Published online Feb 28, 2013. doi: 10.3748/wjg.v19.i8.1200
Revised: October 30, 2012
Accepted: November 6, 2012
Published online: February 28, 2013
Processing time: 209 Days and 22.7 Hours
AIM: To investigate the role of the pelvic nerve pathway in stress-induced acceleration of colorectal transit and defecation in rats.
METHODS: Surgical transection of rectal nerves (rectal branches of the pelvic nerve), vagotomy (Vag) or adrenalectomy (Adx) were performed bilaterally in rats. Number of fecal pellet output of these rats was measured during 1-h water avoidance stress (WAS). To evaluate the colonic transit, rats were given phenol red through the catheter indwelled in the proximal colon and subjected to WAS. After WAS session, entire colon and rectum were isolated and distribution of phenol red was measured. Distal colonic and rectal transit was evaluated using glass bead. Rats were inserted the glass bead into the distal colon and evacuation rate of the bead was measured. Neural activation was assessed by immunohistochemical staining of c-Fos and PGP9.5 in colonic whole-mount preparations of longitudinal muscle myenteric plexus (LMMP).
RESULTS: In the sham-operated rats (sham op), WAS significantly increased defecation and accelerated colorectal transit with marked elevation of plasma corticosterone level. Compared with sham-operated rats, increase in the excretion of fecal pellets during WAS was significantly reduced by rectal nerve transection (RNT) (sham op: 6.9 ± 0.8 vs RNT: 4.3 ± 0.6, P < 0.05) or Vag (sham op: 6.4 ± 0.8 vs Vag: 3.7 ± 1.1, P < 0.05), although corticosterone level remained elevated. Adx-rats significantly increased the defecation despite the lower corticosterone level. Distribution pattern of phenol red showed RNT inhibited distal colonic and rectal transit accelerated by WAS, while Vag inhibited proximal colonic transit. Suppression of distal colonic and rectal transit by RNT was further confirmed by the bead evacuation rate (sham op: 80.0% vs RNT: 53.8%). WAS significantly increased the number of c-Fos-immunoreactive neural cells in the LMMP of the proximal and distal colon, whereas c-Fos expression was decreased by RNT in the distal colon (sham op: 9.0 ± 2.0 vs RNT: 4.4 ± 1.0, P < 0.05) and decreased by Vag in the proximal colon.
CONCLUSION: Pelvic nerve conveys WAS stimuli from the brain to the distal colon, and directly activate the myenteric neurons, followed by the increase of its motility.
- Citation: Suda K, Setoyama H, Nanno M, Matsumoto S, Kawai M. Involvement of parasympathetic pelvic efferent pathway in psychological stress-induced defecation. World J Gastroenterol 2013; 19(8): 1200-1209
- URL: https://www.wjgnet.com/1007-9327/full/v19/i8/1200.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i8.1200
Stress is a key factor in causing abnormal bowel habits, such as constipation and diarrhea, and has been known to exacerbate these symptoms[1-4]. Accumulating evidence suggests that various stressors stimulate colonic motor functions in humans and animals[5-7]. In patients with irritable bowel syndrome, psychological and physical stressors increase colonic slow-wave, contractile, and motor activities[8]. In laboratory animals, acute exposure to restraint, cold restraint, or water avoidance stress (WAS) enhances colonic motor activity (e.g., colonic motility, colonic transit, and defecation)[9-11]. The stress response is a complicated process that involves the endocrine and nervous systems. Corticotropin-releasing factor (CRF) level is increased in the hypothalamic paraventricular nuclei (PVN), amygdala, and Barrington’s nucleus after acute exposure to stressful stimuli[12-15]. CRF is released in the PVN, resulting in the secretion of adrenocorticotropic hormone (ACTH) from the anterior pituitary into the bloodstream. ACTH then increases the release of glucocorticoid from the cortex of the adrenal gland, which is called the hypothalamic-pituitary-adrenal axis (HPA-axis)[16]. Another endocrine system, the sympathetic-adrenal-medullary axis (SAM-axis), is activated by stress and subsequently induces the adrenal medulla to release adrenaline and noradrenaline[17]. These two major bodily pathways cause metabolic, cardiovascular, immune, and behavioral responses[18-20]. The gastrointestinal tract from the esophagus to the distal colon is innervated by parasympathetic vagal efferent fibers from the dorsal motor nucleus in the medulla oblongata[21]. Recently, it was demonstrated that vagotomy (Vag) reduced the restraint stress-induced increase in colonic transit in rats[22,23]. This evidence suggests that physical stress causes colonic dysmotility via parasympathetic vagal activation. On the other hand, the efferent fiber from Barrington’s nucleus projects to the sacral spinal cord and terminates in the region of the preganglionic pelvic nerves[24,25]. This pelvic efferent is also classified as the parasympathetic nerve and projects to the distal colon and rectum[26]. In rats, activation of the pelvic nerve regulates distal colonic and rectal motility under physiological conditions[27], and electrical nerve stimulation elicits contractions in the rectum[28]. Million et al[29] demonstrated that WAS activates the sacral parasympathetic nucleus. However, it is still unclear whether activation of the pelvic nerve is involved in altered colonic and rectal motilities under stressed conditions.
In this study, we examined the role of the pelvic nerve pathway in the activation of defecation, colorectal transit, and myenteric neurons under psychological stress by surgically transecting the rectal branches of the pelvic nerve in rats. Additionally, we compared the differences between colorectal functional changes in pelvic nerve transected rats and vagotomized rats.
