Published online Feb 28, 2013. doi: 10.3748/wjg.v19.i8.1292
Revised: December 24, 2012
Accepted: January 5, 2013
Published online: February 28, 2013
AIM: To determine an optimal cutoff value for abnormal splenic artery diameter/proper hepatic artery diameter (S/P) ratio in cirrhosis-induced portal hypertension.
METHODS: Patients with cirrhosis and portal hypertension (n = 770) and healthy volunteers (n = 31) underwent volumetric computed tomography three-dimensional vascular reconstruction to measure the internal diameters of the splenic artery and proper hepatic artery to calculate the S/P ratio. The cutoff value for abnormal S/P ratio was determined using receiver operating characteristic curve analysis, and the prevalence of abnormal S/P ratio and associations between abnormal S/P ratio and major complications of portal hypertension were studied using logistic regression.
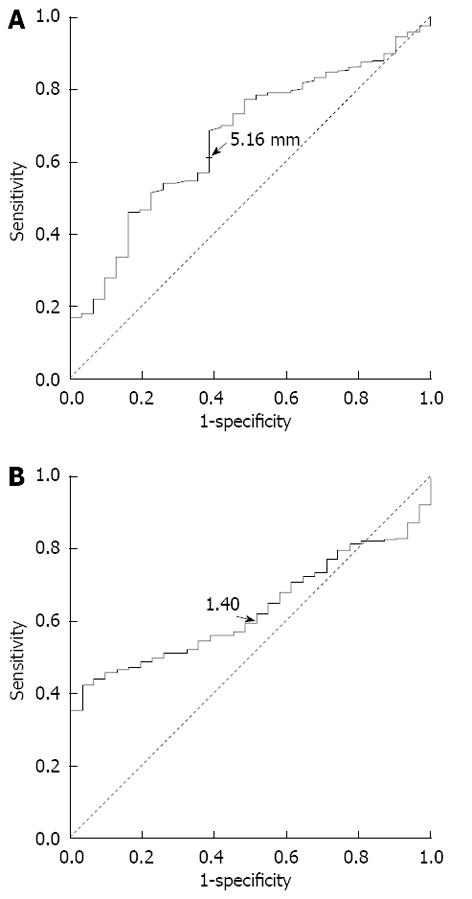
RESULTS: The receiver operating characteristic analysis showed that the cutoff points for abnormal splenic artery internal diameter and S/P ratio were > 5.19 mm and > 1.40, respectively. The sensitivity, specificity, positive predictive value, and negative predictive value were 74.2%, 45.2%, 97.1%, and 6.6%, respectively. The prevalence of an abnormal S/P ratio in the patients with cirrhosis and portal hypertension was 83.4%. Patients with a higher S/P ratio had a lower risk of developing ascites [odds ratio (OR) = 0.708, 95%CI: 0.508-0.986, P = 0.041] and a higher risk of developing esophageal and gastric varices (OR = 1.483, 95%CI: 1.010-2.175, P = 0.044) and forming collateral circulation (OR = 1.518, 95%CI: 1.033-2.230, P = 0.034). After splenectomy, the portal venous pressure and maximum and mean portal venous flow velocities were reduced, while the flow rate and maximum and minimum flow velocities of the hepatic artery were increased (P < 0.05).
CONCLUSION: The prevalence of an abnormal S/P ratio is high in patients with cirrhosis and portal hypertension, and it can be used as an important marker of splanchnic hemodynamic disturbances.
- Citation: Zeng DB, Dai CZ, Lu SC, He N, Wang W, Li HJ. Abnormal splenic artery diameter/hepatic artery diameter ratio in cirrhosis-induced portal hypertension. World J Gastroenterol 2013; 19(8): 1292-1298
- URL: https://www.wjgnet.com/1007-9327/full/v19/i8/1292.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i8.1292
Splanchnic and systemic hemodynamic disorders are common in patients with cirrhosis[1,2]. The systemic hyperdynamic circulation manifests as increased cardiac output and heart rate, and decreased arterial blood pressure and systemic vascular resistance[3,4]. The splanchnic hemodynamic disorder mainly manifests as increased vascular resistance and comparative visceral ischemia, which are associated with hepatic encephalopathy, hepatorenal syndrome, and portal hypertension[5]. Currently, portal hypertension has been found to be attributed to abnormally high portal venous inflow and high resistance in the portal system[6].
