Copyright
©2013 Baishideng Publishing Group Co.
World J Gastroenterol. Oct 28, 2013; 19(40): 6849-6856
Published online Oct 28, 2013. doi: 10.3748/wjg.v19.i40.6849
Published online Oct 28, 2013. doi: 10.3748/wjg.v19.i40.6849
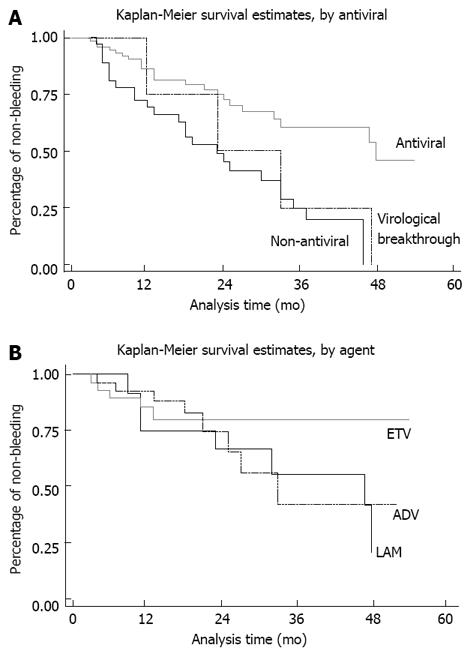
Figure 1 Kaplan-Meier analysis of non-bleeding duration.
A: Non-bleeding duration in antiviral and control cases was compared using the Kaplan-Meier survival model. Bleeding was defined as a failed event. The occurrence of bleeding was reduced and delayed in the antiviral treatment group compared to the control group. The curve of virological breakthrough cases (dashed line) was close to that of the control group, demonstrating reduced efficacy when virological breakthrough occurred; B: Non-bleeding durations for entecavir (ETV), adefovir (ADV) and lamivudine (LAM) therapy were compared using the Kaplan-Meier survival model. Bleeding was defined as a failed event. The non-bleeding durations for ETV, ADV and LAM were similar in the first 2 years, however, differences became clear following long-term treatment, which may have been due to cumulative resistance and bleeding.
- Citation: Li CZ, Cheng LF, Li QS, Wang ZQ, Yan JH. Antiviral therapy delays esophageal variceal bleeding in hepatitis B virus-related cirrhosis. World J Gastroenterol 2013; 19(40): 6849-6856
- URL: https://www.wjgnet.com/1007-9327/full/v19/i40/6849.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i40.6849