Male Sprague-Dawley rats (Japan SLC, Shizuoka, Japan) weighing 300-400 g were maintained under a 12-h/12-h light dark cycle (8:00-20:00) with free access to food and water. All animal experiments were approved by the ethical committee for animal experiments of Yakult Institute. All animals were kept in individual cages in a controlled environment with constant temperature (23 °C ± 2 °C) for at least 1 wk before experiments.
Rats were placed on a rectangular platform (11 cm × 7 cm × 8 cm) in the center of a plastic cage (41 cm × 25 cm × 20 cm) for a 1-h or 2-h period. The cage was filled with water kept at room temperature to the height of 1 cm from the top of the platform. Control rats were placed on the same platform for 1 or 2 h in a cage without water (sham stress). After loading stress, the number of fecal pellets excreted in the cage was counted. WAS and sham stress tests were carried out between 9:00 and 12:00. The animals were placed in a plastic cage for 1 h the day before the experiments, and the rats that excreted more than 5 fecal pellets were excluded from the stress experiment. Each rat was exposed to WAS only once.
The number of fecal pellets found in the tank was counted and blood samples were collected into heparinized tubes from the tail vein immediately after the 1-h WAS or sham stress session. Blood samples were centrifuged and plasma was stored at -80 °C until measurement. The plasma corticosterone concentration was analyzed by the Correlate-EIA corticosterone enzyme immunoassay kit (Enzo Life Sciences, Farmingdale, NY, United States) according to the manufacturer’s instructions.
Rectal nerve transection (RNT), subdiaphragmatic Vag, or adrenalectomy (Adx) was performed in rats that were anesthetized by intraperitoneal injection (ip) of pentobarbital sodium (50 mg/kg; Somnopentyl, Kyoritsu Seiyaku, Tokyo, Japan). We transected the rectal nerve branches from the pelvic plexus to avoid the impairment of the micturition reflex in the present study. A midline incision was made in the abdomen and the rectal nerves were bilaterally transected at a short distance from the major pelvic ganglion. Anterior and posterior branches of the Vag were resected above the hepatic and celiac branches, and the adrenal glands were removed bilaterally. These procedures were performed using forceps under a microscope. Sham-operated animals underwent the same procedure without the above-described resection and removal. The abdominal wall and skin were sutured after completion of surgery. Rats that did not show elevated body weight during the recovery period were excluded from the experiments. Experiments were performed 7-10 d after the surgery.
Rats were anesthetized by pentobarbital sodium (50 mg/kg, ip). A polyethylene catheter (PE-50, Becton, Dickinson and Company, Franklin Lakes, NJ, United States) was inserted from the cecum, and the tip was positioned in the proximal colon 1 cm distal to the cecocolonic junction. The other side of the catheter was tunneled subcutaneously to the posterior neck. Rats were given phenol red (10 mg/mL in a volume of 0.4 mL, Sigma-Aldrich, St Louis, MO, United States) through the catheter that was positioned in the proximal colon and subjected to WAS or sham stress for 1-h at 7-10 d after surgery. After the stress session, rats were euthanized with an ip overdose of pentobarbital sodium. The entire colon and rectum were isolated and divided into 8 equal segments, where the number of the segments from 1 to 8 was consistent with each segment that was isolated from the start of the proximal colon to the rectum. Segments 1-5 were defined as proximal colon while segments 6-8 were defined as distal colon and rectum. The number of fecal pellets in segments 6-8 was counted. Each segment was transferred into tubes and washed 3 times in phosphate buffered saline (PBS), to which followed the addition of 5 mol/L NaOH by one fifth volume. The absorbance was measured at 560 nm to calculate the volume of phenol red. The geometric center was calculated using the following equation.
Geometric center = Σ (fraction of phenol red per segment × segment number)
The rats were fasted overnight with water ad libitum before the experiment. A single 5-mm glass bead was inserted into the distal colon (3 cm proximal to the anus). The rat was subjected to WAS or sham stress for 2-h immediately after bead insertion and the evacuation rate was monitored over a 2-h period.
Rats were euthanized with an ip overdose of pentobarbital sodium 1 h after the WAS or sham stress session. The proximal colon (2 cm distal to the cecocolonic junction) and distal colon (4 cm proximal to the anus) were isolated and placed in 0.01 mol/L PBS (pH 7.4). The isolated colon was opened along the mesenteric border, stretched and pinned flat on a Silicon-coated petri dish (Shin-Etsu Chemical, Tokyo, Japan), and fixed overnight in 4% paraformaldehyde in 0.1 mol/L phosphate buffer at 4 °C. The following day, tissues were washed (3 × 10 min) in PBS, the mucosa, submucosa, and circular muscle were removed, and whole mount preparations of longitudinal muscle myenteric plexus (LMMP) were prepared. The preparations were washed (3 × 10 min) in PBS and incubated in 5% normal donkey serum (Jackson Immuno Research Laboratories, West Grove, PA, United States) in PBS containing 0.3% Triton X-100 (PBS-T) for 30 min at room temperature. The preparations were incubated with rabbit anti-c-Fos (1:10000; Ab-5; Merck Calbiochem, Darmstadt, Germany) and mouse anti-protein gene product 9.5 (PGP9.5, a marker of enteric neurons, 1:50; ab8189; Abcam, Cambridge, United Kingdom) overnight at 4 °C. The preparations were washed (3 × 10 min) in PBS-T and incubated with donkey anti-rabbit Alexa 488 (Molecular probes, Eugene, OR, United States) and donkey anti-mouse IgG Cy3-conjugated antibody (Millipore, Billerica, MA, United States). After washing (3 × 10 min) in PBS-T, the preparations were mounted on glass slides using VECTASHIELD (Vector Laboratories, Burlingame, CA, United States) and analyzed by the Zeiss LSM 510 confocal microscope with LSM Image Browser software (Carl Zeiss, Jena, Germany). The number of c-Fos immunoreactive (IR) cells was counted in 10 randomly selected ganglia in each preparation per rat and the means of c-Fos-IR cells per rat were used to calculate the mean of each group.