The splanchnic hemodynamic disorder in portal hypertension has been studied in a few studies, and some results support the significance of splenic and hepatic artery changes in portal hypertension. As early as in 1970, Yamauchi et al[7] observed an increase in the radius of the splenic artery in patients with portal hypertension, and such increase was not present in splenomegaly without portal hypertension. They found an association between the radius of the splenic artery and portal pressure and the degree of reduction in the portal pressure after splenectomy in patients with portal hypertension, and the dilatation of the splenic artery was accompanied by an increase in the diameter of the proper hepatic artery in those patients[7]. Subsequent studies confirmed the increases in the diameters of the splenic and hepatic arteries in patients with liver cirrhosis as compared with healthy controls[8,9], and further found that changes in the splenic artery occur prior to the onset of clinically evident portal hypertension[8].
Splenic and hepatic arterial blood flow distribution abnormalities are also very common in patients who have received liver transplantation. A study by Quintini et al[10] confirmed that hepatic artery hypoperfusion in liver transplant recipients can be induced by increased portal venous blood flow because over-perfusion of the portal vein cleared adenosine at the terminal end of the hepatic artery, resulting in hepatic artery contraction, i.e., “hepatic arterial buffer response”. Persistence of this response may cause further deterioration of splenic and hepatic arterial blood flow abnormalities, and result in a series of related complications known as splenic artery steal syndrome (SASS) or splenic artery syndrome that includes liver dysfunction, ischemic bile duct injury, and graft dysfunction or loss. Kirbas et al[11] performed multi-detector computed tomography (CT) in post-liver transplanted patients, and the authors proposed that a splenic artery diameter > 4 mm or a splenic artery diameter/hepatic artery diameter ratio > 1.5 indicates presence of SASS, thus establishing the importance of splenic artery diameter and the splenic artery diameter/hepatic artery diameter ratio in the clinical diagnosis of splenic and hepatic arterial blood flow distribution abnormalities.
Small-for-size syndrome (SFSS) is a common clinical complication that occurs after segmental liver transplantation[12,13]. Although the pathogenesis of the SFSS is still controversial, it is assumed that portal hypoperfusion with high intravascular shear stress is a causative factor[14-16]. In 2003, a group of researchers reported that portal flow should be modulated perioperatively in adult living-donor liver transplantation if a suboptimal graft-to-recipient body weight ratio is accompanied by high recipient portal inflow[17]. The authors found that splenic artery ligation could decrease recipient portal inflow, increase recipient hepatic arterial flow, and resolve refractory ascites, and neither SFSS nor vascular complications occurred in recipients undergoing splenic artery ligation[17]. Gruttadauria et al[18] obtained similar findings when they applied splenic artery embolization (SAE) for the treatment of SFSS after living-related donor liver transplantation. Their results showed that clinical conditions in patients with SFSS rapidly improved after SAE, and they thought that early SAE can protect the segmental graft against the harmful effects of the portal overflow[18]. Other animal and clinical studies of patients with liver cirrhosis and idiopathic portal hypertension showed that splenectomy can reduce portal venous blood flow and portal venous pressure, and increase hepatic arterial blood flow[19,20].
In this study, we examined whether splenic artery diameter and the splenic artery internal diameter/proper hepatic artery internal diameter (S/P) ratio are of clinical significance in patients with cirrhosis and portal hypertension. We determined the optimal cutoff values for abnormal splenic artery diameter and S/P ratio, observed the prevalence of an abnormal S/P ratio, and studied the associations between the S/P ratio and major complications of portal hypertension in patients with cirrhosis. The preoperative and postoperative portal vein hemodynamic indexes were further compared in patients undergoing splenectomy to explore the significance of splenectomy in the treatment of portal hypertension and its complications.
A total of 1000 patients with liver cirrhosis and portal hypertension who sought medical treatment at our hospital from January 2011 to May 2011 were recruited. All patients had a pathological diagnosis of liver cirrhosis. Clinical diagnosis of portal hypertension was made in the setting of end-stage liver disease, and in the presence of ascites and/or varices. Of the 1000 patients, 230 were excluded: 109 with hepatic artery variations, 66 with post-splenectomy, 51 with partial splenic artery embolization, and 4 with transjugular intrahepatic portosystemic stent shunting. Thus, a total of 770 patients with liver cirrhosis and portal hypertension were enrolled (554 males and 216 females), of whom 558 had biochemical examination results. Thirty-one healthy volunteers who participated in the donor screening for the living-related liver transplantation were also enrolled. This study was approved by the Institutional Review Boards of our hospital, and all the participants provided written informed consent.
