Published online Feb 14, 2013. doi: 10.3748/wjg.v19.i6.936
Revised: October 22, 2012
Accepted: October 30, 2012
Published online: February 14, 2013
AIM: To systematically assess the association between diabetes and incidence of gastric cancer.
METHODS: We searched MedLine (PubMed), EMBASE, and the Cochrane Library without any limitations with respect to publication date or language, we also searched the references of qualifying articles. Case-control studies and cohort studies comparing the risk of gastric cancer between diabetic patients and control subjects were included. We excluded studies reporting only standardized incidence ratios without control groups and those that investigated only mortality but not incidence. Seventeen studies met our criteria, and the qualities of these studies were assessed using the Newcastle-Ottawa Quality Assessment Scale. We performed a meta-analysis of pre-existing diabetes and gastric cancer incidence using the DerSimonian-Laird method for random-effects. For subgroup analyses, we separated the studies by study type, region, sex and method to determine confounding factors and reliability. We also conducted subgroup analyses to examine the effects of smoking, Helicobacter pylori (H. pylori) infection, and cancer site. Publication bias was evaluated using Begg’s test.
RESULTS: A random-effects model meta-analysis showed an increased gastric cancer risk in diabetic patients [relative risk (RR) = 1.19; 95%CI: 1.08-1.31]. Subgroup analyses indicated that this result persisted in cohort studies (RR = 1.20; 95%CI: 1.08-1.34), in studies on populations of both Western (RR = 1.18; 95%CI: 1.03-1.36) and Eastern countries (RR = 1.19; 95%CI: 1.02-1.38), in a female subgroup (RR=1.24; 95%CI: 1.01-1.52), and in highly qualified studies (RR = 1.17; 95%CI: 1.05-1.31). Moreover, these results persisted when the analysis was confined to studies adjusted for well-known gastric cancer risk factors such as smoking (RR = 1.17; 95%CI: 1.01-1.34) and H. pylori infection (RR = 2.35; 95%CI: 1.24-4.46).
CONCLUSION: Pre-existing diabetes mellitus may increase the risk of gastric cancer by approximately 19%. This effect seems to be unrelated to geographical region.
- Citation: Yoon JM, Son KY, Eom CS, Durrance D, Park SM. Pre-existing diabetes mellitus increases the risk of gastric cancer: A meta-analysis. World J Gastroenterol 2013; 19(6): 936-945
- URL: https://www.wjgnet.com/1007-9327/full/v19/i6/936.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i6.936
Diabetes mellitus is currently an epidemic. Approximately 171 million individuals worldwide had diabetes mellitus in 2000, and it is estimated that 366 million people will have diabetes by 2030, which corresponds to nearly 4.5% of the global population[1]. The estimated prevalence of diabetes among adults in the United States currently ranges from 5.9% to 12.4% (median 8.3%)[2], and some cancers, such as colorectal, endometrial, breast, liver, and pancreatic cancer, are reportedly more prevalent in diabetes patients[3-7]. Diabetes mellitus has also been associated with an increased risk of long-term and all-cause mortality in cancer patients[8].
Although the incidence and associated mortality of gastric cancer is decreasing, it remains the fourth most common cancer and the second leading cause of cancer-related mortality worldwide[9]. Despite the success in gastric cancer control, the persistence of the public health burden of gastric cancer indicates the need to expand efforts to identify and address the individual and social determinants of the disease. The public health significance of a potential causal link between diabetes mellitus and gastric cancer highlights the need for a systematic assessment of the association between these 2 diseases.
Therefore, several systematic reviews have recently been performed to examine the above mentioned associations. One review suggested that diabetic individuals have an increased risk of gastric cancer[10]. However, this review had some limitations as it included studies without control groups[11-14] and excluded appropriate studies[15,16]. Two other reviews showing inconclusive results[17,18] did not include any recent studies[19,20], and did not differentiate between “incidence” and “mortality” in terms of describing the risk[17]. Such methodological weaknesses of the previous meta-analysis made it difficult to understand the association between preexisting diabetes mellitus and the risk of gastric cancer.
In the present study, we aimed to clarify the association between diabetes and gastric cancer through an extensive search of the literature, which we reviewed using strict criteria.
The procedures performed in this meta-analysis are in accordance with recent guidelines for the reporting of meta-analysis (PRISMA guidelines).
We conducted a systematic search of electronic databases and the bibliographies of all eligible studies to identify all relevant studies. We initiated the search on February 7, 2012 without any limitations with respect to publication date or language. The electronic databases searched included MedLine (PubMed), EMBASE, and the Cochrane Library. The search strategy included terms for diabetes (glucose, diabetes, or hyperglycemia), gastric cancer (stomach cancer, gastric cancer, stomach malignant neoplasm, or gastric malignant neoplasm), and risk (incidence, prevalence, or risk). We also searched the references of included articles.
Case-control studies, cohort studies, and randomized controlled trials comparing the risk of gastric cancer between diabetic patients and control subjects were eligible for inclusion. We included studies evaluating self-reported diabetes, registered diabetes, and high HbA1c and blood glucose levels. To be included in our meta-analysis, articles had to contain both of the following: (1) a risk estimate (hazard ratio, relative risk, or odds ratio relating preexisting diabetes to subsequent occurrences of gastric cancer); and (2) an estimate of precision (standard error or 95%CI). We also included articles that failed to report precision directly, but from which we could reconstruct a precision estimate using the data described[21,22]. We excluded studies reporting only standardized incidence ratios without control groups. We also excluded studies that investigated only mortality without incidence and studies in which the classification of the subjects’ diabetes status (diabetic or non-diabetic) was not possible due to insufficient ranges in blood glucose levels. Studies were excluded if either the abstract[23] or the full text[24] was unavailable after author contact was made.
The titles, abstracts, and full articles were reviewed independently by two authors (Yoon JM and Son KY). Yoon JM performed a full abstraction of the data, and Son KY verified the accuracy. Disagreements were resolved by discussion, consensus, and arbitration by a third author (Park SM). Abstracted data included type of study, study population characteristics, criteria for diabetes mellitus or hyperglycemia, duration of follow-up, incidence of cancer, adjustment variables, and study quality. Quality was assessed using the Newcastle-Ottawa Quality Assessment Scale (NOS) for case-control or cohort studies.
To avoid overlapping patient populations, we compared data sources and geographic locations. If a patient population was found to overlap, we included the article with the most comprehensive population or the most adjusted risk estimate associated with preexisting diabetes. If an article reported estimates separately by sex[15,19,25-29] or by different cohorts[16], we regarded the study as 2 independent studies. If an article did not report the risk ratio and confidence interval, we estimated the risk using Fisher’s exact test without adjustment[20-22], and the estimates were rounded off to the nearest hundredth. In case-control studies, odds ratios were regarded as relative risks because of the low prevalence of gastric cancer[9,30,31].