All results are presented as mean ± SE. Comparisons between 2 groups were evaluated using the unpaired Student’s t test or χ2 test. Comparisons between the mean values of multiple groups were performed with one-way analysis of variance followed by Dunnett’s multiple comparison test. P values of < 0.05 were considered statistically significant.
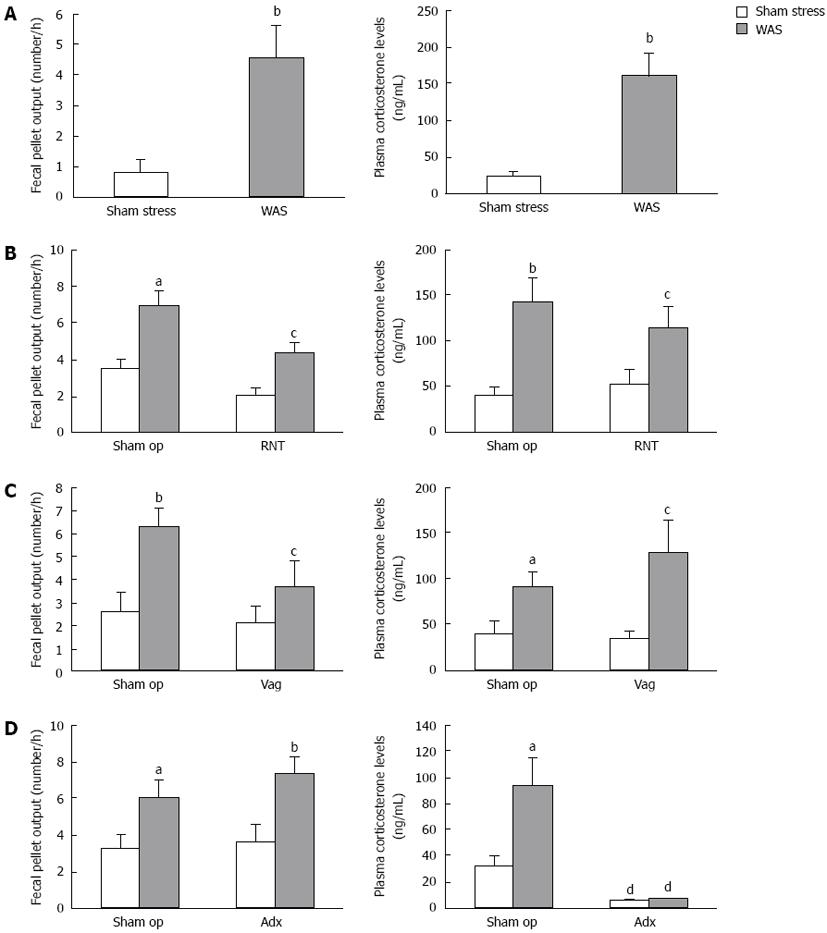
When rats were subjected to acute WAS for 1-h, the number of excreted fecal pellets was significantly increased compared to rats under sham stress (4.6 ± 1.0 vs 0.8 ± 0.4, P < 0.01). Plasma corticosterone levels markedly rose in rats just after WAS compared with those under sham stress (161.6 ± 29.5 ng/mL vs 24.2 ± 5.3 ng/mL, P < 0.01) (Figure 1A).
The sham-operated rats in RNT or Vag (Figure 1B-C) evacuated more fecal pellets and showed higher level of corticosterone in plasma after WAS treatment. The WAS-induced increase in fecal pellet output was significantly suppressed by treating RNT beforehand (sham op-WAS: 6.9 ± 0.8, RNT-WAS: 4.3 ± 0.6, P < 0.05), although the elevation of corticosterone was almost equally observed in RNT-rats and sham-operated rats (Figure 1B). Defecation under the condition of sham stress did not differ in RNT-rats and sham-operated rats. Similarly, Vag significantly reduced the WAS-induced increase in fecal pellet output (sham op-WAS: 6.4 ± 0.8, Vag-WAS: 3.7 ± 1.1, P < 0.05), but did not suppress the increase in corticosterone levels induced by WAS (Figure 1C).
On the other hand, WAS significantly increased the number of fecal pellets in Adx-rats compared with sham stress (7.4 ± 0.8 vs 3.6 ± 0.9). The baseline levels of plasma corticosterone were lower in Adx-rats than in sham-operated rats, and an obvious change in plasma corticosterone level was not observed after WAS in Adx-rats (Figure 1D).
