Copyright
©2014 Baishideng Publishing Group Co.
World J Gastroenterol. Jan 7, 2014; 20(1): 64-77
Published online Jan 7, 2014. doi: 10.3748/wjg.v20.i1.64
Published online Jan 7, 2014. doi: 10.3748/wjg.v20.i1.64
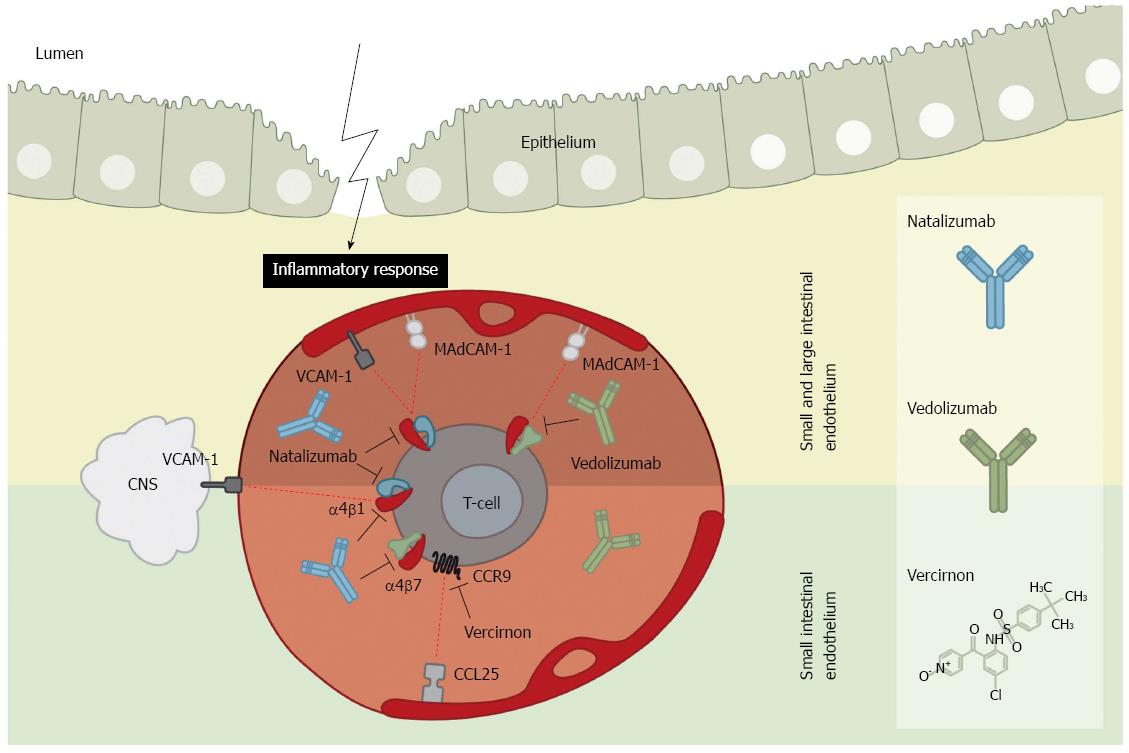
Figure 2 Integrin and chemokine inhibitors in intestinal endothelium.
Mucosal addressin cell adhesion molecule-1 (MAdCAM-1) and vascular adhesion molecule-1 (VCAM-1) adhesion molecules become up-regulated on the endothelium during intestinal inflammation. In the small and large intestine (upper panel) the adhesion molecule MAdCAM-1 functions as docking site for integrins α4β1 and α4β7 expressed by T-cells. Contrary, the adhesion molecule VCAM-1 also acts as docking molecule for α4β1. Addition of the inhibitory α4β1 antibody, natalizumab, thus blocks adhesion of T-cells to the entire intestinal mucosa. As VCAM-1 is additionally expressed within the central nervous system (CNS), natalizumab also blocks T-cell extravasation and hence limits immune surveillance of the CNS which might lead to progressive multifocal leukoencephalopathy. Using the α4β7 inhibitory antibody, vedolizumab, only the adhesion of T-cells to the intestine specific MAdCAM-1 is blocked, and thus does not limit immune surveillance of the CNS while dampening the inflammatory response. In the endothelium of the small intestine (lower panel) the chemokine ligand 25 (CCL25) adhesion molecule is predominantly expressed during inflammation. It acts as a ligand for the chemokine receptor 9 (CCR9) on T-cells. The inhibitor vercirnon blocks the CCL25-CCR9 chemotaxis thus inhibiting T-cells adhesion to the small intestinal mucosa. Vercirnon was recently withdrawn following a phase III trial.
- Citation: Pedersen J, Coskun M, Soendergaard C, Salem M, Nielsen OH. Inflammatory pathways of importance for management of inflammatory bowel disease. World J Gastroenterol 2014; 20(1): 64-77
- URL: https://www.wjgnet.com/1007-9327/full/v20/i1/64.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i1.64