All the patients received routine blood, coagulation, and liver function tests in a fasting state. The routine blood tests were performed with an SYSMES-2100 automatic blood cell analyzer and reagents (SYSMES Co., Japan). Coagulation tests were performed with a CA-7000 automatic coagulation instrument and ancillary reagents (SYSMES Co., Japan). Liver function tests were performed with an AU5400 automatic biochemical analyzer and related reagents (Mishima Olympus Co., Japan).
All the patients underwent abdominal enhanced computed tomography (CT) scanning with a General Electric (GE) Lightspeed VCT 64-slice scanner (GE, Milwaukee, WIS, United States). For scanning, the collimator width was 0.625 mm, the tube current was 380 mA, the tube voltage was 120 kV, the slice thickness was 5 mm, the slice interval was 5 mm, and the reconstruction slice thickness was 0.625 mm. A high-pressure syringe was used to inject 100 mL contrast agent iopromide (370 mg/mL) via the antecubital vein at a flow rate of 2.5-3.5 mL/s, and the scan phases were the arterial phase (20-25 s), venous phase (65-70 s), and delayed phase (180 s). The data were input into a GE ADW 4.3 image workstation, and multiplanar reconstruction, maximum intensity projection, and volume rendering post-processing were performed. The splenic and proper hepatic artery internal diameters were measured in the arterial phase. The internal diameter of the proper hepatic artery was measured within 1 cm from its origin. The internal diameter of the splenic artery was measured at 3 locations: within 1 cm from its origin, at the upper edge of the pancreas, and within 1 cm from the point where the splenic artery branches originate. The mean value was calculated and recorded as the internal diameter of the splenic artery. The S/P ratio was then calculated.
The free portal venous pressure was measured by catheterization via the right gastroepiploic vein before and after splenectomy. Doppler ultrasound (Esaote, Italy) was used to measure the hemodynamic indexes of the portal vein and hepatic artery within 1 wk prior to splenectomy and within 2 wk after splenectomy. Indexes measured were the portal venous flow rate and the maximum and mean portal venous flow velocities, the flow rate and maximum and minimum flow velocities of the hepatic artery, and the resistance index (RI) of the hepatic artery.
Demographic characteristics were presented as number (%) for sex, causes of cirrhosis, and level of liver function, mean ± SD for age, and median [interquartile range (IQR): Q1-Q3] for the internal diameters of the splenic artery and proper hepatic artery and the S/P ratio because data were not normally distributed. Data of patients with cirrhosis and healthy controls were compared using Pearson χ2 test for sex, two-sample t test for age, and Mann-Whitney U test for splenic artery diameter, proper hepatic artery diameter, and the S/P ratio, respectively. Receiver operating characteristic (ROC) curve analysis through binary logistic regression was carried out to identify the optimal cutoff values of abnormal internal diameter of the splenic artery and S/P ratio. The prevalence of an abnormal S/P ratio in patients with cirrhosis and portal hypertension was calculated based on the identified cutoff value. Associations between the S/P ratio and complications of cirrhosis and portal hypertension were analyzed using univariate logistic regression. The preoperative and postoperative portal vein hemodynamic indexes and routine blood test data of patients undergoing splenectomy were presented as mean ± SD or median (IQR: Q1-Q3) if data were not normally distributed. The preoperative and postoperative data were compared using paired t test or Wilcoxon sign-rank test if data were not normally distributed. All statistical assessments were two-tailed and the significance level α was set at 0.05. All statistical analyses were performed using SPSS 17.0 statistics software (SPSS Inc, Chicago, IL, United States).
A total of 770 patients with cirrhosis and portal hypertension (554 males and 216 females) and 31 healthy controls (16 males and 15 females) were enrolled. Their demographic characteristics are summarized in Table 1. The mean age of patients with cirrhosis and portal hypertension was 53.3 ± 11.3 years, and the mean age of healthy controls was 46.0 ± 12.0 years. Among the patients with cirrhosis and portal hypertension, 622 had cirrhosis due to hepatitis B, 61 had alcoholic cirrhosis, 39 had cirrhosis due to hepatitis C, 24 had autoimmune liver disease, 7 had drug-induced liver damage, 4 had Budd-Chiari syndrome, 4 had cirrhosis caused by hepatitis B and C, 3 had alcoholic cirrhosis with hepatitis B virus infection, 2 had idiopathic portal hypertension, 1 had hepatolenticular degeneration, 1 had cryptogenic cirrhosis, 1 had nonalcoholic fatty liver cirrhosis, and 1 had cirrhosis due to unknown cause.