For the meta-analysis, we calculated pooled estimates for all the studies. For the subgroup analyses, we separated studies by study type, region, sex, and method to determine the confounding factors and reliability. We also conducted subgroup analyses to examine the effects of smoking, Helicobacter pylori (H. pylori) infection, and cancer site, which are major factors influencing the risk of gastric cancer[32-34]. We calculated all the pooled estimates using the DerSimonian-Laird method for random effects. We also reported I2 values for heterogeneity assessment. Publication bias was evaluated using Begg’s test. All analyses were conducted using Stata software(version12.1, StataCorp, United States).
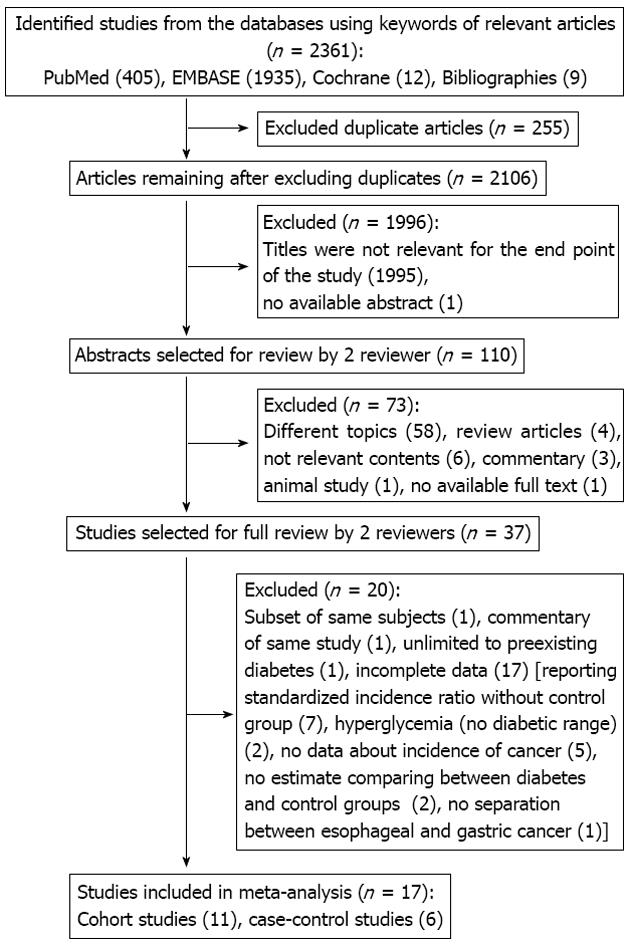
The systematic literature search identified 2361 relevant references (Figure 1). After screening the titles, we excluded 255 duplicated and 1996 non-relevant studies. By reviewing the abstracts, we further excluded 73 articles. The full texts of the 37 remaining articles were retrieved for formal review. After independent review, 20 studies were excluded; 2 studies[35,36] used the same populations as another trial[37], and 1 did not limit the exposure group to individuals with preexisting diabetes[38].
Seventeen studies reported only limited data (uncertain diabetes diagnosis[39,40], usage of a standardized incidence ratio[11,13,14,41-44], lack of an acceptable control group[45,46], reporting of only mortality data[47-51], and uncertain gastric cancer diagnosis[52]). Table 1 provides the details of the 17 studies that met our predefined inclusion and exclusion criteria. In total, 11 cohort[15,16,19,22,26-28,37,53-55] and 6 case-control[20,21,25,29,56,57] studies were used. We were unable to find any suitable randomized control trials.
| Study | Study type | Country | DM criteria | Age (yr) | Follow up (yr) | Least interval1 (yr) | Sex | DM prevalence | RR or OR (95%CI) | Adjustment variables/matched variable of selection | NOS | |
| Atchison et al[53] | CS | United States (veterans) | Registration | 18-100 | 27 | 1 | Male | 13.2% | 0.95 (0.89-1.02) | Age, time, latency, race, number of visits, diagnoses of alcohol–related conditions, obesity and COPD | 8 | |
| Carstensen et al[19] | CS | Denmark | Registration | No limit | 15 | Not mentioned | Male | 1.28 (1.15-1.43) | Age, calendar time, date of birth | 8 | ||
| Female | 1.34 (1.14-1.58) | |||||||||||
| Jiang et al[20] | CC | United States | Self reported | 30-74 | 1 | Both | 1.51 (1.10-2.07)2 | Age, sex, race, education, birth place, smoking and BMI | 7 (5)5 | |||
| Lin et al[22] | CS | United States | Self reported | 50-71 | 10 | 2 | Male | 9.9% | 1.52 (1.21-1.92)2 | Age, calories, alcohol consumption, smoking, fruit consumption, vegetable consumption, ethnicity, education, and physical activity | 8 (6)5 | |
| Female | 7.2% | 1.62 (0.98-2.68)2 | ||||||||||
| Wotton et al[16] | ORLS1 | CS | England | Registration | ≥ 30 | 35 | 0 | Both | 5.5% | 1.11 (0.89-1.37) | Sex, age in 5-year bands, time and district of residence | 8 |
| ORLS2 | 9 | 4.0% | 2.05 (1.30-3.10) | |||||||||
| Chodick et al[54] | CS | Israel | Registration | ≥ 21 | 8 | 0 | Male | 1.44 (0.98-2.11) | Age, region, SES level, use of healthcare, BMI, and history of CVD/matched for age, sex | 8 | ||
| Female | 0.99 (0.55-1.80) | |||||||||||
| Ikeda et al[37] | CS | Japan | HbA1c (5.0%-5.9% vs 7.0%) | ≥ 40 | 14 | Not mentioned | Both | 3.9% | 2.69 (1.24-5.85) | Age, sex, H. pylori, history of peptic ulcer, BMI, TC, alcohol intake, smoking, dietary factor4 | 8 | |
| Ogunleye et al[55] | CS | Scotland | Registration (only type 2 DM) | No limit | 11 | 1 | Both | 0.77 (0.36-1.66) | Deprivation deciles of Carstairs/matched for age, sex and doctor practice | 8 | ||