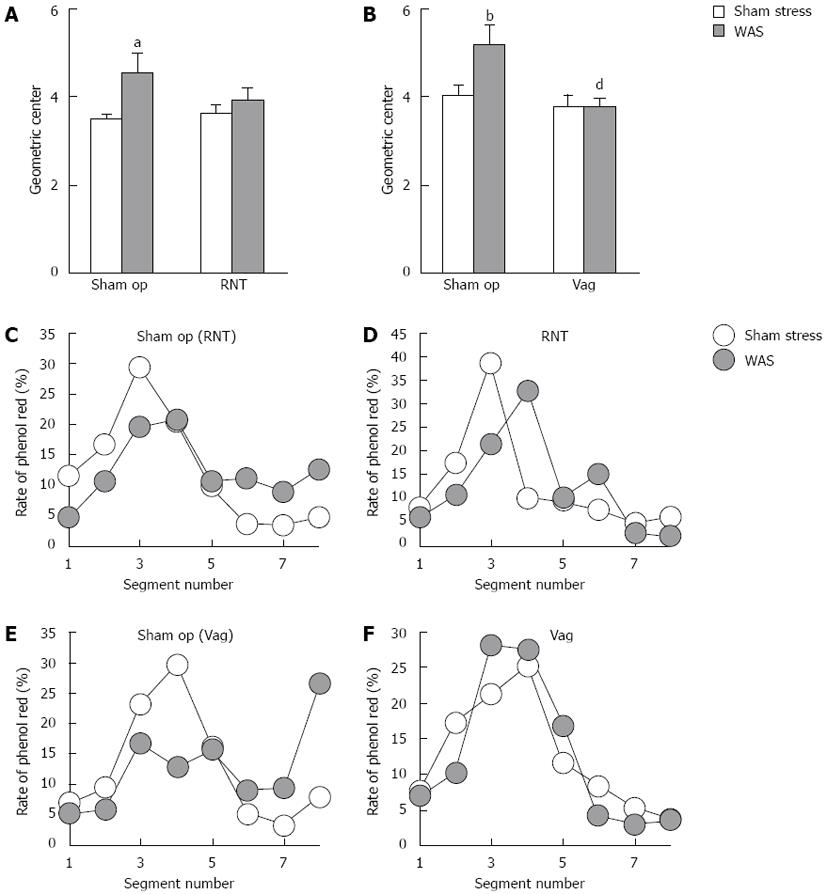
We evaluated whether the pelvic nerve pathway is involved in the colonic transit during the stress session. Phenol red, a non-absorbable marker, was injected into the proximal colon via a catheter before stress, and geometric center was measured in the rats under the different stress conditions. In sham-operated rats, WAS significantly increased the geometric center (4.6 ± 0.4 vs 3.5 ± 0.1, P < 0.05, Figure 2A), and also RNT-rats were found to exhibit the higher geometric center after WAS treatment although the difference was not statistically significant (Figure 2A). In contrast, WAS-induced increase of geometric center was completely suppressed in Vag-rats (sham op-WAS: 5.2 ± 0.4, Vag-WAS: 3.8 ± 0.2, P < 0.001, Figure 2B).
In sham-stressed RNT- and Vag-rats, the phenol red was distributed over a wide range from segments 1-8 with peaks at segments 3-4. The phenol red contents decreased in segments 1-4 and increased in segments 6-8 in sham-operated rats after WAS loading compared with sham stress (Figure 2C and E). In RNT-rats, the peak of phenol red at segment 3 during sham stress shifted to segments 4-6 after WAS loading (Figure 2D). However, the distribution pattern of phenol red did not change in Vag-rats even after WAS was treated (Figure 2F).
The number of luminal feces in the distal colon and rectum was higher in RNT-rats and Vag-rats than in sham-operated rats during sham stress (baseline values) [sham op (RNT): 2.3 ± 0.5, RNT-rats: 4.1 ± 0.4, sham op (Vag): 2.1 ± 0.4, Vag-rats: 3.0 ± 0.5, Table 1]. WAS tended to reduce luminal feces in sham-operated and Vag-rats, with differences of -0.6 and -1.8 between luminal feces in sham stress and in WAS, respectively (Table 1). The number of fecal pellets was increased by WAS in RNT-rats, but was decreased in sham op- and Vag-rats. (B)-(A) indicates the differences of the influx and efflux of fecal pellets from the distal colon and rectum. However, the difference between sham stress and WAS was 0.1 feces in RNT-rats, indicating that the luminal feces in segments 6-8 did not decrease under WAS in these rats.
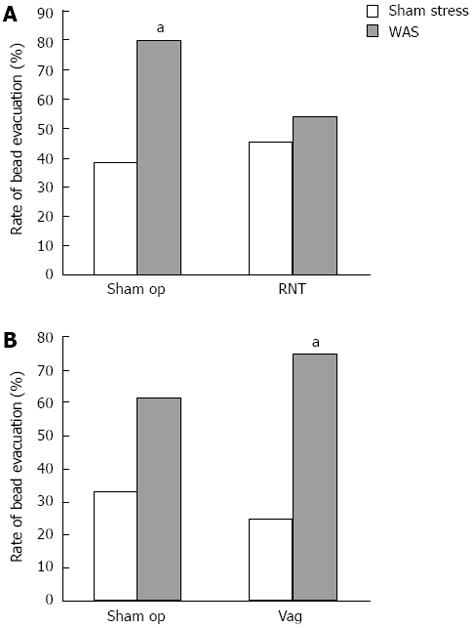
Preganglionic parasympathetic pelvic nerves project from the sacral spinal cord to the distal colon and rectum[26]. Thus, to investigate the effect of RNT on WAS-induced distal colonic and rectal transit, a glass bead was inserted until 3 cm depth from the anus into the distal colon, and the evacuation rate of the bead was monitored over a 2-h period.
In sham-operated rats, WAS significantly increased the rate of bead evacuation to 80% compared to 38% in sham stress, but this increase was not observed in RNT-rats subjected to WAS (Figure 3A). On the other hand, Vag did not affect the WAS-induced increase in the bead evacuation rate (Figure 3B).