| Variables | Patients with cirrhosis and portal hypertension | Healthy controls | P value |
| (n = 770) | (n = 31) | ||
| Sex | 0.0251 | ||
| Male | 554 (71.9) | 16 (51.6) | |
| Female | 216 (28.1) | 15 (48.4) | |
| Age (yr) | 53.3 ± 11.3 | 46.0 ± 12.0 | < 0.0011 |
| Causes of cirrhosis | NA | ||
| Hepatitis B | 622 (80.8) | - | |
| Hepatitis C | 39 (5.1) | - | |
| Alcoholic cirrhosis | 61 (7.9) | - | |
| Autoimmune liver disease | 24 (3.1) | - | |
| Others | 24 (3.1) | - | |
| Level of liver function | NA | ||
| I | 230 (29.9) | - | |
| II | 209 (27.1) | - | |
| III | 119 (15.5) | - | |
| Undefined | 212 (27.5) | - | |
| Splenic artery internal diameter (mm) | 5.35 (4.67-6.18) | 4.60 (4.32-5.32) | 0.0021 |
| Proper hepatic artery internal diameter (mm) | 3.63 (2.97-4.43) | 3.72 (3.14-4.19) | 0.830 |
| S/P ratio | 1.48 (1.19-1.82) | 1.36 (1.13-1.48) | 0.0121 |
Patients with cirrhosis and portal hypertension had a higher internal diameter of the splenic artery and S/P ratio than healthy controls (both, P < 0.05). The median internal diameter of the splenic artery was 5.35 mm (IQR: 4.67-6.18 mm) in patients with cirrhosis and portal hypertension and 4.60 mm (IQR: 4.32-5.32 mm) in healthy controls. The median S/P ratio was 1.48 (IQR: 1.19-1.82) in patients with cirrhosis and portal hypertension and 1.36 (1.13-1.48) in healthy controls (Table 1). The internal diameter of the proper hepatic artery was not significantly different between patients with cirrhosis and portal hypertension and healthy controls.
ROC curve analysis showed that the optimal cutoff values of abnormal internal diameter of the splenic artery and S/P ratio were > 5.19 mm and > 1.40, respectively (Figure 1). The sensitivity, specificity, positive predictive value, and negative predictive value were 74.2%, 45.2%, 97.1%, and 6.6%, respectively. Using the cutoff value of 1.40, 640 patients (83.4%) with cirrhosis and portal hypertension had an abnormal S/P ratio, and 28 healthy controls (90.3%) had a normal S/P ratio.
Results of univariate logistic regression are presented in Table 2. The results showed that patients with a higher S/P ratio had a lower risk of developing ascites [odds ratio (OR) = 0.708, 95%CI: 0.508-0.986, P = 0.041]. However, patients with a higher S/P ratio had a higher risk of developing esophageal and gastric varices (OR = 1.483, 95%CI: 1.010-2.175, P = 0.044) and forming collateral circulation (OR = 1.518, 95%CI: 1.033-2.230, P = 0.034).
| Complication | Total | Patients with normal S/P ratio | Patients with abnormal S/P ratio | OR (95%CI) | P value |
| (n = 770) | (n = 130) | (n = 640) | |||
| Ascites | |||||
| Yes | 426 (55.7) | 304 (53.5) | 122 (61.9) | 0.708 (0.508-0.986) | 0.0411 |
| No | 339 (44.3) | 264 (46.5) | 75 (38.1) | Reference | |
| Hepatic encephalopathy | |||||
| Yes | 77 (10.1) | 58 (10.2) | 19 (9.6) | 1.065 (0.617-1.838) | 0.820 |
| No | 688 (89.9) | 510 (89.8) | 178 (90.4) | Reference | |
| Esophageal and gastric varices | |||||
| Yes | 209 (27.4) | 166 (29.3) | 43 (21.8) | 1.483 (1.010-2.175) | 0.0441 |
| No | 555 (72.6) | 401 (70.7) | 154 (78.2) | Reference | |
| Upper gastrointestinal bleeding | |||||
| Yes | 111 (14.5) | 90 (15.9) | 21 (10.7) | 1.581 (0.954-2.621) | 0.076 |
| No | 653 (85.5) | 477 (84.1) | 176 (89.3) | Reference | |
| Collateral circulation formation | |||||
| Yes | 611 (79.9) | 464 (81.7) | 147 (74.6) | 1.518 (1.033-2.230) | 0.0341 |
| No | 154 (20.1) | 104 (18.3) | 50 (25.4) | Reference | |
A total of 17 patients with cirrhosis and portal hypertension underwent splenectomy. Table 3 presents the comparison of the preoperative and postoperative portal vein hemodynamic indexes in these patients. After splenectomy, the portal venous pressure and maximum and mean portal venous flow velocities were significantly reduced compared with the preoperative values (all, P < 0.05). The postoperative flow rate, maximum flow velocity, and minimum flow velocity of the hepatic artery were significantly higher than the preoperative values (all, P < 0.05). The RI of the hepatic artery was significantly reduced after splenectomy (P < 0.001).