| Kuriki et al[29] | CC | Japan | Self reported | 40-80 | Not mentioned | Male | 1.16 (0.93-1.44) | Age, BMI, drinking, smoking, exercise, bowel movement, FHx of cancer and DM, dietary restriction, vegetable intake, greasy food, snack | 6 | |||
| Female | 1.70 (1.16-2.48) | |||||||||||
| Inoue et al[27] | CS | Japan | Self reported | 40-69 | 13 | 5 | Male | 6.7% | 1.09 (0.79-1.5) | Age, study area, history of CVD and IHD, smoking, BMI, physical activity, alcohol, vegetable, coffee | 9 | |
| Female | 3.0% | 1.92 (1.06-3.47) | ||||||||||
| Jun et al[57] | NCC | Korea | FBS (< 99 mg/dL vs≥ 126 mg/dL) | ≥ 35 | Case 2.4/control 6.5 h | 2 | Both | 1.77 (0.57-5.45) | H. pylori, smoking, drinking, and education level/matched on age, sex, year and area of enrollment, and follow-up duration | 9 | ||
| Khan et al[28] | CS | Japan | Self reported | 40-79 | 19 | 2 | Male | 7.5% | 0.72 (0.40-1.09) | Age, BMI, smoking, drinking | 8 | |
| Female | 4.6% | 0.26 (0.08-0.82) | ||||||||||
| Rapp et al[15] | CS | Austria | FBS (4.2-5.2 mmol/L vs 7.0 mmol/L) | > 19 | 14 | 1 | Male | 3.9% | 0.84 (0.38-1.87) | Age, smoking, occupation, BMI | 8 | |
| Female | 3.0% | -3 | ||||||||||
| Rousseau et al[56] | CC | Canada | Self reported | 35-70 | 2 | Male | 1.0 (0.5-1.8) | Age, income, education, ethnicity, proxy status, BMI, smoking, beta-carotene, alcohol | 7 | |||
| Jee et al[26] | CS | South Korea | FBS (< 90 mg/dL vs≥ 126 mg/dL) or DM medication | 30-95 | 10 | 1 | Male | 5.1% | 1.11 (1.04-1.2) | Age, age squared, smoking, alcohol | 8 | |
| Female | 4.5% | 1.15 (0.99-1.34) | ||||||||||
| La Vecchia et al[25] | CC | Italy | Self reported | ≤ 75 | Not mentioned | Male | 0.6 (0.4-1.0) | Age | 6 | |||
| Female | 0.7 (0.4-1.2) | |||||||||||
| O'Mara et al[21] | CC | USA (only white) | Self reported | 30-89 | 1 | Male | 0.91 (0.28-2.27)2 | Age | 4 (3)5 | |||
| Female | 1.57 (0.48-3.94) | |||||||||||
The 17 articles included in our analysis were heterogeneous in many respects. Geographically, 6 studies were conducted in East Asia, 1 in West Asia, 5 in North America, and 5 in Europe. According to the cohort studies, the prevalence of diabetes varied from 3.0% to 13.2%. Four studies used measurement criteria to define diabetes mellitus, and 2 studies differentiated between gastric cardia and non-cardia cancer. The reporting of age and follow-up time varied widely. In 13 studies, a time interval of more than 1 year between the diagnoses of diabetes and cancer was required in order to minimize reverse causality, although 2 studies showed only the estimates without time intervals[16,54]. Quality varied from 4 to 9 stars according to the NOS, but 3 studies were downgraded because we used recalculated estimates without adjusting for pooled analysis[20-22]. After downgrading, the mean NOS score was 7.3. We therefore conducted subgroup analysis according to methodological quality (with “high-quality” defined as a score of ≥ 8).
Fifteen studies included both sexes and 10 reported data separated by sex. In one of the latter, fewer than 5 female patients were diagnosed with both diabetes mellitus and subsequent gastric cancer[15]; the estimate was therefore not reported. In total, we found 28 independent groups separated by sex and cohort, and we yielded 27 eligible estimates. Only 2 studies described the specific risk ratios of cardia and non-cardia gastric cancers[20,22].
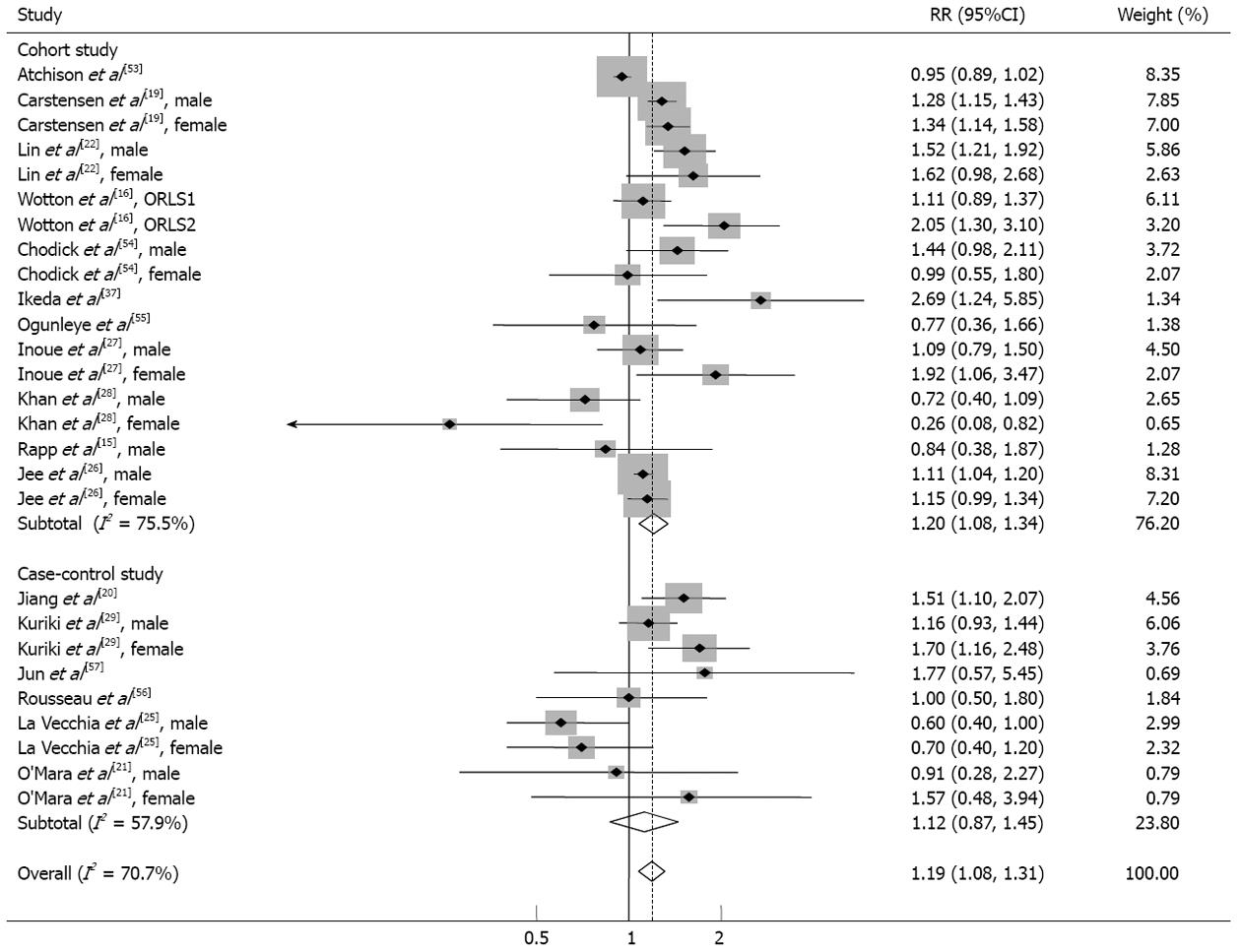
As shown in Figure 2, diabetes mellitus was associated with a significantly increased risk of gastric cancer in all included studies using random effects model analysis [relative risk (RR) = 1.19; 95%CI: 1.08-1.31; I2 = 70.7%; 95%CI: 56.8%-80.2%].