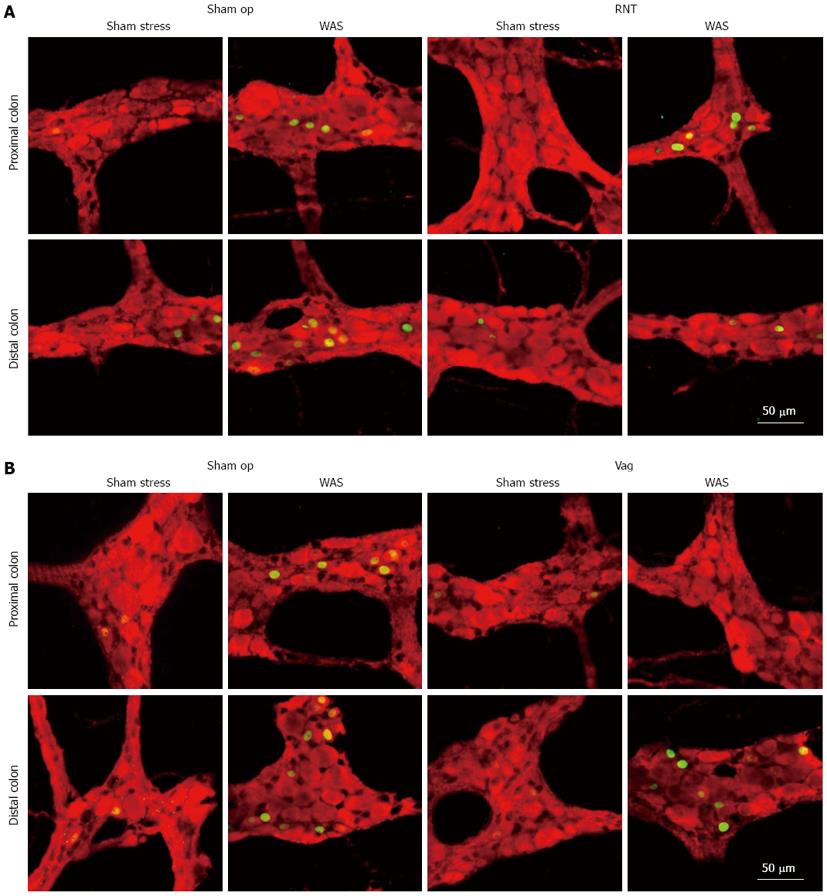
After rats were exposed to WAS for 1 h, the number of c-Fos-IR cells was examined in whole mount preparations of LMMP from the distal colon at 0, 1, 2 and 4 h later. The maximum increase after WAS was found at 0 h, and this number returned to a normal level within 4 h (data not shown). Therefore, we measured c-Fos expression immediately after the WAS session (1-h) in all of the following experiments using denervated rats.
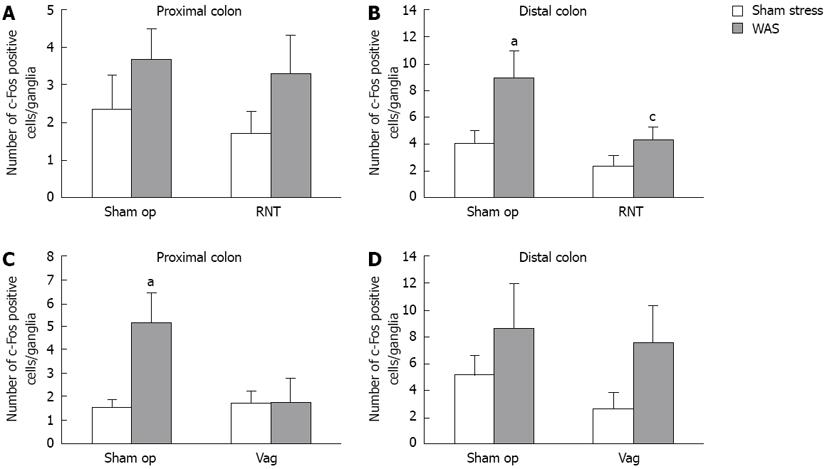
The number of c-Fos-IR cells per ganglia in the LMMP of both the proximal and distal colon (Figures 4 and 5) was increased by WAS compared with sham stress. RNT significantly reduced the WAS-induced increase of c-Fos-IR cells in the LMMP by 52% in the distal colon compared with sham-operated rats (4.4 ± 1.0 vs 9.0 ± 2.0, P < 0.05, Figure 5B). In contrast, Vag inhibited the increase in c-Fos-IR cells after WAS in the LMMP of proximal colon but not of distal colon (1.7 ± 1.0 vs 5.2 ± 1.3, Figure 5C and D).
In the present study, we demonstrated the functional importance of the pelvic nerve pathway in the response to psychological stress by showing that transection of the rectal branches of the pelvic nerve (RNT) inhibited the WAS-induced increase in fecal pellet output in rats. This inhibitory effect is supported further by the observation that the RNT inhibited the increased propulsive activity of the distal colon and rectum as shown in the analysis of the colorectal excretion rate of glass beads. Therefore, it is likely that WAS accelerates distal colonic and rectal motilities via activation of the pelvic nerve, resulting in increased defecation in the rat. Lenz[22] and Nakade et al[23] showed that Vag reduced the restraint stress-induced increase in colonic motility in rats, suggesting that physical stress causes colonic dysmotility via parasympathetic vagal activation. Vag also suppressed the WAS-induced increase in fecal pellet output in our study, which is consistent with their results. Our study also confirmed that proximal part of the colonic transit was accelerated in RNT-rats, whereas it was inhibited in Vag-rats under stressed conditions. In fact, many hard fecal pellets remained in the distal colon and rectum of RNT-rats in spite of WAS loading. In contrast, luminal fecal pellets in the distal part of the colon and rectum were decreased in sham-operated and Vag-rats after WAS. These results suggest that pelvic nerve activation by WAS accelerates motor activities of distal colon and rectum, but not in those of proximal colon. On the other hand, Vagus nerve activation accelerates the proximal colonic transit, but not the distal colonic and rectal transit. Therefore, the difference in projection sites of the gut between the pelvic and vagal efferent nerves may be responsible for the differential actions in the gut[21,26]. The pelvic nerve innervates the distal colon and rectum, and regulates rectal motility in the physiological conditions[27]. In this study, a larger number of fecal pellets remained in the distal colon and rectum in RNT-rats without stress, compared with sham-operated rats. These observations suggest that the pelvic nerve pathway may be involved in colorectal motility in the non-stressed conditions. In addition, our findings demonstrate that acute psychological stress stimulates colorectal transit via the pelvic nerve pathway.
