Copyright
©2013 Baishideng Publishing Group Co.
World J Gastroenterol. Dec 14, 2013; 19(46): 8531-8542
Published online Dec 14, 2013. doi: 10.3748/wjg.v19.i46.8531
Published online Dec 14, 2013. doi: 10.3748/wjg.v19.i46.8531
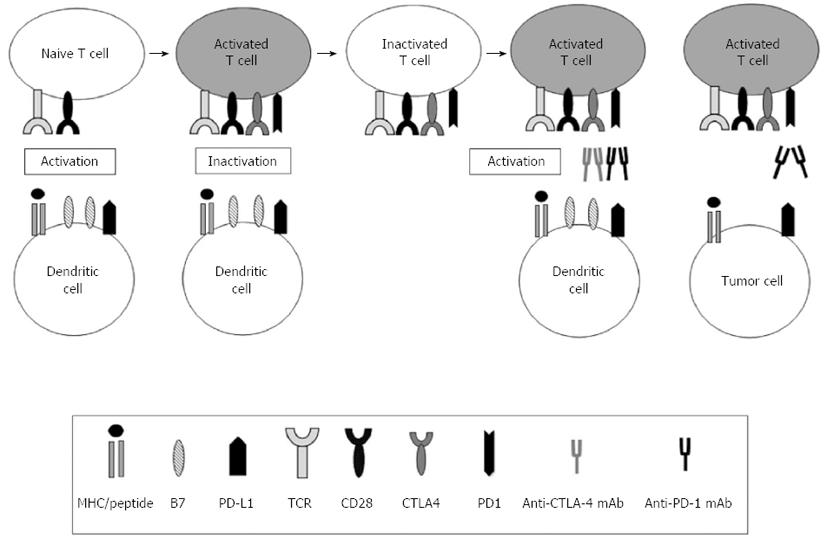
Figure 3 Immune regulatory checkpoints in cancer immunotherapy.
Cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and programmed cell death protein 1 (PD1) are two well-described co-inhibitory molecules that are expressed on naïve or memory T cells and decrease antitumor immune responses. The CTLA-4-mediated immune checkpoint is induced in T cells at the initial response to antigen (early activation phase). After the T cell receptor (TCR) is triggered by antigen encounter, CTLA-4 is transported to the surface of naïve or memory T cells. In contrast, the major role of the PD1 pathway is not at the initial T cell activation stage but rather the regulation of inflammatory responses by effector T cells that recognize antigen in peripheral tissue cells. Thus, PD-1 is highly expressed by antigen-specific cytotoxic T lymphocytes (CTLs) in malignancies and is associated with impaired T-cell function. The best-characterized signal for PD1 ligand 1 (PD-L1) induction is interferon-γ (IFN-γ), which is predominantly produced by Th1 cells. Although PD-L2 expression is limited to dendritic cells (DCs) and macrophages, PD-L1 is broadly expressed in tissues and is considered a molecular shield that protects cells from auto-reactive attack. In some tumors, PDL1 is not constitutively expressed but is induced in response to inflammatory signals that are produced by an active antitumor immune response. Loading DCs with soluble PD1 decreases their function. Therefore, antibodies can be used to block inhibitory ligand:receptor interactions by acting on tumor cells, DCs (e.g., anti-PD-L1) or T cells (e.g., anti-CTLA-4 or anti-PD1). Combining the blockade of multiple inhibitory pathways synergistically decreases T cell anergy and improves T cell responsiveness against tumors.
- Citation: Koido S, Ohkusa T, Homma S, Namiki Y, Takakura K, Saito K, Ito Z, Kobayashi H, Kajihara M, Uchiyama K, Arihiro S, Arakawa H, Okamoto M, Gong J, Tajiri H. Immunotherapy for colorectal cancer. World J Gastroenterol 2013; 19(46): 8531-8542
- URL: https://www.wjgnet.com/1007-9327/full/v19/i46/8531.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i46.8531