We examined methodological differences when determining the quality of the studies. According to Figure 2, subgroup meta-analysis by study design revealed significant associations in cohort studies (RR = 1.20; 95%CI: 1.08-1.34), while the results of case-control studies were not statistically significant. Positive associations were observed in more reliable subgroup studies using the registration record or laboratory measurements as diabetes criteria and studies that were highly graded by NOS. A subgroup meta-analysis of studies designed to minimize reverse causality, including the shortest cancer-free interval, indicated that diabetes seemed to increase subsequent gastric cancer (Table 2).
| Studies (n) | RR (95%CI) | I2% (95%CI) | |
| Overall | 27 | 1.19 (1.08-1.31) | 70.7 (56.8-80.2) |
| Countries | |||
| East Asian countries1 | 10 | 1.19 (1.02-1.38) | 60.1 (20.0-80.1) |
| Western countries2 | 17 | 1.18 (1.03-1.36) | 75.6 (61.0-84.8) |
| Study type | |||
| Cohort study | 18 | 1.20 (1.08-1.34) | 75.5 (61.2-84.5) |
| Case control study | 9 | 1.12 (0.87-1.45) | 57.9 (11.8-79.9) |
| Sex | |||
| Male | 12 | 1.10 (0.97-1.24) | 74.9 (55.7-85.8) |
| Female | 9 | 1.24 (1.01-1.52) | 58.9 (14.1-80.3) |
| DM criteria | |||
| Registration | 8 | 1.21 (1.02-1.44) | 83.0 (67.8-91.0) |
| Measured3 | 5 | 1.15 (1.01-1.32) | 35.5 (0.0-75.8) |
| Self report | 14 | 1.13 (0.93-1.39) | 65.2 (38.6-80.3) |
| Interval4 | |||
| One and more years | 15 | 1.15 (1.02-1.31) | 67.5 (44.3-81.0) |
| Quality (NOS) | |||
| High quality (8 and more) | 17 | 1.17 (1.05-1.31) | 73.4 (57.0-83.6) |
| Low quality (7 and less) | 10 | 1.20 (0.97-1.49) | 61.2 (22.6-80.5) |
| Adjustment for risk factor | |||
| Smoking5 | 12 | 1.17 (1.01-1.34) | 52.6 (8.7-75.4) |
| H. pylori5 | 2 | 2.35 (1.24-4.46) | 0.0 (UC) |
| Cancer site | |||
| Cardia cancer | 2 | 1.39 (0.72-2.69) | 82.3 (UC) |
| Noncardia cancer | 2 | 1.19 (0.80-1.77) | 59.6 (UC) |
Regional and gender-based differences are presented in Table 2. East Asian (RR = 1.19; 95%CI: 1.02-1.38) and Western studies (RR = 1.18; 95%CI: 1.03-1.36) showed similar increased risks. In the sexual subgroup, only women exhibited statistical significance (RR = 1.24; 95%CI: 1.01-1.52).
Pooled estimates adjusted for well-known gastric cancer risk factors (cigarette smoking and H. pylori infection) were also significant. The pooled estimate adjusted for cigarette smoking status was similar to the overall results (RR = 1.17; 95%CI: 1.01-1.34). The pooled estimate adjusted for H. pylori infection was greater than the overall estimate (RR = 2.35; 95%CI: 1.24-4.46). Nevertheless, subgroup analyses on either cardia or noncardia cancer did not produce significant results.
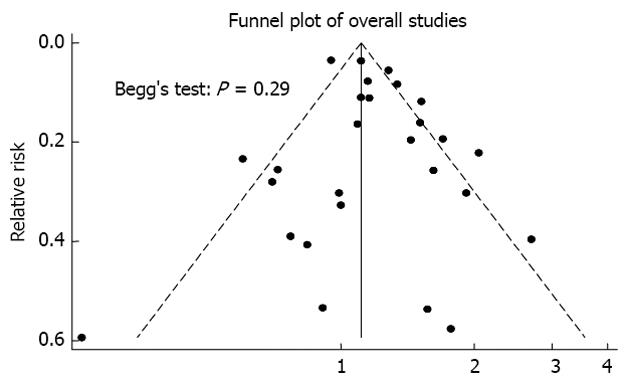
Begg’s test indicated no publication bias in our analysis (Figure 3).
We found a significant association between preexisting diabetes and gastric cancer incidence. In diabetic patients, we found that the risk of subsequent gastric cancer incidence was increased by approximately 19%, and this effect was significant in women. The subgroups of cohort, Western and Eastern studies, showed similar results. These results persisted in studies using self-reported criteria and those adjusted for known risk factors such as cigarette smoking and H. pylori infection.
We observed some discrepancy between our analysis and previous meta-analysis. Three previous meta-analysis have explored the association between diabetes and gastric cancer incidence. One of these, a meta-analysis of cohort studies[18], produced a misleading result due to the exclusion of appropriate cohort studies[16,19,54] and the inclusion of a study using a glucose level that was not clearly defined and an unrepresentative range of diabetes mellitus[40]. The study also presented an incomprehensible method for summarizing 2 estimates of cardia and noncardia cancer from the same control group. A different study[17] used the term “risk” without a distinction between incidence and mortality. This methodological weakness made the result heterogeneous and biologically implausible. Another meta-analysis[10] also excluded several recent studies[15,16,19,20] and included studies reporting a standardized incidence ratio without quality assessment.
To supplement the previous insufficient meta-analyses, we restricted inclusion in our analysis to studies reporting a risk ratio compared to a control group, and we assessed the quality of these studies using various criteria. As a result, we found that diabetes and the risk of gastric cancer were positively associated with each other. This positive association may be reliable based on consistent results from well-designed studies including cohort studies, studies of high quality based on assessment with the NOS, studies examining the time interval between onset of diabetes and subsequent gastric cancer, and studies using objective diabetic criteria (diabetes registry or laboratory measurements) that were adjusted based on important factors (smoking or H. pylori infection). Although a time interval of 1 year between diabetes and gastric cancer may be insufficient evidence of a causal relationship, we were able to minimize the reverse causality.
Regarding biological plausibility, the results of this study are consistent with previous studies in which preexisting diabetes was associated with an increased risk of a variety of cancers. The oncogenic properties of diabetes have been well documented. The increased insulin concentration in early diabetes can stimulate cell proliferation through activation of the insulin receptor or insulin-like growth factor-I receptor (IGF-IR) and the inhibition of IGF binding proteins, which may result in increased free and bioavailable IGF-I[58]. Additionally, a high level of serum IGF-I has been demonstrated to increase the risk for development of several carcinomas including gastrointestinal carcinomas[59]. Exogenous IGFs stimulate the proliferation of gastric cancer cells, while the blocking of IGF-IR inhibits tumor development[60,61].