Recently, c-Fos protein expression has been used as a marker of neural cell activation[30]. c-Fos expression was significantly increased in PGP9.5-positive myenteric neurons in the proximal and distal colon after WAS. This finding is similar to that of a study by Miampamba et al[31], who reported increased c-Fos expression in the myenteric ganglia of the proximal and distal colon after WAS. Enhanced c-Fos expression was correlated with the number of fecal pellets in the rats and was significantly suppressed in the distal, but not proximal, colon by the denervation of pelvic nerve branches after WAS. On the other hand, Vag suppressed c-Fos expression in the proximal colon but had no effect in the distal colon. These results concerning c-Fos expression are consistent with the differences in the functional site of action between the vagus and pelvic nerves, as discussed above. It is yet unclear whether c-Fos expression, which was induced by WAS-loading, is a direct or indirect response to pelvic efferent nerve activation. As a direct effect, c-Fos expression could be due to inputs from the preganglionic efferent fibers to the myenteric neurons[21,26]. As indirect effect, this may be due to mechanisms such as colonic motility and secretory responses, sensory neurons and interneurons in enteric nervous systems, or the release of gastrointestinal hormones and other factors that activate myenteric neurons. Although we did not clarify the types of neurons that expressed c-Fos protein in these experiments, myenteric neurons are generally classified as motor neurons, sensory neurons, and interneurons based on histological and morphological properties[31,32]. A double-labeling study revealed that 24%-25% of the total peripheral-choline acetyltransferase-positive neurons, which are mostly motor neurons in the myenteric plexus[32], were c-Fos positive in the proximal and distal colon after WAS[31]. Furthermore, the muscarinic receptor antagonist atropine inhibits the WAS-induced increase in fecal pellets without reducing the elevation of c-Fos-IR nuclei in the proximal and distal colonic LMMP[31,33]. Taken together, these results suggest that myenteric neurons in the distal and proximal colon are activated directly via the pelvic and vagal efferent, respectively, followed by acceleration of colonic transit and defecation.
The HPA-axis is also known as a key stress response pathway. HPA-axis activation ultimately induces corticosterone release from the adrenal gland[16]. In this study, the failure to release corticosterone by bilateral Adx did not affect the WAS-induced increase in fecal pellets. Furthermore, Lenz[22] showed that Adx or hypophysectomy did not alter the restraint stress-induced acceleration of colonic transit. These results indicate that the HPA-axis is not mainly involved in acute stress-induced acceleration of colorectal transit and defecation. Our results from adrenalectomized rats in this study also imply that the SAM-axis is of little importance in acute stress-induced colonic dysmotility because Adx inhibits the SAM-axis in addition to the HPA-axis[34]. It was shown recently that a corticoid-receptor antagonist inhibits chronic stress-induced defecation, indicating that the HPA-axis could be involved in the acceleration of colonic motor function by stress[35]. It is not clear why this corticoid-receptor antagonist decreased stress-induced colonic motor function, but this might be due to the different mechanisms that are involved in acute and chronic stress. In fact, it has been reported that chronic exposure of the amygdala to corticosterone delayed colorectal transit after WAS[36]. The current study has only examined the involvement of the parasympathetic nerves in stress-induced acceleration of colonic transit and defecation. The colon and rectum also receives sympathetic inputs from the lumbar colonic nerves and hypogastric nerves. It has been shown that stimulation of sympathetic nerves inhibits rectal motility in guinea pigs and cats[37-39]. Furthermore, it has been reported that sympathetic nerve denervation increased the c-Fos immunoreactivity in the LMMP[40] and the rectal motility[27]. These results suggested that sympathetic nerves may negatively regulate the colonic motor function. In the stressed-condition, however, it remains to be determined whether sympathetic nerve activity participates in the acceleration of colorectal transit and defecation induced by stress. Further experimental investigations are needed to understand the involvement of sympathetic nerves in stress-induced colonic dysmotility.
In conclusion, we have shown that WAS-induced acceleration of distal colonic transit and defecation is mediated by the parasympathetic pelvic efferent. The pelvic nerve may convey stress stimuli from the brain to the distal colon and rectum, directly activating myenteric neurons and subsequently accelerating distal colonic motility. In contrast, WAS-induced acceleration of colonic transit and activation of myenteric neurons in proximal colon is mediated by parasympathetic vagal efferent.
We thank Dr. Mamoru Tanida from the Department of Biomedical Sciences, College of Life Sciences, Ritsumeikan University, for his technical advice on the subdiaphragmatic vagotomy, and the entire staff at the animal care facility of Yakult Central Institute for Microbiological Research.