| Variables | Before splenectomy | After splenectomy | P value |
| Portal vein | |||
| Pressure (cm H2O) | 39.19 ± 5.87 | 27.04 ± 3.03 | < 0.0011 |
| Flow rate (mL/min) | 1005.50 (833.00-1811.25) | 868.00 (539.50-1215.75) | 0.116 |
| Maximum flow velocity (cm/s) | 23.85 (18.53-31.20) | 15.85 (12.68-20.58) | 0.0091 |
| Mean flow velocity (cm/s) | 20.85 (15.90-25.95) | 12.25 (10.48-16.40) | 0.0111 |
| Hepatic artery | |||
| Flow rate (mL/min) | 153.50 (128.75-188.77) | 365.00 (190.00-511.00) | 0.0481 |
| Minimum flow velocity (cm/s) | 16.25 ± 15.23 | 32.60 ± 15.23 | 0.0011 |
| Resistance index | 0.74 ± 0.05 | 0.64 ± 0.07 | < 0.0011 |
Table 4 presents the comparison of the results of the preoperative and postoperative routine blood tests in patients with cirrhosis and portal hypertension who underwent splenectomy. After splenectomy, total bilirubin, direct bilirubin, white blood cell (WBC) count, and platelet count were significantly increased (all, P < 0.05), and creatinine levels were significantly decreased (P < 0.05).
| Variable | Before splenectomy | After splenectomy | P value |
| ALT (U/L) | 29.39 ± 16.86 | 36.47 ± 28.9 | 0.439 |
| AST (U/L) | 33 (21.9-45.4) | 37 (26.65-48.45) | 0.653 |
| Total bilirubin (μmol/L) | 16.8 (12.95-24.9) | 21.4 (16.2-39.55) | 0.0051 |
| Direct bilirubin (μmol/L) | 4.74 ± 2.13 | 7.56 ± 4.31 | 0.0031 |
| Albumin (g/L) | 37.67 ± 3.73 | 36.74 ± 3.28 | 0.439 |
| Creatinine (μmol/L) | 70.44 ± 22.28 | 58.69 ± 14.78 | 0.0361 |
| WBC count (E + 09/L) | 1.73 (1.26-2.24) | 9.05 (6.43-12.84) | < 0.0011 |
| RBC count (E + 12/L) | 3.45 ± 0.73 | 3.42 ± 0.47 | 0.87 |
| Platelet count (E + 09/L) | 47.41 ± 24.48 | 307 ± 132.4 | < 0.0011 |
The results of this study showed that patients with cirrhosis and portal hypertension had a significantly greater internal diameter of the splenic artery and higher S/P ratio than healthy controls, but not a significantly different internal diameter of the proper hepatic artery. The finding of an increased diameter of the splenic artery in patients with cirrhosis and portal hypertension is consistent with the findings of prior studies[7-9]. The results indicate that excessive perfusion of the splenic artery increases the return blood flow in the splenic vein, thereby increasing the blood flow in the portal venous system resulting in portal hypertension. However, an increase in the diameter of the hepatic artery was observed in patients with portal hypertension in some early studies[7-9], a finding not observed in our study. A possible reason for this discrepancy is that different imaging methods were used to measure the arterial internal diameter and current enhanced CT scanning is more accurate than the imaging methods used in earlier studies.