Vascular endothelial growth factor (VEGF), the levels of which are increased in the blood of diabetic patients[62], is another cytokine that is related to the oncogenic properties of diabetes. VEGF is correlated with tumor vascularity and the frequency of hepatic metastasis, and it induces the proliferation and dilation of lymphatics culminating in node metastasis[63-65]. Moreover, the IGF/IGF-IR axis has also been shown to interact with the VEGF/VEGFR system in various tumors including gastrointestinal malignancies[66,67].
DNA damage in diabetic patients is another potential mechanism of oncogenesis. The increased production of reactive oxygen species could result in greater oxidative damage to DNA[68]. Furthermore, in an experimental study, high blood glucose levels were shown to directly induce DNA damage[69].
While we found that the association between diabetes and gastric cancer persisted irrespective of H. pylori infection, it nevertheless seems probable that diabetes and H. pylori infection, which is a known risk factor for gastric cancer, work synergistically to increase the risk of gastric cancer. Indeed, a previous cohort study in Japan demonstrated that a high HbA1c level and a concomitant H. pylori infection increased the gastric cancer risk synergistically[37]. It is possible that reactive oxygen-dependent DNA damage enhances the modifying effect of H. pylori on epithelial cell proliferation.
To confirm this synergistic effect, we also performed subgroup analyses by cancer site. Although noncardia gastric cancers were known to be strongly associated with H. pylori infection[70,71], we failed to show a similar relationship between diabetes mellitus and specific sites of gastric cancer. As further studies dealing with site-specific data are performed, a reassessment will be required.
A positive association between diabetes and gastric cancer incidence was observed among women but not among men. These results must be interpreted carefully as there were only a few studies differentiating men and women. Sex hormones could represent one possible reason for this difference. In breast cancer, it is well established that estrogen and IGF-1 interactively affect cell proliferation[72]. Gastrointestinal tissues, whether normal or cancerous, contain estrogen receptor β, and estrogen can bind to them[73,74]. Through an undefined mechanism, estrogen levels in diabetic women could influence or stimulate the proliferation of gastric cancer cells.
This gender-based difference could also be due to disparate methods of diabetes intervention. Metformin[46,75], aspirin[76,77], and statins[78] are known to protect against a number of cancers, while insulin is reported to increase the risk of several cancers[75]. Although gender differences in this matter were not explored in many previous studies, clues were obtained from the following studies: One study suggested that gender differences in adherence to diabetes management were minimal[79]. However, an Italian study reported that diabetic women were more likely to use insulin than men[80]. According to an American study, men use statins more frequently in type 2 diabetes mellitus[81]. These factors, however small, might contribute to the above-mentioned gender differences.
Our study has several limitations. First, the studies we examined were highly heterogeneous. In particular, diabetes mellitus was defined by various methods and cut-off values. Diabetic prevalence and subject age also differed. Second, dietary food factors such as salt, nitrite[82-84], and fresh vegetable[85] intake could not be considered in the analysis despite being potential confounders. Finally, the selected studies contained no details regarding diabetes interventions that were sufficient to adjust for the effects of diabetes treatments.
In conclusion, despite the limitations, our meta-analysis suggests that preexisting diabetes mellitus may increase the risk of gastric cancer. Further prospective studies assessing confounders such as diabetes interventions are needed to specifically test the effects of diabetes mellitus on gastric cancer risk.
The burden of diabetes mellitus is increasing globally. Gastric cancer is the fourth most common cancer and the second leading cause of cancer-related mortality worldwide. Although the association between diabetes mellitus and a variety of cancers is well established, the association between diabetes mellitus and gastric cancer is currently unclear.
Over the past 3 decades, many studies have been performed to understand the associations between preexisting diabetes mellitus and subsequent gastric cancer incidence. Moreover, several systematic reviews were recently performed to investigate these associations. However, these reviews were methodologically insufficient and thus could not achieve a comprehensive conclusion.
Based on this meta-analysis, preexisting diabetes mellitus may increase the risk of gastric cancer by approximately 19%. Similar associations were indicated in subgroup analyses of East Asian, Western, cohort, and high-quality studies. These findings were not presented clearly in previous systematic reviews.
Diabetes mellitus appears to be either directly or indirectly associated with the risk of gastric cancer. An exploration of the mechanism for this association may help them to reduce the gastric cancer risk.
Insulin-like growth factors (IGFs) are proteins similar to insulin. IGF is a part of the cellular communication system. Recently, IGFs were proposed to play a role in aging. Vascular endothelial growth factor (VEGF) is a protein related to angiogenesis. In diabetes mellitus, the level of VEGF in the blood is high.
This is a well-performed meta-analysis of currently available studies on the potential role of diabetes mellitus in the development of gastric cancer. The authors found that pre-existing diabetes mellitus may increase the risk of gastric cancer by approximately 19%. This finding was found to be unrelated to geographical region. This is a good meta-analysis and the authors have included many relevant issues missed by other research groups.
P- Reviewer Lindberg G S- Editor Gou SX L- Editor A E- Editor Li JY
| 1. | Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047-1053. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 139] [Cited by in F6Publishing: 107] [Article Influence: 5.4] [Reference Citation Analysis (1)] |
| 2. | Hughes E, Kilmer G, Li Y, Valluru B, Brown J, Colclough G, Geathers S, Roberts H, Elam-Evans L, Balluz L. Surveillance for certain health behaviors among states and selected local areas - United States, 2008. MMWR Surveill Summ. 2010;59:1-221. [PubMed] [Cited in This Article: ] |
| 3. | Friberg E, Orsini N, Mantzoros CS, Wolk A. Diabetes mellitus and risk of endometrial cancer: a meta-analysis. Diabetologia. 2007;50:1365-1374. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 351] [Cited by in F6Publishing: 351] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 4. | Larsson SC, Mantzoros CS, Wolk A. Diabetes mellitus and risk of breast cancer: a meta-analysis. Int J Cancer. 2007;121:856-862. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 598] [Cited by in F6Publishing: 610] [Article Influence: 35.9] [Reference Citation Analysis (0)] |
| 5. | Larsson SC, Orsini N, Wolk A. Diabetes mellitus and risk of colorectal cancer: a meta-analysis. J Natl Cancer Inst. 2005;97:1679-1687. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 719] [Cited by in F6Publishing: 714] [Article Influence: 37.6] [Reference Citation Analysis (0)] |
