, Talebipour B, Sharifian A, Khoram Z, Haj-sheikh-oleslami F, Gholamreza-shirazi M, Zali MR. Clinical features of hepatopulmonary syndrome in cirrhotic patients. World J Gastroenterol. 2006;12:1954-1956. [PubMed] [Cited in This Article: ] |
| 50. | Sood G, Fallon MB, Niwas S, Tutton T, van Leeuwen DJ, Bloomer JR. Utility of a dyspnea-fatigue index for screening liver transplant candidates for hepatopulmonary syndrome (Abstract). Hepatology. 1998;28:742. [Cited in This Article: ] |
| 51. | Machicao VI, Fallon MB. Hepatopulmonary syndrome. Semin Respir Crit Care Med. 2012;33:11-16. [PubMed] [DOI] [Cited in This Article: ] |
| 52. | Tumgor G, Ozkan T, Ulger Z, Kilic M, Aydogdu S. Liver transplantation of a child with child a cirrhosis and severe hepatopulmonary syndrome. Transplant Proc. 2006;38:1432-1434. [PubMed] [Cited in This Article: ] |
| 53. | Krowka MJ, Cortese DA. Hepatopulmonary syndrome: an evolving perspective in the era of liver transplantation. Hepatology. 1990;11:138-142. [PubMed] [DOI] [Cited in This Article: ] |
| 54. | Krowka MJ, Wiseman GA, Burnett OL, Spivey JR, Therneau T, Porayko MK, Wiesner RH. Hepatopulmonary syndrome: a prospective study of relationships between severity of liver disease, PaO(2) response to 100% oxygen, and brain uptake after (99m)Tc MAA lung scanning. Chest. 2000;118:615-624. [PubMed] [DOI] [Cited in This Article: ] |
| 55. | Köksal D, Kaçar S, Köksal AS, Tüfekçioğlu O, Küçükay F, Okten S, Saşmaz N, Arda K, Sahin B. Evaluation of intrapulmonary vascular dilatations with high-resolution computed thorax tomography in patients with hepatopulmonary syndrome. J Clin Gastroenterol. 2006;40:77-83. [PubMed] [Cited in This Article: ] |
| 56. | Shub C, Tajik AJ, Seward JB, Dines DE. Detecting intrapulmonary right-to-left shunt with contrast echocardiography. Observations in a patient with diffuse pulmonary arteriovenous fistulas. Mayo Clin Proc. 1976;51:81-84. [PubMed] [Cited in This Article: ] |
| 57. | Herve P, Le Pavec J, Sztrymf B, Decante B, Savale L, Sitbon O. Pulmonary vascular abnormalities in cirrhosis. Best Pract Res Clin Gastroenterol. 2007;21:141-159. [PubMed] [DOI] [Cited in This Article: ] |
| 58. | Rodríguez-Roisin R, Krowka MJ, Hervé P, Fallon MB. Pulmonary-Hepatic vascular Disorders (PHD). Eur Respir J. 2004;24:861-880. [PubMed] [DOI] [Cited in This Article: ] |
| 59. | Palma DT, Fallon MB. The hepatopulmonary syndrome. J Hepatol. 2006;45:617-625. [PubMed] [DOI] [Cited in This Article: ] |
| 60. | Aller R, Moya JL, Moreira V, Boixeda D, Cano A, Picher J, García-Rull S, de Luis DA. Diagnosis of hepatopulmonary syndrome with contrast transesophageal echocardiography: advantages over contrast transthoracic echocardiography. Dig Dis Sci. 1999;44:1243-1248. [PubMed] [DOI] [Cited in This Article: ] |
| 61. | Pouriki S, Alexopoulou A, Chrysochoou C, Raftopoulos L, Papatheodoridis G, Stefanadis C, Pectasides D. Left ventricle enlargement and increased systolic velocity in the mitral valve are indirect markers of the hepatopulmonary syndrome. Liver Int. 2011;31:1388-1394. [PubMed] [DOI] [Cited in This Article: ] |
| 62. | Robin ED, Laman D, Horn BR, Theodore J. Platypnea related to orthodeoxia caused by true vascular lung shunts. N Engl J Med. 1976;294:941-943. [PubMed] [DOI] [Cited in This Article: ] |
| 63. | Cheng TO. Mechanisms of platypnea-orthodeoxia: what causes water to flow uphill? Circulation. 2002;105:e47. [PubMed] [Cited in This Article: ] |
| 64. | Gómez FP, Martínez-Pallí G, Barberà JA, Roca J, Navasa M, Rodríguez-Roisin R. Gas exchange mechanism of orthodeoxia in hepatopulmonary syndrome. Hepatology. 2004;40:660-666. [PubMed] [DOI] [Cited in This Article: ] |