The optimal cutoff values for the abnormal internal diameter of the splenic artery and S/P ratio were identified to be > 5.19 mm and > 1.40, respectively, by the ROC curve analysis. When the abnormal S/P ratio > 1.40 was used, 83.4% of patients with cirrhosis and portal hypertension had an abnormal S/P ratio. The prevalence of an abnormal S/P ratio is very high in patients with cirrhosis and portal hypertension, indicating that an abnormal S/P ratio can be used as a marker of splanchnic hemodynamic disturbances in these patients. Machálek et al[21] summarized measurements of the splenic artery internal diameter of 30 normal spleens, and found that splenic artery thickening was indicated by an internal diameter of the splenic artery greater than 5.6 ± 1.3 mm. In our study, we found that an internal diameter of the splenic artery greater than 5.19 mm is the best cutoff value to predict cirrhosis and portal hypertension, which is consistent with the finding of Machálek et al[21]. Kirbas et al[11] proposed that a splenic artery diameter > 4 mm or a splenic artery diameter/hepatic artery diameter ratio > 1.5 indicates presence of SASS after liver transplantation, which is similar to the S/P ratio cutoff point of 1.40 for patients with cirrhosis and portal hypertension determined in our study. However, Kirbas et al[11] reported a much lower abnormal value for the internal diameter of the splenic artery than the value reported by Machálek et al[21] and our study, which may be due to the a different measurement method used by them.
Our results indicated associations between the S/P ratio and major complications in patients with portal hypertension. Patients with a higher S/P ratio had a lower risk of developing ascites (OR = 0.708, 95%CI: 0.508-0.986, P = 0.041) and a higher risk of developing esophageal and gastric varices (OR = 1.483, 95%CI: 1.010-2.175, P = 0.044) and forming collateral circulation (OR = 1.518, 95%CI: 1.033-2.230, P = 0.034). The results indicate that the S/P ratio has a great clinical significance for predicting major complications of portal hypertension in patients with cirrhosis.
Studies have also shown that splenectomy can reduce portal venous blood flow and portal venous pressure, increase hepatic arterial blood flow, and improve liver function, thereby decreasing major complications of portal hypertension[22,23]. Our study showed that splenectomy significantly reduced portal venous pressure and maximum and mean portal venous flow velocities, and increased the flow rate, maximum flow velocity, and minimum flow velocity of the hepatic artery. The study of SASS after liver transplantation by Mogl et al[24] found a significant elevation of the RI of the hepatic artery (0.79 ± 0.14) in patients with SASS, which was significantly reduced (0.65 ± 0.09) after splenic artery embolization. In our study, the preoperative and postoperative RIs of the hepatic artery were 0.74 ± 0.05 and 0.64 ± 0.07, respectively, which is consistent with the findings of Mogl et al[24], Our results also showed that postoperative WBC count and platelet levels were significantly increased compared with the preoperative values, indicating that these indexes can be restored to normal values. These results indicate that the major complications of cirrhosis and portal hypertension and splanchnic hemodynamic disturbances can be improved by splenectomy.
The hepatic-venous pressure gradient (HVPG) is the gold standard for the evaluation of the presence and severity of portal hypertension[25]. A limitation of this study is that portal hypertension was diagnosed using clinical criteria, but not based on HVPG results. In addition, the mean age of our healthy controls was younger than that of patients with cirrhosis and portal hypertension. However, the diameter of the splenic artery was not significantly affected by age or gender in adults.
In conclusion, the prevalence of an abnormal S/P ratio is high in patients with cirrhosis and portal hypertension, and it can be used as an important marker of splanchnic hemodynamic disturbances in these patients. The S/P ratio is closely related to major complications of portal hypertension including ascites, esophageal and gastric varices, and collateral circulation formation. Splenectomy is effective for decreasing portal venous pressure and delaying the progress of liver cirrhosis and portal hypertension.
We would like to thank Dr. Mao J for her help in writing and editing this paper.
The splanchnic hemodynamic disorder in portal hypertension has been studied, and the significance of splenic and hepatic artery changes in portal hypertension has been noted. In patients who have received liver transplantation, the importance of the splenic artery diameter and the splenic artery diameter/hepatic artery diameter ratio in the clinical diagnosis of splenic and hepatic arterial blood flow distribution abnormalities has been established.
Splenic and hepatic arterial blood flow abnormalities are common in patients with cirrhosis and portal hypertension, but the clinical significance of the splenic artery diameter and splenic artery internal diameter/proper hepatic artery internal diameter (S/P) ratio in those patients has not been clearly elucidated.