| 6. | El-Serag HB, Hampel H, Javadi F. The association between diabetes and hepatocellular carcinoma: a systematic review of epidemiologic evidence. Clin Gastroenterol Hepatol. 2006;4:369-380. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 581] [Cited by in F6Publishing: 553] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 7. | Huxley R, Ansary-Moghaddam A, Berrington de González A, Barzi F, Woodward M. Type-II diabetes and pancreatic cancer: a meta-analysis of 36 studies. Br J Cancer. 2005;92:2076-2083. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 758] [Cited by in F6Publishing: 737] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 8. | Barone BB, Yeh HC, Snyder CF, Peairs KS, Stein KB, Derr RL, Wolff AC, Brancati FL. Long-term all-cause mortality in cancer patients with preexisting diabetes mellitus: a systematic review and meta-analysis. JAMA. 2008;300:2754-2764. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 608] [Cited by in F6Publishing: 633] [Article Influence: 39.6] [Reference Citation Analysis (0)] |
| 9. | Crew KD, Neugut AI. Epidemiology of gastric cancer. World J Gastroenterol. 2006;12:354-362. [PubMed] [Cited in This Article: ] |
| 10. | Tian T, Zhang LQ, Ma XH, Zhou JN, Shen J. Diabetes mellitus and incidence and mortality of gastric cancer: a meta-analysis. Exp Clin Endocrinol Diabetes. 2012;120:217-223. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 11. | Adami HO, McLaughlin J, Ekbom A, Berne C, Silverman D, Hacker D, Persson I. Cancer risk in patients with diabetes mellitus. Cancer Causes Control. 1991;2:307-314. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 220] [Cited by in F6Publishing: 235] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 12. | Azfar RS, Ogunleye T, Treat J. Annular blisters on the arm. Arch Dermatol. 2009;145:497. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Wideroff L, Gridley G, Mellemkjaer L, Chow WH, Linet M, Keehn S, Borch-Johnsen K, Olsen JH. Cancer incidence in a population-based cohort of patients hospitalized with diabetes mellitus in Denmark. J Natl Cancer Inst. 1997;89:1360-1365. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 453] [Cited by in F6Publishing: 484] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 14. | Zendehdel K, Nyrén O, Ostenson CG, Adami HO, Ekbom A, Ye W. Cancer incidence in patients with type 1 diabetes mellitus: a population-based cohort study in Sweden. J Natl Cancer Inst. 2003;95:1797-1800. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 190] [Cited by in F6Publishing: 203] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 15. | Rapp K, Schroeder J, Klenk J, Ulmer H, Concin H, Diem G, Oberaigner W, Weiland SK. Fasting blood glucose and cancer risk in a cohort of more than 140,000 adults in Austria. Diabetologia. 2006;49:945-952. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 195] [Cited by in F6Publishing: 207] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 16. | Wotton CJ, Yeates DG, Goldacre MJ. Cancer in patients admitted to hospital with diabetes mellitus aged 30 years and over: record linkage studies. Diabetologia. 2011;54:527-534. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 17. | Ge Z, Ben Q, Qian J, Wang Y, Li Y. Diabetes mellitus and risk of gastric cancer: a systematic review and meta-analysis of observational studies. Eur J Gastroenterol Hepatol. 2011;23:1127-1135. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 62] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 18. | Marimuthu SP, Vijayaragavan P, Moysich KB, Jayaprakash V. Diabetes mellitus and gastric carcinoma: Is there an association? J Carcinog. 2011;10:30. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 19. | Carstensen B, Witte DR, Friis S. Cancer occurrence in Danish diabetic patients: duration and insulin effects. Diabetologia. 2012;55:948-958. [PubMed] [Cited in This Article: ] |
| 20. | Jiang X, Bernstein L, Tseng CC, Wu AH. Diabetes and risk of esophageal and gastric adenocarcinomas. Int J Cancer. 2012;131:1417-1422. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | O’Mara BA, Byers T, Schoenfeld E. Diabetes mellitus and cancer risk: a multisite case-control study. J Chronic Dis. 1985;38:435-441. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 145] [Cited by in F6Publishing: 163] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 22. | Lin SW, Freedman ND, Hollenbeck AR, Schatzkin A, Abnet CC. Prospective study of self-reported diabetes and risk of upper gastrointestinal cancers. Cancer Epidemiol Biomarkers Prev. 2011;20:954-961. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 23. | Ishida T. So called “diabetic cancer risk” in the inpatient’s health examination (Japanese). Teishin Igaku. 1973;25:107-115. [Cited in This Article: ] |
| 24. | Svacina S, Matoulek M, Svobodová S, Visokai V, Lipská L, Topolcan O, Zvárová J, Plavcová M. Gastrointestinal tract cancer and diabetes mellitus. Vnitr Lek. 2004;50:386-391. [PubMed] [Cited in This Article: ] |
| 25. | La Vecchia C, Negri E, Franceschi S, D’Avanzo B, Boyle P. A case-control study of diabetes mellitus and cancer risk. Br J Cancer. 1994;70:950-953. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 218] [Cited by in F6Publishing: 235] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 26. | Jee SH, Ohrr H, Sull JW, Yun JE, Ji M, Samet JM. Fasting serum glucose level and cancer risk in Korean men and women. JAMA. 2005;293:194-202. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 682] [Cited by in F6Publishing: 686] [Article Influence: 36.1] [Reference Citation Analysis (0)] |
| 27. | Inoue M, Iwasaki M, Otani T, Sasazuki S, Noda M, Tsugane S. Diabetes mellitus and the risk of cancer: results from a large-scale population-based cohort study in Japan. Arch Intern Med. 2006;166:1871-1877. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 384] [Cited by in F6Publishing: 392] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 28. | Khan M, Mori M, Fujino Y, Shibata A, Sakauchi F, Washio M, Tamakoshi A. Site-specific cancer risk due to diabetes mellitus history: evidence from the Japan Collaborative Cohort (JACC) Study. Asian Pac J Cancer Prev. 2006;7:253-259. [PubMed] [Cited in This Article: ] |
| 29. | Kuriki K, Hirose K, Tajima K. Diabetes and cancer risk for all and specific sites among Japanese men and women. Eur J Cancer Prev. 2007;16:83-89. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 113] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 30. | Boyle P. World Cancer Report. International Agency for Research on Cancer Press. 2008;344-349 Available from: http: //www.iarc.fr/en/publications/pdfs-online/wcr/2008/index.php. [Cited in This Article: ] |
| 31. | Forman D, Burley VJ. Gastric cancer: global pattern of the disease and an overview of environmental risk factors. Best Pract Res Clin Gastroenterol. 2006;20:633-649. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 285] [Cited by in F6Publishing: 295] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 32. | Compare D, Rocco A, Nardone G. Risk factors in gastric cancer. Eur Rev Med Pharmacol Sci. 2010;14:302-308. [PubMed] [Cited in This Article: ] |
| 33. | Trédaniel J, Boffetta P, Buiatti E, Saracci R, Hirsch A. Tobacco smoking and gastric cancer: review and meta-analysis. Int J Cancer. 1997;72:565-573. [PubMed] [Cited in This Article: ] |