Stress is a key factor in causing abnormal bowel habits, such as constipation and diarrhea, and has been known to exacerbate these symptoms. It has been described that various stressors stimulate colonic motor functions. Although it is reported that the activation of colonic motor function is mediated via the parasympathetic vagus nerve, there are few studies that have reported the involvement of the parasympathetic pelvic nerve in stress-induced gut dysmotility.
The pelvic efferent projects from the sacral parasympathetic nucleus to the distal colon and rectum. It is reported that water avoidance stress (WAS) activate the sacral parasympathetic nucleus, however, it is still unclear whether activation of the pelvic nerve is involved in altered colonic and rectal motilities under stressed conditions.
This study demonstrated the importance of the pelvic nerve in the response to WAS-induced acceleration of distal colonic transit and increase of defecation. Furthermore, the results confirmed the involvement of vagus nerve to WAS-induced acceleration of proximal colonic transit. This is the first study to report the differences in the functional site of action between two nerves under stressed condition.
By understanding how stress stimuli cause the colonic dysmotility, these results would be useful for the therapeutic applications of stress-related colonic motor dysfunction, such as irritable bowel syndrome.
Longitudinal muscle myenteric plexus (LMMP): LMMP is generally used as immunohistochemical study of the myenteric plexus; c-Fos: c-Fos protein is the product of immediate-early gene, c-fos, which is used as a marker of neural activation.
The authors clearly demonstrated involvement of pelvic nerve in the psychological stress-induced acceleration of distal colonic transit and defecation. Additionally this study suggested that pelvic nerve directly activate the myenteric neurons and subsequently accelerating distal colonic motility. This is an interesting study, that clarify the role of the pelvic and vagus nerve in the stress-induced colonic dysmotility.
P- Reviewer Lohsiriwat V S- Editor Wen LL L- Editor A E- Editor Li JY
| 1. | Hislop IG. Psychological significance of the irritable colon syndrome. Gut. 1971;12:452-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 140] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 2. | Guthrie E, Creed F, Dawson D, Tomenson B. A controlled trial of psychological treatment for the irritable bowel syndrome. Gastroenterology. 1991;100:450-457. [PubMed] |
| 3. | Chan AO, Cheng C, Hui WM, Hu WH, Wong NY, Lam KF, Wong WM, Lai KC, Lam SK, Wong BC. Differing coping mechanisms, stress level and anorectal physiology in patients with functional constipation. World J Gastroenterol. 2005;11:5362-5366. [PubMed] |
| 4. | Murray CD, Flynn J, Ratcliffe L, Jacyna MR, Kamm MA, Emmanuel AV. Effect of acute physical and psychological stress on gut autonomic innervation in irritable bowel syndrome. Gastroenterology. 2004;127:1695-1703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 104] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 5. | Rao SS, Hatfield RA, Suls JM, Chamberlain MJ. Psychological and physical stress induce differential effects on human colonic motility. Am J Gastroenterol. 1998;93:985-990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Wittmann T, Crenner F, Angel F, Hanusz L, Ringwald C, Grenier JF. Long-duration stress. Immediate and late effects on small and large bowel motility in rat. Dig Dis Sci. 1990;35:495-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Stam R, Croiset G, Akkermans LM, Wiegant VM. Effects of novelty and conditioned fear on small intestinal and colonic motility and behaviour in the rat. Physiol Behav. 1995;58:803-809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Welgan P, Meshkinpour H, Hoehler F. The effect of stress on colon motor and electrical activity in irritable bowel syndrome. Psychosom Med. 1985;47:139-149. [PubMed] |
| 9. | Miyata K, Kamato T, Nishida A, Ito H, Yuki H, Yamano M, Tsutsumi R, Katsuyama Y, Honda K. Role of the serotonin3 receptor in stress-induced defecation. J Pharmacol Exp Ther. 1992;261:297-303. [PubMed] |
| 10. | Barone FC, Deegan JF, Price WJ, Fowler PJ, Fondacaro JD, Ormsbee HS. Cold-restraint stress increases rat fecal pellet output and colonic transit. Am J Physiol. 1990;258:G329-G337. [PubMed] |
| 11. | Bonaz B, Taché Y. Water-avoidance stress-induced c-fos expression in the rat brain and stimulation of fecal output: role of corticotropin-releasing factor. Brain Res. 1994;641:21-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 117] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 12. | Kalin NH, Takahashi LK, Chen FL. Restraint stress increases corticotropin-releasing hormone mRNA content in the amygdala and paraventricular nucleus. Brain Res. 1994;656:182-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 181] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 13. | Merlo Pich E, Lorang M, Yeganeh M, Rodriguez de Fonseca F, Raber J, Koob GF, Weiss F. Increase of extracellular corticotropin-releasing factor-like immunoreactivity levels in the amygdala of awake rats during restraint stress and ethanol withdrawal as measured by microdialysis. J Neurosci. 1995;15:5439-5447. [PubMed] |
| 14. | Yamano Y, Yoshioka M, Toda Y, Oshida Y, Chaki S, Hamamoto K, Morishima I. Regulation of CRF, POMC and MC4R gene expression after electrical foot shock stress in the rat amygdala and hypothalamus. J Vet Med Sci. 2004;66:1323-1327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 52] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 15. | Imaki T, Nahan JL, Rivier C, Sawchenko PE, Vale W. Differential regulation of corticotropin-releasing factor mRNA in rat brain regions by glucocorticoids and stress. J Neurosci. 1991;11:585-599. [PubMed] |