For the first time, the authors determined the optimal cutoff values of abnormal internal diameter of the splenic artery and S/P ratio in patients with cirrhosis and portal hypertension, which were > 5.19 mm and > 1.40, respectively. A high prevalence of an abnormal S/P ratio and close associations between the S/P ratio and the major complications of portal hypertension including ascites, esophageal and gastric varices, and collateral circulation formation were found in patients with cirrhosis and portal hypertension.
The study results suggest that an abnormal internal diameter of the splenic artery and S/P ratio are of clinical significance in patients with cirrhosis and portal hypertension, and an abnormal S/P can be used as an important marker of splanchnic hemodynamic disturbances in these patients.
In this study, the splenic and proper hepatic artery internal diameters refer to the values measured in the arterial phase of abdominal enhanced computed tomographic (CT) scanning. The internal diameter of the proper hepatic artery was measured within 1 cm from its origin. The internal diameter of the splenic artery was measured at 3 places: within 1 cm from its origin, at the upper edge of the pancreas, and within 1 cm from the point that the splenic artery branches originate. The mean value was calculated and recorded as the internal diameter of the splenic artery. The S/P ratio was then calculated.
It is a nice study which sought and found correlations between splenic artery internal diameter/proper hepatic artery internal diameter ratio, calculated by a non-invasive tool (3D CT scan) and clinical complications of portal hypertension. The study is original and the manuscript is well written.
P- Reviewer Gruttadauria S S- Editor Gou SX L- Editor Ma JY E- Editor Zhang DN
| 1. | Møller S, Bendtsen F, Henriksen JH. Splanchnic and systemic hemodynamic derangement in decompensated cirrhosis. Can J Gastroenterol. 2001;15:94-106. [PubMed] [Cited in This Article: ] |
| 2. | Groszmann RJ, Abraldes JG. Portal hypertension: from bedside to bench. J Clin Gastroenterol. 2005;39:S125-S130. [PubMed] [DOI] [Cited in This Article: ] |
| 3. | Iwakiri Y, Groszmann RJ. The hyperdynamic circulation of chronic liver diseases: from the patient to the molecule. Hepatology. 2006;43:S121-S131. [PubMed] [DOI] [Cited in This Article: ] |
| 4. | Møller S, Henriksen JH. Cardiovascular complications of cirrhosis. Gut. 2008;57:268-278. [PubMed] [DOI] [Cited in This Article: ] |
| 5. | La Villa G, Gentilini P. Hemodynamic alterations in liver cirrhosis. Mol Aspects Med. 2008;29:112-118. [PubMed] [DOI] [Cited in This Article: ] |
| 6. | Sikuler E, Groszmann RJ. Interaction of flow and resistance in maintenance of portal hypertension in a rat model. Am J Physiol. 1986;250:G205-G212. [PubMed] [Cited in This Article: ] |
| 7. | Yamauchi H, Suda Y, Yamamoto K, Sato T. Arteriographic studies of splenic and hepatic arteries in portal hypertension. Tohoku J Exp Med. 1970;101:363-374. [PubMed] [DOI] [Cited in This Article: ] |
| 8. | Toni R, Bolondi L, Gaiani S, Re G, Calabrese L, Cavalli G, Labò G. Accessory ultrasonographic findings in chronic liver disease: diameter of splenic and hepatic arteries, fasting gallbladder volume, and course of left portal vein. J Clin Ultrasound. 1985;13:611-618. [PubMed] [Cited in This Article: ] |
| 9. | Aoki H, Hasumi A, Hashizume M, Kato H, Moriyasu F, Idezuki Y. Hemodynamic analysis of findings in patients with portal hypertension: multicenter analysis in Japan. Japan Portal Hypertension Study Group. Hepatogastroenterology. 1995;42:1030-1038. [PubMed] [Cited in This Article: ] |
| 10. | Quintini C, Hirose K, Hashimoto K, Diago T, Aucejo F, Eghtesad B, Vogt D, Pierce G, Baker M, Kelly D. “Splenic artery steal syndrome” is a misnomer: the cause is portal hyperperfusion, not arterial siphon. Liver Transpl. 2008;14:374-379. [PubMed] [DOI] [Cited in This Article: ] |