| 34. | González CA, Pera G, Agudo A, Palli D, Krogh V, Vineis P, Tumino R, Panico S, Berglund G, Simán H. Smoking and the risk of gastric cancer in the European Prospective Investigation Into Cancer and Nutrition (EPIC). Int J Cancer. 2003;107:629-634. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 158] [Cited by in F6Publishing: 159] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 35. | Lippert E, Endlicher E. Hyperglycemia: a risk factor for gastric cancer development? Z Gastroenterol. 2009;47:850-853. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 36. | Yamagata H, Kiyohara Y, Nakamura S, Kubo M, Tanizaki Y, Matsumoto T, Tanaka K, Kato I, Shirota T, Iida M. Impact of fasting plasma glucose levels on gastric cancer incidence in a general Japanese population: the Hisayama study. Diabetes Care. 2005;28:789-794. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 71] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 37. | Ikeda F, Doi Y, Yonemoto K, Ninomiya T, Kubo M, Shikata K, Hata J, Tanizaki Y, Matsumoto T, Iida M. Hyperglycemia increases risk of gastric cancer posed by Helicobacter pylori infection: a population-based cohort study. Gastroenterology. 2009;136:1234-1241. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 99] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 38. | Koppert LB, Janssen-Heijnen ML, Louwman MW, Lemmens VE, Wijnhoven BP, Tilanus HW, Coebergh JW. Comparison of comorbidity prevalence in oesophageal and gastric carcinoma patients: a population-based study. Eur J Gastroenterol Hepatol. 2004;16:681-688. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 30] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 39. | Stocks T, Rapp K, Bjørge T, Manjer J, Ulmer H, Selmer R, Lukanova A, Johansen D, Concin H, Tretli S. Blood glucose and risk of incident and fatal cancer in the metabolic syndrome and cancer project (me-can): analysis of six prospective cohorts. PLoS Med. 2009;6:e1000201. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 176] [Cited by in F6Publishing: 184] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 40. | Stattin P, Björ O, Ferrari P, Lukanova A, Lenner P, Lindahl B, Hallmans G, Kaaks R. Prospective study of hyperglycemia and cancer risk. Diabetes Care. 2007;30:561-567. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 246] [Cited by in F6Publishing: 253] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 41. | Shu X, Ji J, Li X, Sundquist J, Sundquist K, Hemminki K. Cancer risk among patients hospitalized for Type 1 diabetes mellitus: a population-based cohort study in Sweden. Diabet Med. 2010;27:791-797. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 87] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 42. | Ekbom A, McLaughlin JK, Karlsson BM, Nyrén O, Gridley G, Adami HO, Fraumeni JF. Pancreatitis and pancreatic cancer: a population-based study. J Natl Cancer Inst. 1994;86:625-627. [PubMed] [Cited in This Article: ] |
| 43. | Ragozzino M, Melton LJ, Chu CP, Palumbo PJ. Subsequent cancer risk in the incidence cohort of Rochester, Minnesota, residents with diabetes mellitus. J Chronic Dis. 1982;35:13-19. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 151] [Cited by in F6Publishing: 162] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 44. | Hjalgrim H, Frisch M, Ekbom A, Kyvik KO, Melbye M, Green A. Cancer and diabetes--a follow-up study of two population-based cohorts of diabetic patients. J Intern Med. 1997;241:471-475. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 106] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 45. | Bershteĭn LM, Merabishvili VM, Semenova NV, Karpova IA, Kovalevskiĭ AIu. Registry-based analysis of cancer and diabetes combination: prevalence and features. Vopr Onkol. 2007;53:285-290. [PubMed] [Cited in This Article: ] |
| 46. | Lee MS, Hsu CC, Wahlqvist ML, Tsai HN, Chang YH, Huang YC. Type 2 diabetes increases and metformin reduces total, colorectal, liver and pancreatic cancer incidences in Taiwanese: a representative population prospective cohort study of 800,000 individuals. BMC Cancer. 2011;11:20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 314] [Cited by in F6Publishing: 340] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 47. | Tseng CH. Diabetes conveys a higher risk of gastric cancer mortality despite an age-standardised decreasing trend in the general population in Taiwan. Gut. 2011;60:774-779. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 48. | Coughlin SS, Calle EE, Teras LR, Petrelli J, Thun MJ. Diabetes mellitus as a predictor of cancer mortality in a large cohort of US adults. Am J Epidemiol. 2004;159:1160-1167. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 49. | Batty GD, Shipley MJ, Marmot M, Smith GD. Diabetes status and post-load plasma glucose concentration in relation to site-specific cancer mortality: findings from the original Whitehall study. Cancer Causes Control. 2004;15:873-881. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 101] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 50. | Levine W, Dyer AR, Shekelle RB, Schoenberger JA, Stamler J. Post-load plasma glucose and cancer mortality in middle-aged men and women. 12-year follow-up findings of the Chicago Heart Association Detection Project in Industry. Am J Epidemiol. 1990;131:254-262. [PubMed] [Cited in This Article: ] |
| 51. | Smith GD, Egger M, Shipley MJ, Marmot MG. Post-challenge glucose concentration, impaired glucose tolerance, diabetes, and cancer mortality in men. Am J Epidemiol. 1992;136:1110-1114. [PubMed] [Cited in This Article: ] |
| 52. | Rubenstein JH, Davis J, Marrero JA, Inadomi JM. Relationship between diabetes mellitus and adenocarcinoma of the oesophagus and gastric cardia. Aliment Pharmacol Ther. 2005;22:267-271. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 53. | Atchison EA, Gridley G, Carreon JD, Leitzmann MF, McGlynn KA. Risk of cancer in a large cohort of U.S. veterans with diabetes. Int J Cancer. 2011;128:635-643. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 166] [Cited by in F6Publishing: 181] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 54. | Chodick G, Heymann AD, Rosenmann L, Green MS, Flash S, Porath A, Kokia E, Shalev V. Diabetes and risk of incident cancer: a large population-based cohort study in Israel. Cancer Causes Control. 2010;21:879-887. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 90] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 55. | Ogunleye AA, Ogston SA, Morris AD, Evans JM. A cohort study of the risk of cancer associated with type 2 diabetes. Br J Cancer. 2009;101:1199-1201. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 110] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 56. | Rousseau MC, Parent ME, Pollak MN, Siemiatycki J. Diabetes mellitus and cancer risk in a population-based case-control study among men from Montreal, Canada. Int J Cancer. 2006;118:2105-2109. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 119] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 57. | Jun JK, Gwack J, Park SK, Choi YH, Kim Y, Shin A, Chang SH, Shin HR, Yoo KY. Fasting serum glucose level and gastric cancer risk in a nested case-control study. J Prev Med Public Health. 2006;39:493-498. [PubMed] [Cited in This Article: ] |
| 58. | Giovannucci E. Insulin, insulin-like growth factors and colon cancer: a review of the evidence. J Nutr. 2001;131:3109S-3120S. [PubMed] [Cited in This Article: ] |