| 65. | Arguedas MR, Singh H, Faulk DK, Fallon MB. Utility of pulse oximetry screening for hepatopulmonary syndrome. Clin Gastroenterol Hepatol. 2007;5:749-754. [PubMed] [DOI] [Cited in This Article: ] |
| 66. | Abrams GA, Sanders MK, Fallon MB. Utility of pulse oximetry in the detection of arterial hypoxemia in liver transplant candidates. Liver Transpl. 2002;8:391-396. [PubMed] [DOI] [Cited in This Article: ] |
| 67. | Krowka MJ, Mandell MS, Ramsay MA, Kawut SM, Fallon MB, Manzarbeitia C, Pardo M, Marotta P, Uemoto S, Stoffel MP. Hepatopulmonary syndrome and portopulmonary hypertension: a report of the multicenter liver transplant database. Liver Transpl. 2004;10:174-182. [PubMed] [DOI] [Cited in This Article: ] |
| 68. | Abrams GA, Jaffe CC, Hoffer PB, Binder HJ, Fallon MB. Diagnostic utility of contrast echocardiography and lung perfusion scan in patients with hepatopulmonary syndrome. Gastroenterology. 1995;109:1283-1288. [PubMed] [DOI] [Cited in This Article: ] |
| 69. | Lima BL, França AV, Pazin-Filho A, Araújo WM, Martinez JA, Maciel BC, Simões MV, Terra-Filho J, Martinelli AL. Frequency, clinical characteristics, and respiratory parameters of hepatopulmonary syndrome. Mayo Clin Proc. 2004;79:42-48. [PubMed] [Cited in This Article: ] |
| 70. | Rodriguez-Roisin R, Roca J, Agusti AG, Mastai R, Wagner PD, Bosch J. Gas exchange and pulmonary vascular reactivity in patients with liver cirrhosis. Am Rev Respir Dis. 1987;135:1085-1092. [PubMed] [Cited in This Article: ] |
| 71. | Lee KN, Lee HJ, Shin WW, Webb WR. Hypoxemia and liver cirrhosis (hepatopulmonary syndrome) in eight patients: comparison of the central and peripheral pulmonary vasculature. Radiology. 1999;211:549-553. [PubMed] [Cited in This Article: ] |
| 72. | Maddrey WC, Van Thiel DH. Liver transplantation: an overview. Hepatology. 1988;8:948-959. [PubMed] [DOI] [Cited in This Article: ] |
| 73. | Schenk P, Madl C, Rezaie-Majd S, Lehr S, Müller C. Methylene blue improves the hepatopulmonary syndrome. Ann Intern Med. 2000;133:701-706. [PubMed] [DOI] [Cited in This Article: ] |
| 74. | Salem O, Dindzans VJ, Freeman J, O’Dorisio T, Ruthardt F, Van Thiel DH. Liver transplantation following preoperative closure of intrapulmonary shunts. J Okla State Med Assoc. 1994;87:53-55. [PubMed] [Cited in This Article: ] |
| 75. | Krowka MJ, Cortese DA. Severe hypoxemia associated with liver disease: Mayo Clinic experience and the experimental use of almitrine bismesylate. Mayo Clin Proc. 1987;62:164-173. [PubMed] [DOI] [Cited in This Article: ] |
| 76. | Vercelino R, Tieppo J, Dias AS, Marroni CA, Garcia E, Meurer L, Picada JN, Marroni NP. N-acetylcysteine effects on genotoxic and oxidative stress parameters in cirrhotic rats with hepatopulmonary syndrome. Basic Clin Pharmacol Toxicol. 2008;102:370-376. [PubMed] [DOI] [Cited in This Article: ] |
| 77. | Abrams GA, Fallon MB. Treatment of hepatopulmonary syndrome with Allium sativum L. (garlic): a pilot trial. J Clin Gastroenterol. 1998;27:232-235. [PubMed] [Cited in This Article: ] |
| 78. | Najafi Sani M, Kianifar HR, Kianee A, Khatami G. Effect of oral garlic on arterial oxygen pressure in children with hepatopulmonary syndrome. World J Gastroenterol. 2006;12:2427-2431. [PubMed] [Cited in This Article: ] |
| 79. | Moreira Silva H, Reis G, Guedes M, Cleto E, Vizcaíno JR, Kelly D, Gennery AR, Santos Silva E. A case of hepatopulmonary syndrome solved by mycophenolate mofetil (an inhibitor of angiogenesis and nitric oxide production). J Hepatol. 2013;58:630-633. [PubMed] [DOI] [Cited in This Article: ] |
| 80. | Tanikella R, Philips GM, Faulk DK, Kawut SM, Fallon MB. Pilot study of pentoxifylline in hepatopulmonary syndrome. Liver Transpl. 2008;14:1199-1203. [PubMed] [DOI] [Cited in This Article: ] |