| 11. | Kirbas I, Tutar NU, Emiroglu FK, Coskun M, Haberal M. Multidetector computed tomography angiography in detection of active bleeding in renal and liver transplant recipients. Transplant Proc. 2007;39:1111-1115. [PubMed] [DOI] [Cited in This Article: ] |
| 12. | Lo CM, Liu CL, Fan ST. Portal hyperperfusion injury as the cause of primary nonfunction in a small-for-size liver graft-successful treatment with splenic artery ligation. Liver Transpl. 2003;9:626-628. [PubMed] [DOI] [Cited in This Article: ] |
| 13. | Tucker ON, Heaton N. The ‘small for size’ liver syndrome. Curr Opin Crit Care. 2005;11:150-155. [PubMed] [DOI] [Cited in This Article: ] |
| 14. | Dahm F, Georgiev P, Clavien PA. Small-for-size syndrome after partial liver transplantation: definition, mechanisms of disease and clinical implications. Am J Transplant. 2005;5:2605-2610. [PubMed] [Cited in This Article: ] |
| 15. | Glanemann M, Eipel C, Nussler AK, Vollmar B, Neuhaus P. Hyperperfusion syndrome in small-for-size livers. Eur Surg Res. 2005;37:335-341. [PubMed] [Cited in This Article: ] |
| 16. | Demetris AJ, Kelly DM, Eghtesad B, Fontes P, Wallis Marsh J, Tom K, Tan HP, Shaw-Stiffel T, Boig L, Novelli P. Pathophysiologic observations and histopathologic recognition of the portal hyperperfusion or small-for-size syndrome. Am J Surg Pathol. 2006;30:986-993. [PubMed] [DOI] [Cited in This Article: ] |
| 17. | Troisi R, Cammu G, Militerno G, De Baerdemaeker L, Decruyenaere J, Hoste E, Smeets P, Colle I, Van Vlierberghe H, Petrovic M. Modulation of portal graft inflow: a necessity in adult living-donor liver transplantation? Ann Surg. 2003;237:429-436. [PubMed] [DOI] [Cited in This Article: ] |
| 18. | Gruttadauria S, Mandala’ L, Miraglia R, Caruso S, Minervini MI, Biondo D, Volpes R, Vizzini G, Marsh JW, Luca A. Successful treatment of small-for-size syndrome in adult-to-adult living-related liver transplantation: single center series. Clin Transplant. 2007;21:761-766. [PubMed] [Cited in This Article: ] |
| 19. | Kawasaki S, Kidokoro A, Sugiura M, Sanjo K, Idezuki Y. Effects of nonshunting operations on portal venous pressure and hepatic blood flow. Am J Surg. 1987;153:295-299. [PubMed] [DOI] [Cited in This Article: ] |
| 20. | Eipel C, Abshagen K, Ritter J, Cantré D, Menger MD, Vollmar B. Splenectomy improves survival by increasing arterial blood supply in a rat model of reduced-size liver. Transpl Int. 2010;23:998-1007. [PubMed] [DOI] [Cited in This Article: ] |
| 21. | Machálek L, Holibková A, Tůma J, Houserková D. The size of the splenic hilus, diameter of the splenic artery and its branches in the human spleen. Acta Univ Palacki Olomuc Fac Med. 1998;141:45-48. [PubMed] [Cited in This Article: ] |
| 22. | Zhang Y, Wen T, Yan L, Chen Z, Yang H, Deng X, Liang G, Li G, Zhang X, Ran S. The changes of hepatic hemodynamics and functional hepatic reserve after splenectomy with periesophagogastric devascularization. Hepatogastroenterology. 2009;56:835-839. [PubMed] [Cited in This Article: ] |
| 23. | Shi CJ, Zhang DW, Wang CF, Tian H. Impact of devascularization on liver blood flow dynamics and hepatic function in patients with portal hypertension. Zhonghua Putong Waike Zazhi. 1999;3:185-187. [Cited in This Article: ] |
| 24. | Mogl MT, Nüssler NC, Presser SJ, Podrabsky P, Denecke T, Grieser C, Neuhaus P, Guckelberger O. Evolving experience with prevention and treatment of splenic artery syndrome after orthotopic liver transplantation. Transpl Int. 2010;23:831-841. [PubMed] [Cited in This Article: ] |
| 25. | Snowdon VK, Guha N, Fallowfield JA. Noninvasive evaluation of portal hypertension: emerging tools and techniques. Int J Hepatol. 2012;2012:691089. [PubMed] [Cited in This Article: ] |