| 59. | Ma J, Pollak MN, Giovannucci E, Chan JM, Tao Y, Hennekens CH, Stampfer MJ. Prospective study of colorectal cancer risk in men and plasma levels of insulin-like growth factor (IGF)-I and IGF-binding protein-3. J Natl Cancer Inst. 1999;91:620-625. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 757] [Cited by in F6Publishing: 718] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 60. | Thompson MA, Cox AJ, Whitehead RH, Jonas HA. Autocrine regulation of human tumor cell proliferation by insulin-like growth factor II: an in-vitro model. Endocrinology. 1990;126:3033-3042. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 41] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 61. | Pavelić K, Kolak T, Kapitanović S, Radosević S, Spaventi S, Kruslin B, Pavelić J. Gastric cancer: the role of insulin-like growth factor 2 (IGF 2) and its receptors (IGF 1R and M6-P/IGF 2R). J Pathol. 2003;201:430-438. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 57] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 62. | Mahdy RA, Nada WM. Evaluation of the role of vascular endothelial growth factor in diabetic retinopathy. Ophthalmic Res. 2011;45:87-91. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 63. | Tanigawa N, Amaya H, Matsumura M, Shimomatsuya T. Correlation between expression of vascular endothelial growth factor and tumor vascularity, and patient outcome in human gastric carcinoma. J Clin Oncol. 1997;15:826-832. [PubMed] [Cited in This Article: ] |
| 64. | Maeda K, Chung YS, Ogawa Y, Takatsuka S, Kang SM, Ogawa M, Sawada T, Sowa M. Prognostic value of vascular endothelial growth factor expression in gastric carcinoma. Cancer. 1996;77:858-863. [PubMed] [Cited in This Article: ] |
| 65. | Yonemura Y, Endo Y, Fujita H, Fushida S, Ninomiya I, Bandou E, Taniguchi K, Miwa K, Ohoyama S, Sugiyama K. Role of vascular endothelial growth factor C expression in the development of lymph node metastasis in gastric cancer. Clin Cancer Res. 1999;5:1823-1829. [PubMed] [Cited in This Article: ] |
| 66. | Warren RS, Yuan H, Matli MR, Ferrara N, Donner DB. Induction of vascular endothelial growth factor by insulin-like growth factor 1 in colorectal carcinoma. J Biol Chem. 1996;271:29483-29488. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 180] [Cited by in F6Publishing: 188] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 67. | Akagi Y, Liu W, Zebrowski B, Xie K, Ellis LM. Regulation of vascular endothelial growth factor expression in human colon cancer by insulin-like growth factor-I. Cancer Res. 1998;58:4008-4014. [PubMed] [Cited in This Article: ] |
| 68. | Dandona P, Thusu K, Cook S, Snyder B, Makowski J, Armstrong D, Nicotera T. Oxidative damage to DNA in diabetes mellitus. Lancet. 1996;347:444-445. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 564] [Cited by in F6Publishing: 541] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 69. | Lorenzi M, Montisano DF, Toledo S, Barrieux A. High glucose induces DNA damage in cultured human endothelial cells. J Clin Invest. 1986;77:322-325. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 180] [Cited by in F6Publishing: 197] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 70. | González CA, Megraud F, Buissonniere A, Lujan Barroso L, Agudo A, Duell EJ, Boutron-Ruault MC, Clavel-Chapelon F, Palli D, Krogh V. Helicobacter pylori infection assessed by ELISA and by immunoblot and noncardia gastric cancer risk in a prospective study: the Eurgast-EPIC project. Ann Oncol. 2012;23:1320-1324. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 82] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 71. | Kamangar F, Dawsey SM, Blaser MJ, Perez-Perez GI, Pietinen P, Newschaffer CJ, Abnet CC, Albanes D, Virtamo J, Taylor PR. Opposing risks of gastric cardia and noncardia gastric adenocarcinomas associated with Helicobacter pylori seropositivity. J Natl Cancer Inst. 2006;98:1445-1452. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 221] [Cited by in F6Publishing: 239] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 72. | Lanzino M, Morelli C, Garofalo C, Panno ML, Mauro L, Andò S, Sisci D. Interaction between estrogen receptor alpha and insulin/IGF signaling in breast cancer. Curr Cancer Drug Targets. 2008;8:597-610. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 64] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 73. | Matsuyama S, Ohkura Y, Eguchi H, Kobayashi Y, Akagi K, Uchida K, Nakachi K, Gustafsson JA, Hayashi S. Estrogen receptor beta is expressed in human stomach adenocarcinoma. J Cancer Res Clin Oncol. 2002;128:319-324. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 87] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 74. | Enmark E, Pelto-Huikko M, Grandien K, Lagercrantz S, Lagercrantz J, Fried G, Nordenskjöld M, Gustafsson JA. Human estrogen receptor beta-gene structure, chromosomal localization, and expression pattern. J Clin Endocrinol Metab. 1997;82:4258-4265. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 303] [Cited by in F6Publishing: 315] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 75. | Currie CJ, Poole CD, Gale EA. The influence of glucose-lowering therapies on cancer risk in type 2 diabetes. Diabetologia. 2009;52:1766-1777. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 859] [Cited by in F6Publishing: 826] [Article Influence: 55.1] [Reference Citation Analysis (1)] |
| 76. | Yang P, Zhou Y, Chen B, Wan HW, Jia GQ, Bai HL, Wu XT. Aspirin use and the risk of gastric cancer: a meta-analysis. Dig Dis Sci. 2010;55:1533-1539. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 77. | Tian W, Zhao Y, Liu S, Li X. Meta-analysis on the relationship between nonsteroidal anti-inflammatory drug use and gastric cancer. Eur J Cancer Prev. 2010;19:288-298. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 78. | Kuoppala J, Lamminpää A, Pukkala E. Statins and cancer: A systematic review and meta-analysis. Eur J Cancer. 2008;44:2122-2132. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 207] [Cited by in F6Publishing: 221] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 79. | Fitzgerald JT, Anderson RM, Davis WK. Gender differences in diabetes attitudes and adherence. Diabetes Educ. 1995;21:523-529. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 50] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 80. | Franzini L, Ardigò D, Cavalot F, Miccoli R, Rivellese AA, Trovati M, Zavaroni I, Vaccaro O. Women show worse control of type 2 diabetes and cardiovascular disease risk factors than men: Results from the MIND.IT Study Group of the Italian Society of Diabetology. Nutr Metab Cardiovasc Dis. 2012;Mar 5; Epub ahead of print. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 81. | Fu AZ, Zhang Q, Davies MJ, Pentakota SR, Radican L, Seck T. Underutilization of statins in patients with type 2 diabetes in US clinical practice: a retrospective cohort study. Curr Med Res Opin. 2011;27:1035-1040. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 82. | Huang XE, Tajima K, Hamajima N, Xiang J, Inoue M, Hirose K, Tominaga S, Takezaki T, Kuroishi T, Tokudome S. Comparison of lifestyle and risk factors among Japanese with and without gastric cancer family history. Int J Cancer. 2000;86:421-424. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 83. | Lee JK, Park BJ, Yoo KY, Ahn YO. Dietary factors and stomach cancer: a case-control study in Korea. Int J Epidemiol. 1995;24:33-41. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 110] [Cited by in F6Publishing: 110] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 84. | Brenner H, Rothenbacher D, Arndt V. Epidemiology of stomach cancer. Methods Mol Biol. 2009;472:467-477. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 374] [Cited by in F6Publishing: 395] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 85. | Liu C, Russell RM. Nutrition and gastric cancer risk: an update. Nutr Rev. 2008;66:237-249. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 104] [Article Influence: 6.5] [Reference Citation Analysis (0)] |