| 81. | Lasch HM, Fried MW, Zacks SL, Odell P, Johnson MW, Gerber DA, Sandhu FS, Fair JH, Shrestha R. Use of transjugular intrahepatic portosystemic shunt as a bridge to liver transplantation in a patient with severe hepatopulmonary syndrome. Liver Transpl. 2001;7:147-149. [PubMed] [DOI] [Cited in This Article: ] |
| 82. | Paramesh AS, Husain SZ, Shneider B, Guller J, Tokat I, Gondolesi GE, Moyer S, Emre S. Improvement of hepatopulmonary syndrome after transjugular intrahepatic portasystemic shunting: case report and review of literature. Pediatr Transplant. 2003;7:157-162. [PubMed] [DOI] [Cited in This Article: ] |
| 83. | Martinez-Palli G, Drake BB, Garcia-Pagan JC, Barbera JA, Arguedas MR, Rodriguez-Roisin R, Bosch J, Fallon MB. Effect of transjugular intrahepatic portosystemic shunt on pulmonary gas exchange in patients with portal hypertension and hepatopulmonary syndrome. World J Gastroenterol. 2005;11:6858-6862. [PubMed] [Cited in This Article: ] |
| 84. | Rabiller A, Nunes H, Lebrec D, Tazi KA, Wartski M, Dulmet E, Libert JM, Mougeot C, Moreau R, Mazmanian M. Prevention of gram-negative translocation reduces the severity of hepatopulmonary syndrome. Am J Respir Crit Care Med. 2002;166:514-517. [PubMed] [DOI] [Cited in This Article: ] |
| 85. | Swanson KL, Wiesner RH, Krowka MJ. Natural history of hepatopulmonary syndrome: Impact of liver transplantation. Hepatology. 2005;41:1122-1129. [PubMed] [DOI] [Cited in This Article: ] |
| 86. | Fallon MB, Mulligan DC, Gish RG, Krowka MJ. Model for end-stage liver disease (MELD) exception for hepatopulmonary syndrome. Liver Transpl. 2006;12:S105-S107. [PubMed] [DOI] [Cited in This Article: ] |
| 87. | Iyer VN, Swanson KL, Cartin-Ceba R, Dierkhising RA, Rosen CB, Heimbach JK, Wiesner RH, Krowka MJ. Hepatopulmonary syndrome: favorable outcomes in the MELD exception era. Hepatology. 2013;57:2427-2435. [PubMed] [DOI] [Cited in This Article: ] |
| 88. | Krowka MJ. Hepatopulmonary syndromes. Gut. 2000;46:1-4. [PubMed] [DOI] [Cited in This Article: ] |
| 89. | Gupta S, Castel H, Rao RV, Picard M, Lilly L, Faughnan ME, Pomier-Layrargues G. Improved survival after liver transplantation in patients with hepatopulmonary syndrome. Am J Transplant. 2010;10:354-363. [PubMed] [DOI] [Cited in This Article: ] |
| 90. | Yi HM, Wang GS, Yi SH, Yang Y, Cai CJ, Chen GH. Prospective evaluation of postoperative outcome after liver transplantation in hepatopulmonary syndrome patients. Chin Med J (Engl). 2009;122:2598-2602. [PubMed] [DOI] [Cited in This Article: ] |
| 91. | Ewert R, Mutze S, Schachschal G, Lochs H, Plauth M. High prevalence of pulmonary diffusion abnormalities without interstitial changes in long-term survivors of liver transplantation. Transpl Int. 1999;12:222-228. [PubMed] [DOI] [Cited in This Article: ] |
| 92. | Go▽mez FP, Martı▽nez-Pallı▽ G, Garcı▽a-Valdecasas JC, Barbera` JA, Roca J, Rodriguez-Roisin R. Incomplete gas-exchange resolution after liver transplantation in hepatopulmonary syndrome. Eur Respir J. 2003;22 Suppl 45:19s (abstract). [Cited in This Article: ] |
| 93. | Stanley NN, Williams AJ, Dewar CA, Blendis LM, Reid L. Hypoxia and hydrothoraces in a case of liver cirrhosis: correlation of physiological, radiographic, scintigraphic, and pathological findings. Thorax. 1977;32:457-471. [PubMed] [Cited in This Article: ] |
| 94. | Kim HY, Choi MS, Lee SC, Park SW, Lee JH, Koh KC, Paik SW, Yoo BC, Rhee JC. Outcomes in patients with hepatopulmonary syndrome undergoing liver transplantation. Transplant Proc. 2004;36:2762-2763. [PubMed] [DOI] [Cited in This Article: ] |