| 16. | Lightman SL. The neuroendocrinology of stress: a never ending story. J Neuroendocrinol. 2008;20:880-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 200] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 17. | Buske-Kirschbaum A, Geiben A, Höllig H, Morschhäuser E, Hellhammer D. Altered responsiveness of the hypothalamus-pituitary-adrenal axis and the sympathetic adrenomedullary system to stress in patients with atopic dermatitis. J Clin Endocrinol Metab. 2002;87:4245-4251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 164] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 18. | Charmandari E, Tsigos C, Chrousos G. Endocrinology of the stress response. Annu Rev Physiol. 2005;67:259-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1000] [Cited by in RCA: 1046] [Article Influence: 52.3] [Reference Citation Analysis (0)] |
| 19. | Yang EV, Glaser R. Stress-induced immunomodulation: impact on immune defenses against infectious disease. Biomed Pharmacother. 2000;54:245-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 49] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | De Vente W, Olff M, Van Amsterdam JG, Kamphuis JH, Emmelkamp PM. Physiological differences between burnout patients and healthy controls: blood pressure, heart rate, and cortisol responses. Occup Environ Med. 2003;60 Suppl 1:i54-i61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 129] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 21. | Berthoud HR, Carlson NR, Powley TL. Topography of efferent vagal innervation of the rat gastrointestinal tract. Am J Physiol. 1991;260:R200-R207. [PubMed] |
| 22. | Lenz HJ. Neurohumoral pathways mediating stress-induced changes in rat gastrointestinal transit. Gastroenterology. 1989;97:216-218. [PubMed] |
| 23. | Nakade Y, Fukuda H, Iwa M, Tsukamoto K, Yanagi H, Yamamura T, Mantyh C, Pappas TN, Takahashi T. Restraint stress stimulates colonic motility via central corticotropin-releasing factor and peripheral 5-HT3 receptors in conscious rats. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1037-G1044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 82] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 24. | Loewy AD, Saper CB, Baker RP. Descending projections from the pontine micturition center. Brain Res. 1979;172:533-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 198] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 25. | Blok BF, Holstege G. Ultrastructural evidence for a direct pathway from the pontine micturition center to the parasympathetic preganglionic motoneurons of the bladder of the cat. Neurosci Lett. 1997;222:195-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 77] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 26. | Luckensmeyer GB, Keast JR. Projections of pelvic autonomic neurons within the lower bowel of the male rat: an anterograde labelling study. Neuroscience. 1998;84:263-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 27. | Ridolfi TJ, Tong WD, Takahashi T, Kosinski L, Ludwig KA. Sympathetic and parasympathetic regulation of rectal motility in rats. J Gastrointest Surg. 2009;13:2027-2033; discussion 2033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 28. | Tong WD, Ridolfi TJ, Kosinski L, Ludwig K, Takahashi T. Effects of autonomic nerve stimulation on colorectal motility in rats. Neurogastroenterol Motil. 2010;22:688-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 29. | Million M, Wang L, Martinez V, Taché Y. Differential Fos expression in the paraventricular nucleus of the hypothalamus, sacral parasympathetic nucleus and colonic motor response to water avoidance stress in Fischer and Lewis rats. Brain Res. 2000;877:345-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 55] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 30. | Sagar SM, Sharp FR, Curran T. Expression of c-fos protein in brain: metabolic mapping at the cellular level. Science. 1988;240:1328-1331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1542] [Cited by in RCA: 1605] [Article Influence: 43.4] [Reference Citation Analysis (0)] |
| 31. | Miampamba M, Million M, Yuan PQ, Larauche M, Taché Y. Water avoidance stress activates colonic myenteric neurons in female rats. Neuroreport. 2007;18:679-682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 32. | Gwynne RM, Bornstein JC. Synaptic transmission at functionally identified synapses in the enteric nervous system: roles for both ionotropic and metabotropic receptors. Curr Neuropharmacol. 2007;5:1-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 51] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 33. | Mönnikes H, Schmidt BG, Taché Y. Psychological stress-induced accelerated colonic transit in rats involves hypothalamic corticotropin-releasing factor. Gastroenterology. 1993;104:716-723. [PubMed] |
| 34. | Ismahan G, Parvez H, Parvez S, Youdim MB. Comparative effects of hypophysectomy and adrenalectomy upon plasma and adrenal monoamines in pregnant and non-pregnant rats. Br J Pharmacol. 1977;60:385-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 35. | Hong S, Zheng G, Wu X, Snider NT, Owyang C, Wiley JW. Corticosterone mediates reciprocal changes in CB 1 and TRPV1 receptors in primary sensory neurons in the chronically stressed rat. Gastroenterology. 2011;140:627-637.e4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 75] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 36. | Venkova K, Johnson AC, Myers B, Greenwood-Van Meerveld B. Exposure of the amygdala to elevated levels of corticosterone alters colonic motility in response to acute psychological stress. Neuropharmacology. 2010;58:1161-1167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 37. | Takaki M, Neya T, Nakayama S. Sympathetic activity in the recto-rectal reflex of the guinea pig. Pflugers Arch. 1980;388:45-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 21] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 38. | Neya T, Takaki M, Nakayama S. Mechanism of rectal contraction mediated by sympathetic efferents from rectoanal pelvic afferents in guinea pigs. Acta Med Okayama. 1984;38:21-27. [PubMed] |
| 39. | Carlstedt A, Fasth S, Hultén L, Nordgren S. The sympathetic innervation of the internal anal sphincter and rectum in the cat. Acta Physiol Scand. 1988;133:423-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 24] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 40. | Yuyama N, Mizuno J, Tsuzuki H, Wada-Takahashi S, Takahashi O, Tamura K. Effects of extrinsic autonomic inputs on expression of c-Fos immunoreactivity in myenteric neurons of the guinea pig distal colon. Brain Res. 2002;948:8-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |